Abstract
Electrophysiological, morphological, and biochemical approaches were combined to study the effect of the presynaptic injection of the light chain of botulinum toxin C1 into the squid giant synapse. Presynaptic injection was accompanied by synaptic block that occurred progressively as the toxin filled the presynaptic terminal. Neither the presynaptic action potential nor the Ca2+ currents in the presynaptic terminal were affected by the toxin. Biochemical analysis of syntaxin moiety in squid indicates that the light chain of botulinum toxin C1 lyses syntaxin in vitro, suggesting that this was the mechanism responsible for synaptic block. Ultrastructure of the injected synapses demonstrates an enormous increase in the number of presynaptic vesicles, suggesting that the release rather than the docking of vesicles is affected by biochemical lysing of the syntaxin molecule.
Keywords: exocytosis, botulinum toxin, synaptic vesicles
Calcium-triggered fusion of synaptic vesicles with the presynaptic membrane is one of the most specialized features of neurons and is an essential step for chemical neurotransmission. Molecular mechanisms underlying this process have recently been described (1). The formation of the complex composed of the plasma membrane proteins syntaxin 1, synaptosomal-associated protein of 25 kDa (SNAP-25), and vesicle-associated membrane protein synaptobrevin constitutes a key step in the pathway to exocytosis (2). Indeed, experimental evidence for an essential role of this complex came from the identification of these proteins as substrates of neurotransmission-blocking clostridium neurotoxins (3). Furthermore, cleavage of syntaxin 1 by botulinum toxin C1 (BoNT/C1) impairs synaptic vesicle exocytosis and blocks neurotransmitter release (4). Syntaxin 1 is present at the presynaptic active zone and is mostly associated with calcium channel proteins, in particular N (5, 6) and P/Q (7, 8) type calcium channels, and with a possible calcium sensor, synaptotagmin, in a calcium-dependent manner. Molecular studies on syntaxin 1 revealed two isoforms of this protein in mammalian neuronal tissues, syntaxin 1A and syntaxin 1B, and both isoforms are cleaved by BoNT/C1 (3, 9). The squid Loligo pelaii expresses a presynaptic protein similar to mammalian syntaxin 1 (10). The protein was isolated from squid optic lobes, cloned, and sequenced, and a polyclonal anti-Loligo syntaxin antibody was generated that demonstrated positive immunoblot reaction against the squid syntaxin (10). Presynaptic injection of anti-Loligo syntaxin IgG into the squid giant synapse results in transmitter release block (10). The dynamics of this block were of the type expected with vesicular fusion impairment (11), rather than with a reduction of vesicular availability (12, 13). In the present study, we injected presynaptically the light chain of BoNT/C1 (BoNT/C1-LC), known to lyse the mammalian syntaxins 1A and 1B. Presynaptic BoNT/C1-LC injections were combined with electrophysiological, neurochemical, and ultrastructural studies to determine the effect of such toxin on transmitter release and on presynaptic morphology. We show herein, by using the immunoblot technique, that BoNT/C1-LC lyses the squid syntaxin protein. In addition, ultrastructural findings indicate that after injection of BoNT/C1, vesicular content of the presynaptic terminal increased by almost 3-fold.
MATERIALS AND METHODS
Purification of BTXc-LC.
A pQE3 (Qiagen, Chatsworth, CA) derived expression vector containing the cDNA for BoNT/C1-LC was provided by H. Niemann (Tubingen, Germany). Escherichia coli M15 cells were induced to express the (His)6 fusion protein by addition of isopropyl β-d-thiogalactoside (2 mM, final concentration). Cells were pelleted after 4 hr, resuspended in 50 mM NaH2PO4/300 mM NaCl, pH 8, and sonicated on ice for two 60-sec periods with a probe sonicator. The suspension was centrifuged at 15,000 × g for 20 min, and the clear supernatant was applied to a Ni-NTA-agarose precasted column (Qiagen, Chatsworth, CA). Protein purification was carried out by following the manufacturer’s instruction. Briefly, after a washing step with 50 mM NaH2PO4/300 mM CaCl2/10% glycerol, pH 6, the attached protein was eluted with a 50–300 mM imidazole gradient. One-milliliter fractions were collected and analyzed by SDS/PAGE followed by staining with Coomassie blue. Those fractions containing purified BoNT/C1-LC were concentrated by centrifugation using Ultrafree-MC filtration tubes (Millipore; molecular weight cutoff, 10,000) and stored at −80°C in aliquots.
Electrophysiological Methods.
As in previous studies, the experiments were performed in squid giant synapse at the stellate ganglion. The techniques for dissection and injection were the same as described (14). BoNT/C1-LC was coinjected with dextran-coupled to Texas red, a fluorescent tag. Because BoNT/C1-LC and dextran have similar molecular size, the degree of diffusion into the terminal was continuously monitored by fluorescence imaging, which is a good indicator of the location and quantity of material injected, as well as its mobility into the presynaptic terminal. The electrophysiological methodology included pre- and postsynaptic intracellular recording. In a number of experiments, the presynaptic terminal was voltage-clamped to ascertain whether calcium current was modified by the BoNT/C1-LC.
Generation of Antibodies Against Syntaxin 1.
Antibodies were raised in rabbits against (His)6-syntaxin 1A and (His)6-syntaxin 1B fusion proteins derived from their corresponding cDNA from rat origin. To generate isoform-specific antibodies, we immunized two sets of rabbits with each fusion protein and carried out a purification protocol from crude antisera in which several antibody preparations were obtained (15). For the present study, we checked all those preparations and crude antisera for the detection of a squid syntaxin 1 homolog in Western blot experiments with optic lobe membranes. When these antibodies were used to analyze samples from squid, the crude antiserum was able to detect squid syntaxin 1, and so all Western blot analyses were performed with crude antiserum against rat syntaxin 1 (either 1A or 1B).
Tissue Preparation and Western Blotting.
Squid optic lobes were dissected and homogenized in 20 mM Hepes, pH 7.0/140 mM NaCl/0.05% Triton X-100. Nuclei were removed by centrifugation at 3,000 × g for 10 min at 4°C. The supernatant was subsequently centrifuged at 12,000 × g for 30 min at 4°C to obtain crude membranes that were used to assess the effect of BoNT/C1-LC on syntaxin. SDS/PAGE in 10% gels was carried out as described (16) and proteins were electrotransferred to Immobilon-P membranes (Millipore). Membranes were first soaked in a blocking solution (5% nonfatty milk powder/50 mM Tris⋅HCl, pH 7.4/140 mM NaCl/0.1% Tween-20) for 1 hr at room temperature and were then incubated with peroxidase-conjugated secondary immunoglobulins for 1 hr. After rinsing, peroxidase activity was visualized by using Enhanced Chemiluminescence (ECL; Amersham).
In Vitro Assays of BoNT/C1-LC Activity.
Crude membranes from optic lobes were incubated either in the presence of or in the absence of recombinant BoNT/C1-LC for 90 min at 37°C. The final composition of this reaction medium (16 μl) was 30 μg of protein from squid samples, 20 mM Hepes (pH 7.0), 140 mM NaCl, 0.05% Triton X-100, and 10 mM DTT, with or without the toxin. Samples from rat brain homogenates were used as a control of effective cleavage of syntaxin 1 by the toxin. After this incubation step with or without BoNT/C1-LC, samples from squid or rat brain were supplemented with sample buffer (62.5 mM Tris⋅HC1, pH 6.8/10% glycerol/2% SDS/5% 2-mercaptoethanol/0.1% bromophenol blue) and boiled for 3 min, and proteins were resolved in 10% SDS/PAGE gels. Syntaxin 1 was revealed by Western blotting as described before. These determinations were done twice with samples from three different homogenates of optic lobes.
Ultrastructural Methodologies.
The techniques used have been described (17). The squid stellate ganglion was taken from the recording chamber, fixed in glutaraldehyde (immersion), post-fixed in osmium tetroxide, stained in block with uranium acetate, dehydrated, and embedded in Araldite plastic (CY212). Ultrathin sections were collected on Pyoloform and carbon-coated single-sloth grids and contrasted with uranyl acetate and lead citrate. Morphometry and quantitative analysis of the synaptic vesicles were performed (17) with a Zidas digitizing system (Zeiss) interfaced with a Macintosh II computer. Electron micrographs were taken at an initial magnification of ×14,000 and photographically enlarged to a magnification of ×35,000. Vesicle density at the synaptic active zones was determined as the number of vesicles per section. Processing of the data was performed by using lotus 1-2-3 software for Macintosh.
RESULTS
Electrophysiology.
Electrophysiological results were obtained from the squid giant synapse. Initial experiments consisted of the injection of BoNT/C1-LC in the presynaptic terminal and the testing of transmitter release by direct current stimulation of the presynaptic terminal. Control transmission was tested for 15 min prior to the injection of toxin. After presynpatic injection, transmission was continuously tested for a period of up to 50 min, at which point if no change in the transmission properties were observed, it was concluded that the injected material had no blocking effect. As in previous experiments (18), the speed with which the block of synaptic transmission occurred was related to the distance between the site of injection of the toxin and the site for transmitter release at the terminal digit of the largest of the synapses. Stimuli repeated at 1 min showed a marked decrease of the postsynaptic response with nearly total failure of transmitter release approximately 22 min after injection of BoNT/C1-LC (see Fig. 2A).
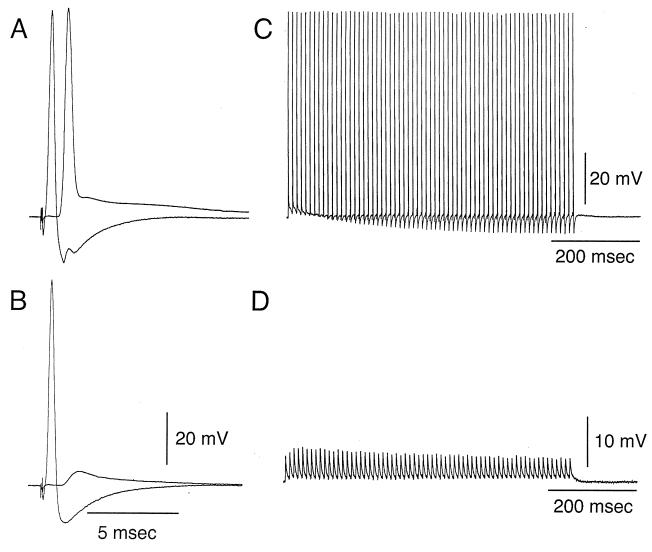
Figure 2.
Transmitter release block as determined by tetanic presynaptic stimulation. (A and B) Presynaptic and postsynaptic response to electrical stimulation of the presynaptic axon. (A) Control. (B) Reduction of postsynaptic response 35 min after presynaptic injection. (C and D) Postsynaptic response to tetanic presynaptic stimulation at 100 Hz. (C) Repetitive postsynaptic response prior to microinjection. (D) Reduction of tetanic postsynaptic response, shown at higher gain (see voltage calibration) 35 min after BoNT/C1-LC microinjection. Note that the amplitude for the postsynaptic potential is maintained close to constant throughout the duration of the tetanus.
A similar set of experiments was performed in eight other synapses in which synaptic transmission was blocked, indicating that in no case injection of BoNT/C1-LC failed to block transmitter release. To determine the kinetics of such block, repetitive stimuli were given in a set of experiments at frequencies of 200 stimuli per second (see Fig. 2B), and tetanic stimulation was performed immediately prior to injection and at different intervals of time after the injection was implemented. As tetanic stimulation was presented at increasing times after injection the amplitude of the postsynaptic response was gradually reduced as shown in Fig. 1 C and D. This gradual reduction was quite similar to that observed after the injection of antibody against syntaxin (10) and with the injection of either a high concentration of inositol phosphate compounds (11) or with the antibody against C2A domain of synaptotagmin (17). The results, therefore, suggest that the type of block resulting from BoNT/C1-LC is more a fusion block rather than a docking block. In the latter case, the reduction of transmitter release occurs abruptly during the tetanic stimulation period.
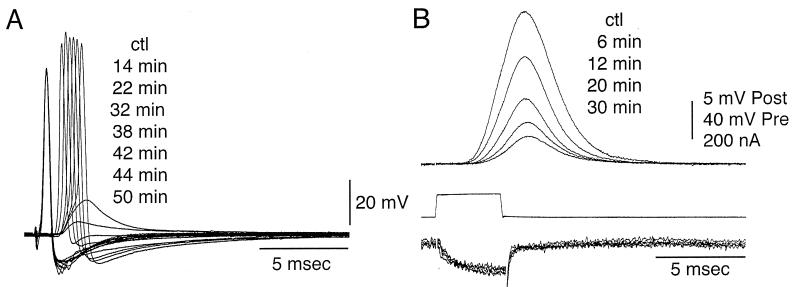
Figure 1.
Synaptic transmission block by preterminal microinjection of BoNT/C1-LC. (A) Blocking of transmitter release as determined by the reduction of the postsynaptic activation (post) with time reported in minutes after injection (numbers to the right starting with the control level, ctl). The presynaptic action potential (pre) was unchanged by the injection. (B) Presynaptic voltage-clamp step (middle trace), presynaptic calcium current (bottom trace), and simultaneously recorded postsynaptic response (top traces) at different times after presynaptic BoNT/C1-LC microinjection. Time, in minutes, after microinjection is on the right (ctl). Note the lack of change in the calcium current.
Effect of BoNT/C1-LC on Presynaptic Calcium Currents.
To assess whether the microinjected BoNT/C1-LC blocking effect is related to a possible regulation of the presynaptic calcium current, a set of experiments (n = 3) was performed to test this possibility directly. This prospect was particularly significant because it has been proposed that syntaxin may regulate the voltage dependence of voltage-gated calcium channels (19). The presynaptic calcium current was directly determined by using a voltage-clamp approach, similar to that used in previous experiments. The presynaptic calcium current and the postsynaptic response were simultaneously recorded at different times after BoNT/C1-LC microinjection (see Fig. 3A). The time to 90% block of the postsynaptic response (Fig. 2B, upper traces) was 46 min, very much in keeping with the site of presynaptic injection at the most proximal end of the preterminal digit. The lack of reduction of calcium current (Fig. 2B, lower trace) indicate that transmitter release was blocked without affecting the presynaptic calcium current.
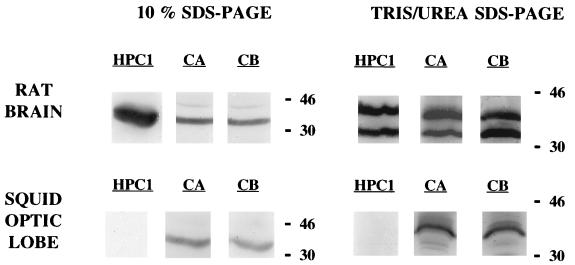
Figure 3.
Western blot detection of syntaxin from rat brain and squid optic lobe homogenates. Two electrophoresis procedures were performed. Syntaxin was detected with three different antibodies: HPC1, monoclonal antibody raised against rat syntaxin 1; CA and CB, crude antisera from rabbits immunized with the rat cDNA fusion proteins (His)6-syntaxin 1A and (His)6-syntaxin 1B, respectively. Total amount of protein loaded was 5 μg for rat brain homogenates and 30 μg for squid optic lobe homogenates.
BoNT/C1-LC Action on Syntaxin in Vitro.
To ascertain that injection of the recombinant BoNT/C1-LC to giant squid synapse was affecting neurotransmitter release by mechanisms similar to that proposed in mammals, that is, through the cleavage of syntaxin, we carried out a set of experiments to determine the effect of BoNT/C1-LC on syntaxin in vitro. Previous Western blot experiments had shown that the antibodies raised against rat syntaxin 1 recognized a single band of 35 kDa in samples from squid optic lobes. This is indeed the molecular weight of rat syntaxin 1 (Fig. 3). It is noteworthy that in Western blots carried out with squid samples resolved by Tris/urea PAGE (18% acrylamide/6 M urea/750 mM Tris⋅HCl, pH 8.85/0.1% SDS) (20), a single band of around 42 kDa was revealed. This technique enabled effective separation of the two syntaxin 1 isoforms of rat, syntaxin 1A and syntaxin 1B, that otherwise appear as a single band of 35 kDa in 10% SDS/PAGE gels. With the former technique these isoforms were detected as distinct bands of 35 and 42 kDa, respectively. In vitro cleavage assays were determined by SDS/PAGE in 10% gels, followed by Western blot with anti-rat syntaxin antibodies. As shown in Fig. 4, syntaxin from squid samples was cleaved by BoNT/C1-LC. BoNT/C1-LC action on rat syntaxin rendered a small fragment of around 25 kDa.
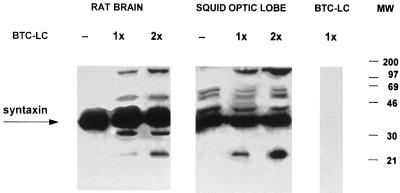
Figure 4.
Syntaxin cleavage by BoNT/C1-LC. In vitro cleavage of syntaxin from rat brain (5 μg of protein) and squid optic lobe homogenates (30 μg of protein), by recombinant BoNT/C1-LC. Two final concentrations of recombinant toxin were tested (1× and 2×). Note the sets of extra bands after the addition of toxin. (A) Rat brain immunoblots. (B) Squid optic lobe immunoblots.
To establish whether BoNT/C1 injection was affecting transmitter release by mechanisms similar to those proposed for synaptic transmission in vitro, protein was obtained from optic lobes from Loligo pealii and blotted (Fig. 3). A Western blot study of protein obtained from such lobes indicated the presence of a band with a molecular weight similar to that found in mouse that could bind syntaxin-specific antibody (Fig. 3 Left). As shown in Fig. 3 (Right), such a band demonstrated about the same amount of lysis as the mouse syntaxin in the presence of BoNT/C1-LC, indicating that this toxin has a similar lysing in these two bands, consistent with the idea that the toxin acts similarly in the syntaxin of mammalian and molluskan origin. Both in squid and rat brain samples the effect of BoNT/C1-LC was not complete because a certain amount of intact syntaxin (35 kDa) was also detected.
Morphological Studies.
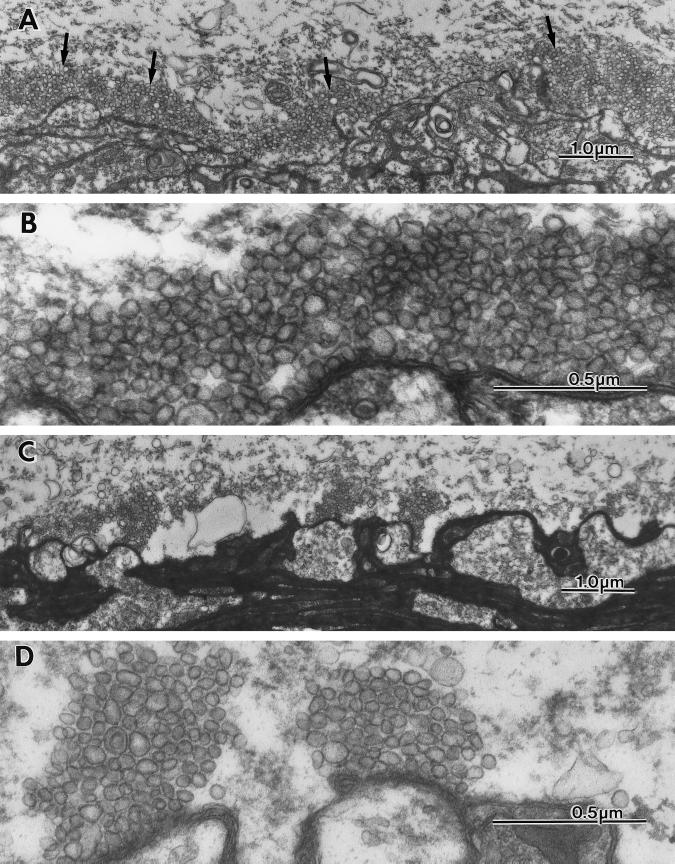
After injection of the presynaptic terminal and total block of presynaptic release, an ultrastructural study was implemented. The results are illustrated in Fig. 5A. In this figure, the typical profile of the squid giant synapse is shown with the presynaptic terminal, demonstrating areas of vesicular confluence immediately in front of the postsynaptic spines that constitute the presynaptic active zone. As in previous studies, the control level for synaptic vesicles is of the order of 200 per active zone, as observed in the plane of section. In four synapses where the ultrastructural results were obtained, a substantial increase in vesicular numbers was observed. Fig. 5 C and D gives a direct picture of such increased at two magnifications. The number of vesicles under this condition was increased manyfold. In fact, the increase is much larger than that observed by any of the other procedures that are known to block synaptic release by simply interfering with vesicular fusion.
Figure 5.
Ultrastructural images of the presynaptic terminal following BoNTC1-LC microinjection. (A and B) Low and high magnification ultrastructural micrographs of the giant synapse showing presynaptic and postsynaptic elements and their spatial relation at the active zones after BoNTC1-LC microinjection. (A) Large numbers of vesicles cluster around the active zones. (B) Higher magnification of the same synapse to illustrate the large number and density of the synaptic vesicles abutting the active zone region. (C and D) Micrographs of a control synapse taken at similar magnification to illustrate the difference in vesicular distribution and number.
DISCUSSION
The present set of results indicate that synaptic transmission block, after BoNT/C1-LC presynaptic microinjection, was not due to change in the presynaptic action potential or the presynaptic calcium current. Furthermore, presynaptic calcium channels are down-regulated by second messenger pathways involving G proteins, and the effects of the G protein are virtually eliminated if syntaxin is first cleaved with BoNT/C1 (21). We conclude, therefore, that this toxin does not affect the ionic mechanisms responsible for spike generation or transmitter release. However, the reduction of transmitter release observed could be consistent with either a reduction in transmitter availability or a defect in the exocytosis step leading to release. To assess this point, tetanic stimulation of the preterminal axon (100 Hz) was implemented. The results clearly demonstrated that after BoNT/C1-LC injection, a gradual reduction of transmitter release occurs, rather than the rapid decrease observed when a depletion of vesicles or vesicular caging is responsible for the transmission block.
On the other hand, as the immunoblot studies indicate that this L chain toxin lyses syntaxin, the findings are consistent with BoNT/C1-LC acting on a syntaxin molecule highly homologous to mouse and rat syntaxin 1A (70% identity) with the presence of an identical amino acid sequence in the cleavage site (9, 10). Cleavage-resulting fragments appearing from rat and squid samples show a major difference: while a fragment of about 33 kDa adjacent to the remnant syntaxin is clearly visible in rat samples, it is not detected in squid optic lobe. Such differences may be due to further proteolysis of that fragment in the squid samples (22). BoNT/C1 causes also a marked loss of the carboxyl terminus of SNAP-25 when the toxin is added to living cultures, whereas it has no action on SNAP-25 in in vitro preparations (23). We conclude that such injection blocks transmitter release by interfering with the integrity of syntaxin and, thus, that syntaxin must be involved with synaptobrevin, SNAP-25, and synaptotagmin (1, 7, 17, 24, 25) in the docking and/or fusion steps of transmitter release in agreement with recent findings in this terminal after presynaptic anti-Loligo syntaxin IgG microinjection (10). The ultrastructural results are also in agreement with a compromise of the release event because synaptic vesicles accumulate in great numbers after BoNT/C1 injection. Interestingly, however, in addition to the large number of synaptic vesicles encountered in the vicinity of the active zone and in the cytoplasm, a very large number of coated vesicles were also observed. This suggests that in addition to a reduction in the amount of transmitter release, BoNT/C1 vesicles may have a proteolytic action on vesicular proteins required for active retrograde vesicles translocation (26), resulting in the large increase of vesicular profiles at the preterminal axon.
Acknowledgments
This work was supported by CICYT (Ministerio de Educaci-n y Ciencia, Spain) to J.M. and J.B., CIRIT (Generalitat de Catalunya) to J.M., and NS13742 to R.L.
ABBREVIATIONS
- BoNT/C1
botulinum toxin C1
- BoNT/C1-LC
light chain of BoNT/C1
- SNAP-25
synaptosomal-associated protein of 25 kDa
References
- 1.Südhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 2.Söllner T, Bennet M K, Whiteheart S V, Scheller R H, Rothman J E. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 3.Niemann H, Blasi J, Jahn R. Trends Cell Biol. 1994;4:179–185. doi: 10.1016/0962-8924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 4.Blasi J, Chapman E R, Yamasaki S, Binz T, Niemann H, Jahn R. EMBO J. 1993;12:4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida A, Oho C, Omori A, Kawahara R, Ito T, Takahashi M. J Biol Chem. 1992;269:24925–24928. [PubMed] [Google Scholar]
- 6.Sheng Z H, Rettig J, Cook T, Catterall W A. Nature (London) 1996;379:451–454. doi: 10.1038/379451a0. [DOI] [PubMed] [Google Scholar]
- 7.Rettig J, Sheng Z H, Kyle Kim D, Hodson C D, Snutch T P, Catterall W A. Proc Natl Acad Sci USA. 1996;93:7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin-Moutot N, Charvin N, Leveque C, Sato K, Nishiki T, Kozaki S, Takahashi M, Seagar M. J Biol Chem. 1996;271:6567–6570. doi: 10.1074/jbc.271.12.6567. [DOI] [PubMed] [Google Scholar]
- 9.Schiavo G, Shone C C, Bennet M K, Scheller R H, Montecucco C. J Biol Chem. 1995;270:10566–10570. doi: 10.1074/jbc.270.18.10566. [DOI] [PubMed] [Google Scholar]
- 10.Sugimori, M., Fukuda, M., Moreira, J. E., Kojima, T., Mikoshiba, K. & Llinás, R. (1997) Neuroscience, in press. [DOI] [PubMed]
- 11.Llinás R, Sugimori M, Lang E J, Morita M, Fukuda M, Niinobe M, Mikoshiba K. Proc Natl Acad Sci USA. 1994;91:12990–12993. doi: 10.1073/pnas.91.26.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llinás R, Sugimori M, Chu D, Morita M, Blasi J, Herreros J, Jahn R, Marsal J. J Physiol (London) 1994;477:129–133. doi: 10.1113/jphysiol.1994.sp020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda M, Moreira J E, Lewis F M T, Sugimori M, Niinobe, Mikoshiba K, Llinás L. Proc Natl Acad Sci USA. 1995;92:10708–10712. doi: 10.1073/pnas.92.23.10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llinás R, Steinberg I Z, Walton K. Biophys J. 1981;33:289–322. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiz-Montasell B, Aguado F, Chapman E R, Canals J M, Marsal J, Blasi J. Eur J Neurosci. 1996;8:2544–2552. doi: 10.1111/j.1460-9568.1996.tb01548.x. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Nature (London) 1970;227:280. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Mikoshiba K, Fukuda M, Moreira J E, Lewis F M T, Sugimori M, Niinobe M, Llinás R. Proc Natl Acad Sci USA. 1995;92:10703–10707. doi: 10.1073/pnas.92.23.10703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llinás R, Sugimori M, Lang E J, Morita M, Fukuda M, Niinobe M, Mikoshiba K. Proc Natl Acad Sci USA. 1994;91:12990–12993. doi: 10.1073/pnas.91.26.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moshida S, Sheng Z H, Baker C, Kobayashi H, Catterall W A. Neuron. 1996;17:781–788. doi: 10.1016/s0896-6273(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 20.Schlensted G, Gudmundsson G H, Boman H G, Zimmermann R. J Biol Chem. 1990;265:13960–13968. [PubMed] [Google Scholar]
- 21.Stanley E F, Mirotznik R R. Nature (London) 1997;385:340–343. doi: 10.1038/385340a0. [DOI] [PubMed] [Google Scholar]
- 22.Link E, Edelmann L, Chou J H, Binz T, Yamasaki S, Eisel U, Baumert M, Südhof T C, Niemann H, Jahn R. Biochem Biophys Res Commun. 1992;189:1017–1023. doi: 10.1016/0006-291x(92)92305-h. [DOI] [PubMed] [Google Scholar]
- 23.Williamson L C, Halpern J L, Montecucco C, Brown J E, Neale E A. J Biol Chem. 1996;271:7694–7699. doi: 10.1074/jbc.271.13.7694. [DOI] [PubMed] [Google Scholar]
- 24.El Far O, Charvin N, Leveque C, Martin-Moutot N, Takahashi M, Seagar M J. FEBS Lett. 1995;361:101–105. doi: 10.1016/0014-5793(95)00156-4. [DOI] [PubMed] [Google Scholar]
- 25.Foran P, Lawrence G W, Shone C, Foster K A, Dolly J O. Biochemistry. 1996;35:2630–2636. doi: 10.1021/bi9519009. [DOI] [PubMed] [Google Scholar]
- 26.Llinás R, Sugimori M, Lin J-W, Leopold P L, Brady S T. Proc Natl Acad Sci USA. 1989;86:5656–5660. doi: 10.1073/pnas.86.14.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]