Abstract
During metamorphosis, ranid frogs shift from a purely aquatic to a partly terrestrial lifestyle. The central auditory system undergoes functional and neuroanatomical reorganization in parallel with the development of new sound conduction pathways adapted for the detection of airborne sounds. Neural responses to sounds can be recorded from the auditory midbrain of tadpoles shortly after hatching, with higher rates of synchronous neural activity and lower sharpness of tuning than observed in postmetamorphic animals. Shortly before the onset of metamorphic climax, there is a brief “deaf” period during which no auditory activity can be evoked from the midbrain, and a loss of connectivity is observed between medullary and midbrain auditory nuclei. During the final stages of metamorphic development, auditory function and neural connectivity are restored. The acoustic communication system of the adult frog emerges from these periods of anatomical and physiological plasticity during metamorphosis.
Keywords: torus semicircularis, auditory brainstem, horseradish peroxidase, audiogram
Many species of anuran amphibians undergo a developmental process called metamorphosis, during which the aquatic larval tadpole transforms into a partly terrestrial frog. Metamorphosis involves considerable alterations in body morphology, peripheral and central nervous system structures, and behaviors (1, 2). This process has been studied extensively in several neural systems, particularly visual, motor, and lateral line systems (3–7). Little is known about the development of central auditory system function across metamorphosis (6–8) even though acoustic cues play a paramount role in regulating social behaviors in adult frogs (9). We report here physiological work characterizing neural responses to sounds in a central auditory nucleus during the period of neuroanatomical rearrangement across the transition from tadpole to frog.
Stages of metamorphosis are classified according to changes in body morphology in the posthatching period (10). Premetamorphic animals (stages 25–30) have no or rudimentary external hind limb buds. Early prometamorphic animals (stages 31–37) show emerging hind limb buds at different degrees of differentiation. Tadpoles in these early stages have neither an external tympanum nor an extratympanic opercularis system for sound conduction to the inner ear organs, and there are several hypotheses regarding the identity of a potential acoustic pathway in these animals (11–13). Late prometamorphic animals (stages 38–41) show final stages of external hind limb differentiation and internal development of forelimbs. By stage 41, the extratympanic sound conduction pathway operating in adults (which includes the opercularis muscle and the operculum cartilage, connecting the shoulder girdle to the oval window in the inner ear) has developed (11). Animals in metamorphic climax (stages 42–45) show emergence of forelimbs, degeneration of the tail, and remodeling of the head, including loss of the oral disk, broadening of the mouth, and change in eye position. The lateral line system has degenerated by climax. In the bullfrog, the tympanic pathway (the external tympanum and its columellar attachment to the oval window) begins developing during metamorphic climax and is complete shortly after climax, when the tympanum appears on the side of the head (14). We have identified changes in central auditory anatomy and function across these stages of metamorphosis that parallel development of peripheral sound conduction pathways, including a striking transient loss of auditory responsiveness and connectivity just before metamorphic climax.
MATERIALS AND METHODS
Using 59 bullfrog (Rana catesbeiana) tadpoles ranging in developmental stage from posthatching (stage 25) to the end of metamorphic climax (stage 45), we recorded multiunit responses to auditory stimuli from the midbrain torus semicircularis (TS), the homologue of the mammalian inferior colliculus. Tadpoles were anesthetized by hypothermia during surgical procedures and immobilized for electrophysiological recordings by i.m. injection of d-tubocurarine chloride (3 mg/ml). They were placed with only the surgical opening above the water line in a custom-built aquarium on a vibration isolation platform in a sound-attenuating chamber maintained at 18–21°C. Multiunit responses were recorded with extra fine tungsten microelectrodes (9–12 MΩ impedance) using standard techniques (14). A maximum of four electrode passes over a period of 1.5 h was made to identify one acoustically responsive site per animal. Activity of the largest amplitude spikes within a multiunit response was isolated through a Schmitt trigger set for a 4:1 signal-to-noise ratio and digitally stored. This trigger level yielded activity from ≈2–10 single units in a given cluster. Ease of unit isolation and number of multiunits sampled in a cluster did not vary across developmental stages. Criterion for determination of neural threshold for a given stimulus was a 30% increase in spike rate in the poststimulus compared with an equal duration prestimulus period, averaged over 16 stimulus presentations. The same threshold criterion was maintained for all animals tested and was chosen to facilitate comparisons with data from postmetamorphic animals (14). Electrode placements were marked by electrolytic lesions (1.5-μA positive current for 10 minutes).
Acoustic stimuli were generated digitally by using standard techniques (14) and consisted of tone bursts (100–3000 Hz), unmodulated broadband noise, and amplitude-modulated (AM) noise (modulation rates ranging from 10 to 300 Hz, all at 100% depth of modulation). Stimulus duration was 200 ms with a 5-ms rise/fall time. Rate of presentation was 1/1800 ms, and order of presentation was pseudorandom. Sounds were presented free field from a loudspeaker suspended in air ≈1 m above the animal’s head at ≈11.3o from the vertical position. This angle of incidence of sound from air to water is within the critical angle for transmission of acoustic energy across the air–water interface (15). Sound levels were calibrated based on voltage levels measured in the tank from a hydrophone (Fish Fone, BioAcoustics, Woods Hole, MA) positioned at approximately the same location as the tadpole’s body. The intensity of each stimulus at the tadpole was adjusted with respect to these calibrated levels and is expressed as dB re: 0.1 Pa.
Manipulation of acoustic input to the inner ear was carried out using custom-fit “earmuffs” constructed of silicon caulk over a central wire placed over, but not touching, the animal’s head. The earmuffs blocked input from the lateral head region to the oval window, which was made visible through the tadpole’s head by a light source placed underneath the body. Attenuation of sounds by the earmuffs was ≈55 dB, as measured by a 6-mm probe tube connected to a Brüel & Kjær Instruments (Marlborough, MA) 1/4" microphone and sound level meter.
Spike activity was sampled digitally and analyzed using custom-written software (14). Midbrain audiograms describing response threshold at different tone frequencies were compiled based on criterion spike rate to pure tone stimuli presented in random order. Synchrony of response was analyzed by compiling period histograms to AM stimuli over each stimulus (modulation rate) period. Vector strength was calculated from period histograms as a measure of phase locking. A vector strength of “0” indicated no synchronization, and a value of “1” indicated that all neural responses fell into a single bin of the histogram. The Rayleigh test was used to test statistical significance of vector strength (16). Modulation transfer functions describing vector strength at different AM rates were compiled from these data.
Connectivity of auditory brainstem nuclei across metamorphosis was characterized in 29 tadpoles by iontophoresis of 10–40% horseradish peroxidase (HRP) (type VI-A or wheat germ agglutinin–HRP complex; Sigma) into the lateral or medial TS. Negative backing current (−0.5 μA) was applied during electrode insertion and withdrawal to prevent leakage. HRP was deposited by using 1.0-μA positive current for 10–20 minutes. After trace deposit (24–72 h), animals were killed with anesthesia with MS-222 and transcardially perfused. Brains were embedded in gelatin albumin and sliced at 50 μm. HRP was visualized by using a modified Hanker–Yates method yielding a brown reaction product (17). Alternate sections were counterstained with cresyl violet. Sections were digitally imaged using image-pro image analysis software.
RESULTS
Electrophysiological data in this report were derived from recording sites whose lesions were all confined to the medial TS, in a region comparable to the principal nucleus of adult bullfrogs.
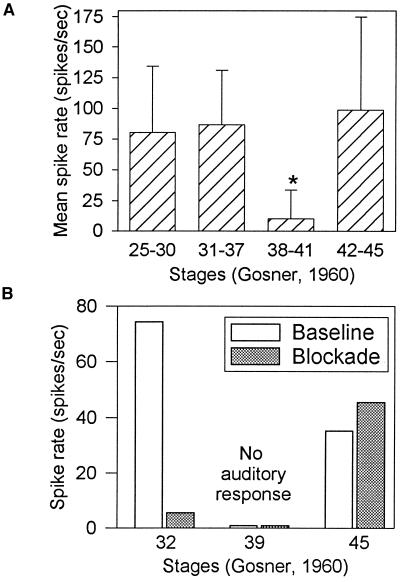
Rates of neural activity to broadband noise were similar in both early (stages 25–37) and metamorphic climax (stages 42–45) tadpoles, but stimulus-evoked activity was reduced significantly in tadpoles in the late prometamorphic period (stages 38–41) (Fig. 1A). These data suggest that there is a “deaf” period between stages 38 and 41 when the animals are insensitive to free field sounds. This transient auditory insensitivity occurs in parallel with developmental changes in sound conduction pathways and reorganization of auditory brainstem nuclei.
Figure 1.
(A) Mean spike rate (spikes/s) in response to 16 repetitions of unmodulated broadband noise (200 ms duration, interstimulus interval 1800 ms), presented at a level of −4 dB (re: 0.1 Pa). Tadpoles in stages 38–41 (n = 6) showed significantly reduced activity (∗) to unmodulated noise compared with tadpoles in other developmental stages (n = 6 for stages 25–30; n = 11 for stages 31–37; n = 7 for stages 42–45). (Error bar = 1 SD.) (B) Spike rates in response to unmodulated noise for four tadpoles with head/oval window regions unblocked (baseline, white fill) vs. blocked by earmuffs (shaded). Data at stage 39 represent means from two animals.
To determine the basis for neural responsiveness to sounds in tadpoles at different developmental stages, TS responses to unmodulated noise were measured before and after manipulation of potential peripheral sound conduction pathways. In stage 25–37 tadpoles, physical blockade of the oval window region by customized earmuffs fitted around, but not touching, the head resulted in significant decreases in auditory responding. Blockade of the head/oval window region in a stage 32 tadpole yielded a reduction in spike rate from 75 spikes/s to 4 spikes/s (Fig. 1B). Blockade of the head/oval window region did not change the rate of responding of tadpoles in the deaf period (n = 2), in which little or no response could be elicited in either baseline or blockade conditions at stimulus presentation levels up to +9 dB, the limit of linearity in our acoustic stimulation system. Similar manipulation in a stage 45 (metamorphic climax) tadpole yielded little difference between baseline and blocked conditions.
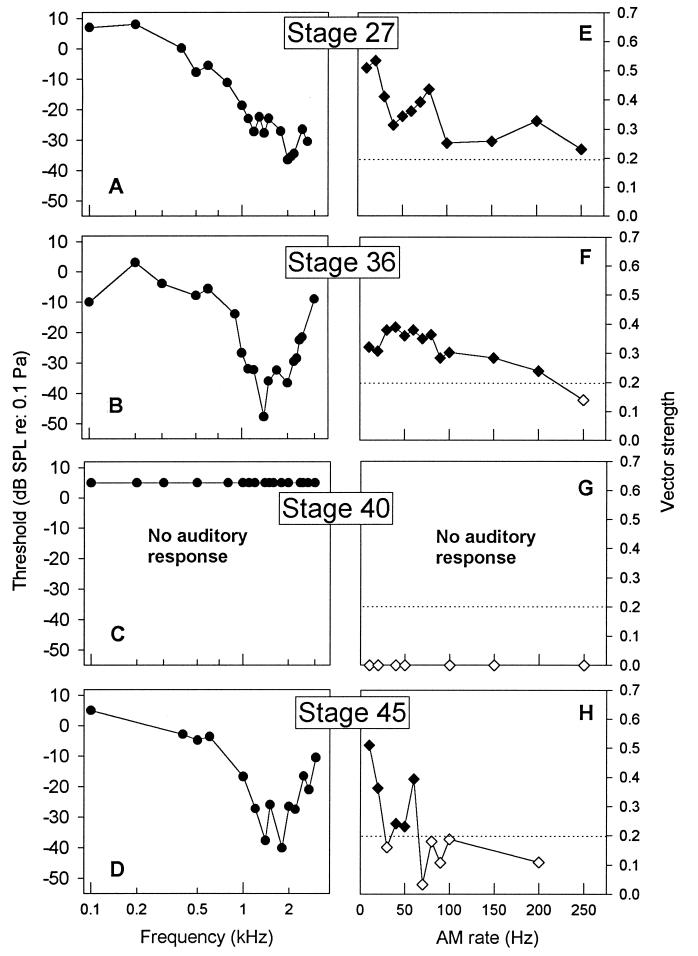
Functional characterization of midbrain auditory sensitivity revealed substantial differences across metamorphic development. Data were based on responses from 29 animals, all with lesions confined to the medial TS. Audiograms of neural responses from stage 25–30 tadpoles showed best sensitivity to tone frequencies around 2000 Hz (Fig. 2A shows a sample audiogram from a stage 27 animal). Audiograms from stage 31–37 tadpoles were “V” shaped and most sensitive to tone frequencies around 1400–1600 Hz (Fig. 2B shows sample data from a stage 36 animal). For animals in these early stages, most sensitive frequency was correlated negatively with developmental stage (r = −0.55, P < 0.05). Threshold at most sensitive frequency was correlated negatively with stage across stages 31–37 (r = −0.84, P = 0.01). From stages 25–37, the oval window increases in diameter from 525 μm to its maximum size of 1000 μm. This increase in size could form the basis for lower neural thresholds and sensitivity to lower sound frequencies, as shown in postmetamorphic froglets (14) and in other vertebrates (18–20). Audiograms from stage 38–41 tadpoles showed 40- to 50-dB higher thresholds when any responses could be elicited, but no auditory responses could be elicited from stage 39–40 tadpoles (Fig. 2C shows data from a stage 40 animal). After this deaf period, vigorous auditory responses could again be recorded from the medial TS. By metamorphic climax (stage 45, Fig. 2D), audiograms were similar in shape and sensitivity to those of stage 31–37 tadpoles. After climax, when the tympanic pathway becomes functional, neural sensitivity undergoes further changes, as described (14).
Figure 2.
(A–D) Midbrain audiograms plotting threshold in dB (re: 0.1 Pa) by tone frequency (kHz) for tadpoles at different metamorphic stages. Plotted are criterion responses to pure tones with frequencies from 100 to 3000 Hz, presented in random order. Each plot represents data from one representative animal. (E–H) Modulation transfer functions plotting vector strength of phase-locked response by AM rate for tadpoles at different metamorphic stages. AM rates were presented in random order. Signal intensity was maintained at −4 dB. Filled symbols (⧫) represent AM rates yielding significant phase locking based on the Rayleigh test (16), and open symbols (◊) represent nonsignificant responses. Horizontal dotted line is criterion for significant phase locking (P < 0.001). Audiograms and modulation transfer functions were derived from the same individual animals.
Synchronous neural activity is critical in both vertebrate sensory development (21–23) and in processing of communication sounds by adult frogs (24). Synchronous activity in the medial TS of 30 tadpoles was examined in response to AM noise at different periodic modulation rates, presented at a level of −4 dB. Responses from premetamorphic tadpoles showed significant phase locking to AM rates as high as 250 Hz (Fig. 2E), with best modulation rates (the AM rate at which vector strength was highest) across all animals in these stages ranging from 10 to 150 Hz. Responses from stage 31–37 tadpoles also showed significant phase locking to modulation rates as high as 200 Hz (Fig. 2F), but, over all animals at these stages, best modulation rates were restricted to a much lower range (10–40 Hz). The range of phase-locked response in tadpoles up to stage 37 was above the maximum range (20–50 Hz) observed in adult frogs (25–27). Tadpoles in the prometamorphic deaf period showed little or no response to AM noise. Significant phase locking to AM noise by tadpoles in metamorphic climax was limited to AM rates below 60 Hz, with best modulation rates between 10 and 20 Hz, similar to that observed in adults (27). There was a significant negative correlation between maximum range of phase locking and developmental stage (r = −0.56, P < 0.01). The sharpness of neural frequency tuning was measured from the audiogram as described (14). Sharpness increased from a mean of 1.29 in the youngest stage tadpoles to 2.20 in metamorphic climax tadpoles and was positively correlated with developmental category (r = 0.41, p = 0.05). These data indicate that, as synchronous activity declined, neural tuning became sharper (Fig. 2 A–D), consistent with observations in the mouse inferior colliculus (28).
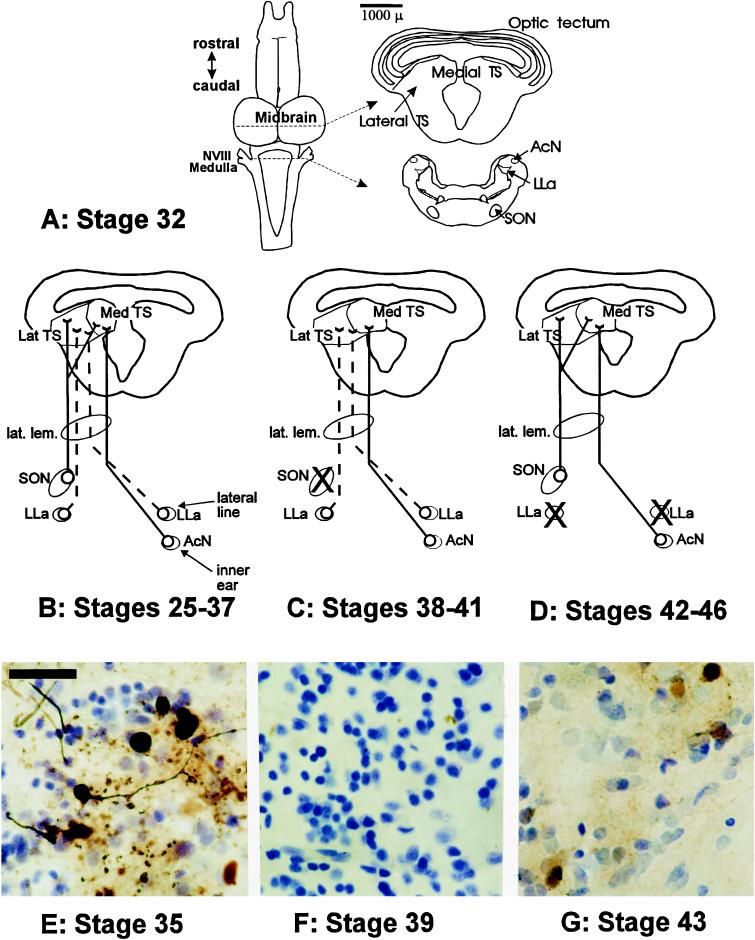
Changes were observed in connectivity of auditory nuclei during metamorphic development, paralleling changes in sound conduction pathways and central auditory function. The TS of early stage tadpoles (Fig. 3A) had less well defined subnuclear organization than that of the adult, with dense clusters of cells found medially near the ventricle and with the lateral region more cell sparse. Iontophoresis of HRP into the medial TS of early stage tadpoles resulted in labeled cell bodies in the ipsilateral superior olivary nucleus (SON) and the contralateral acoustic nucleus (AcN) in the medulla. Injections in the lateral TS resulted in labeled cell bodies in the ipsilateral SON and in the anterior lateral line nucleus (LLa) bilaterally but no label in the AcN (Fig. 3 B and E). During the deaf period (stages 38–41), no labeled cell bodies were observed in the SON after either medial or lateral TS injections (Fig. 3 C and F). There was also a decrease in labeling of the AcN. At metamorphic climax, labeled cell bodies were once again observed in the SON, but labeling of LLa neurons was lost as the lateral line system degenerated (Fig. 3 D and G). By the end of metamorphic climax, the pattern of connectivity and nuclear organization of the TS was similar to that of a postmetamorphic animal (29).
Figure 3.
(A) Schematic representation of major auditory nuclei in the tadpole brainstem as derived from sections from a stage 32 tadpole. Scale bar represents 1000 μm and refers to section outlines on right. Up until approximately stage 38, the tectal ventricle is continuous with the third ventricle. Connectivity pattern is based on retrograde transport of HRP after injections into the medial or lateral TS. Solid lines indicate auditory pathways; dashed lines indicate lateral line pathways. X, loss of retrograde cell filling in medullary brainstem nuclei that previously showed connectivity to the TS. NVIII, eighth (auditory) cranial nerve. (B–D) Changes in afferent connectivity across metamorphic development based on retrograde cell filling of afferents after HRP iontophoresis into the TS. The TS shows changes in overall morphology across metamorphic development, with the two nuclei of the medial torus not joining until stages 38–41. (B) Tadpoles from stage 25–37 (n = 13) showed robust filling of ipsilateral SON cells and cell filling in LLa bilaterally after injections in the lateral TS. HRP injection in the medial TS resulted in cell filling in the SON and in the contralateral AcN. (C) Tadpoles in the deaf period (n = 8) showed labeling of LLa neurons similar to that observed in earlier stage tadpoles but reduced labeling of the AcN and loss of labeling in the SON. (D) Tadpoles in metamorphic climax (n = 8) showed re-establishment of connectivity between the TS and the SON and increased labeling in the AcN but with a stage-dependent decrease in labeling of LLa neurons. By stage 44, no LLa neurons were labeled, and the AcN had expanded dorsally and medially into the region formerly occupied by the lateral line neuropil, coincident with the degeneration of the lateral line system at this stage. (E–G) HRP cell filling of ipsilateral SON neurons derived from injections into TS sites. Data are derived from injections matched for injection site, concentration of HRP, electrode diameter, and survival time. (Scale bar = 40 μm.) (E) Stage 35 tadpole, showing robust labeling of small round cell bodies from the SON. (F) Stage 39 tadpole showing lack of labeling of SON cells. (G) Stage 43 tadpole showing filling of SON cells demonstrating re-establishment of SON–TS afferent connectivity.
DISCUSSION
Our data show that there are significant changes in central auditory sensitivity and connectivity in tadpoles concurrent with the sequential maturation of the peripheral sound conduction pathways found in the postmetamorphic animal. Neural activity in response to sounds can be recorded from the medial TS of tadpoles as early as premetamorphosis (stages 25–30) and into prometamorphosis (stages 31–37) (Fig. 1A). Because neither the external tympanum nor the opercularis system is present in these early stage tadpoles (11–13), we propose that environmental sound induces pressure changes in the inner ear directly through the oval window (11), in a manner similar to that described for the shark (30). We term this mode of conduction a “fenestral” pathway. At these stages, blockade of the head/oval window region reduces neural responsiveness (Fig. 1B). These data suggest that the bronchial columella, which couples the lung to the round window of the otic capsule in early stage tadpoles (12, 13), is not the primary route for sound conduction because its operation presumably would not be affected by this manipulation. The role of the bronchial columella in the auditory system is still unknown; however, one possibility is that it may act as a gain control system for detection of underwater sounds. If the bronchial columella couples pressure changes between the lung and the perilymph of the otic capsule, an air-filled lung would increase pressure at the round window and raise the relative impedance of the perilymph, thus improving the signal-to-noise ratio of an auditory signal. If the lungs were uninflated, as in our experiments, relative impedance and signal-to-noise ratio would decrease, but overall sensitivity might be elevated because of decreased resistance to motion of the hair cells. Further experiments are needed to address this hypothesis.
Blockade of the head/oval window region did not influence TS responding in late prometamorphic (stage 38–41) tadpoles, from whom little or no neural activity could be recorded under either baseline or blocked conditions (Fig. 1 A and B). During this period, the cartilaginous substrate of the developing operculum has condensed over and obstructs the oval window, thereby impeding its ability to act as a direct acoustic receiver. The bronchial columella has begun degenerating by these stages and thus its operation could not affect auditory sensitivity. Later, between stages 42 and 45, blockade of the head/oval window region did not reduce TS activity (Fig. 1B). This suggests that the fenestral pathway no longer forms the primary basis for sound conduction at metamorphic climax. Because the extratympanic opercularis system has developed by climax and because its operation would not be affected by blockade of the head region, this system could now act as the main sound conduction pathway. The tympanic pathway does not become functional until after metamorphic climax, at which point auditory sensitivity undergoes further maturational changes (14).
The most sensitive frequency of midbrain audiograms (frequency at which threshold is lowest) was correlated negatively with developmental stage up to stage 37 (Figs. 2 A and B). Between stages 38 and 41, there is little or no TS response to auditory stimuli at levels up to +9 dB (Fig. 2C). Audiograms from tadpoles in metamorphic climax had most sensitive frequencies and threshold levels similar to those at stages 31–37 (Fig. 2D). Changing the threshold criterion for a response does not change these trends, nor does a more liberal criterion for threshold indicate the presence of significant auditory evoked activity in tadpoles in the deaf period. The changes in frequency sensitivity and threshold of neural audiograms in the tadpole TS across metamorphosis are qualitatively similar to functional changes based on maturation of middle ear structures in other vertebrates (19, 20, 31).
The highest vector strength and broadest AM range of significant phase-locked responses were observed in the youngest tadpoles (Fig. 2E). Vector strength and AM range decreased slightly during stages 31–37 (Fig. 2F). During the deaf period, no significant responses could be evoked to AM stimuli up to levels as high as +9 dB. Phase-locked response re-emerged at the onset of metamorphic climax, but only to rates similar to those observed in adult frogs. The transiently high rates of synchronous activity to AM sounds in the TS of early stage tadpoles suggest that the process of development and maturation might be activity-dependent. This synchronous activity might drive plasticity of auditory neural connections across metamorphosis, similar to the role of activity in development of the vertebrate visual system (21–23). The loss of connectivity between the medullary and midbrain auditory nuclei during the deaf period, concurrent with changes in conduction pathways, might reflect the operation of a Hebbian mechanism, in which the underlying neuroanatomical substrate can be modified by the strength and characteristics of the neural response (32, 33).
The transient deaf period emerges during reorganization of the auditory system in stage 38–41 tadpoles and is passed through before the reorganization of visual and motor systems and the degeneration of the lateral line system during metamorphic climax. During the deaf period, recordings from the lateral TS, which receives both auditory and lateral line input from caudal brainstem nuclei (6), demonstrate continued sensitivity to low frequency lateral line stimulation but show no response to auditory stimulation (13). Thus, the brief loss of auditory sensitivity is specific and does not overlap loss or limitations in other modalities, possibly improving the tadpoles’ chances for survival during a developmentally vulnerable period. It is possible that there are different pathways of sound conduction bypassing the medial TS that might allow tadpoles in the deaf period to still detect sounds. No evidence for such an alternative pathway was found in our tract-tracing experiments.
Several lines of evidence indicate that the metamorphic transition from aquatic to terrestrial lifestyles is a function of specific adaptation to an ecological niche, rather than a recapitulation of any evolutionary shift from water to land (34, 35). The tadpole develops in an aquatic environment with acoustic characteristics similar to that of the mammalian uterine environment, such as low-pass transmission of sound, elevated propagation velocity, and relatively high ambient noise from structurally contiguous sources (36, 37). The metamorphic transition may thus provide a nonfetal model for investigating neural plasticity in developing mammalian and other amniotic auditory systems.
Acknowledgments
This research was supported in part by an National Institutes of Health research grant NS28565 to A.M.S. and a National Science Foundation Graduate Fellowship to S.B.H. Care of animals used in this research was in accordance with guidelines of the Brown University Institutional Animal Care and Use Committee.
ABBREVIATIONS
- AcN
acoustic nucleus
- AM
amplitude-modulated
- HRP
horseradish peroxidase
- LLa
anterior lateral line nucleus
- SON
superior olivary nucleus
- TS
torus semicircularis
References
- 1.Etkin W. In: Physiology of the Amphibia. Moore J A, editor. New York: Academic; 1964. pp. 427–468. [Google Scholar]
- 2.Stehouwer D J. Dev Psychobiol. 1988;21:383–395. doi: 10.1002/dev.420210409. [DOI] [PubMed] [Google Scholar]
- 3.Spaeti U. J Hirnforsch. 1978;19:543–575. [PubMed] [Google Scholar]
- 4.Frank E, Westerfield M. J Physiol (London) 1983;343:595–610. doi: 10.1113/jphysiol.1983.sp014912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant S, Keating M J. Exp Brain Res. 1989;75:99–116. doi: 10.1007/BF00248534. [DOI] [PubMed] [Google Scholar]
- 6.Fritzsch B, Nikundiwe A M, Will U. J Comp Neurol. 1984;229:451–469. doi: 10.1002/cne.902290312. [DOI] [PubMed] [Google Scholar]
- 7.Jacoby J, Rubinson K. J Comp Neurol. 1983;216:152–161. doi: 10.1002/cne.902160204. [DOI] [PubMed] [Google Scholar]
- 8.Jacoby J, Rubinson K. Brain Res. 1984;292:378–381. doi: 10.1016/0006-8993(84)90774-1. [DOI] [PubMed] [Google Scholar]
- 9.Gerhardt H C, Schwartz J J. In: Amphibian Biology: Social Behavior. Heatwole H, Sullivan B K, editors. Vol. 2. Chipping Norton, Australia: Surrey Beatty & Sons; 1995. pp. 603–632. [Google Scholar]
- 10.Gosner K L. Herpetologica. 1960;16:183–190. [Google Scholar]
- 11.Hetherington T E. Zoomorphology. 1987;106:289–300. [Google Scholar]
- 12.Witschi E. Z Naturforsch. 1949;4b:230–242. [Google Scholar]
- 13.Boatright-Horowitz S S. Ph.D. dissertation. Providence, RI: Brown University; 1997. [Google Scholar]
- 14.Boatright-Horowitz S, Simmons A M. J Comp Physiol A. 1995;177:577–590. doi: 10.1007/BF00207187. [DOI] [PubMed] [Google Scholar]
- 15.Horton J W. Fundamentals of Sonar. Annapolis, MD: United States Naval Institute; 1959. [Google Scholar]
- 16.Batschelet E. Circular Statistics in Biology. New York: Academic; 1981. [Google Scholar]
- 17.Hanker J S, Yates P E, Metz C B, Rustioni A. Histochem J. 1977;9:789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- 18.Relkin E M, Saunders J C, Konkle D F. J Acoust Soc Am. 1979;66:133–139. doi: 10.1121/1.383066. [DOI] [PubMed] [Google Scholar]
- 19.Saunders J C, Summers R M. J Comp Physiol. 1982;146:517–525. [Google Scholar]
- 20.Cohen Y E, Hernandez H N, Saunders J C. J Morphol. 1992;212:257–267. doi: 10.1002/jmor.1052120305. [DOI] [PubMed] [Google Scholar]
- 21.Katz L C. Curr Opin Neurobiol. 1993;3:93–99. doi: 10.1016/0959-4388(93)90041-v. [DOI] [PubMed] [Google Scholar]
- 22.Jeffreys J G. Physiol Rev. 1995;75:689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- 23.Shatz C J. Proc Natl Acad Sci USA. 1996;93:602–608. doi: 10.1073/pnas.93.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz J J, Simmons A M. J Comp Physiol A. 1990;166:489–499. doi: 10.1007/BF00192019. [DOI] [PubMed] [Google Scholar]
- 25.Rose G J, Capranica R R. J Neurophysiol. 1985;53:446–465. doi: 10.1152/jn.1985.53.2.446. [DOI] [PubMed] [Google Scholar]
- 26.Gooler D M, Feng A S. J Neurophysiol. 1992;67:1–22. doi: 10.1152/jn.1992.67.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Simmons A M, Buxbaum R. In: Neuroethological Studies of Cognition and Perceptual Processes. Moss C F, Shettleworth S, editors. Boulder, CO: Westview; 1996. pp. 185–228. [Google Scholar]
- 28.Sanes D H, Constantine-Paton M. J Neurosci. 1985;5:1152–1166. doi: 10.1523/JNEUROSCI.05-05-01152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng A S, Lin W. J Comp Neurol. 1991;306:613–630. doi: 10.1002/cne.903060407. [DOI] [PubMed] [Google Scholar]
- 30.Corwin J. J Comp Physiol. 1981;142:379–390. [Google Scholar]
- 31.Mikaelian D O. Laryngoscope. 1979;89:1–15. doi: 10.1288/00005537-197901000-00001. [DOI] [PubMed] [Google Scholar]
- 32.LeVay S, Wiesel T N, Hubel D H. J Comp Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- 33.Reh T A, Constantine-Paton M. J Neurosci. 1985;5:1132–1143. doi: 10.1523/JNEUROSCI.05-05-01132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wassersug R J. Am Zool. 1989;29:65–84. [Google Scholar]
- 35.Fritzsch B. J Neurobiol. 1990;21:1011–1021. doi: 10.1002/neu.480210707. [DOI] [PubMed] [Google Scholar]
- 36.Armitage S E, Baldwin B A, Vince M A. Science. 1980;208:1173–1174. doi: 10.1126/science.7375927. [DOI] [PubMed] [Google Scholar]
- 37.Bench J. J Genet Psychol. 1968;113:85–87. doi: 10.1080/00221325.1968.10533811. [DOI] [PubMed] [Google Scholar]