Abstract
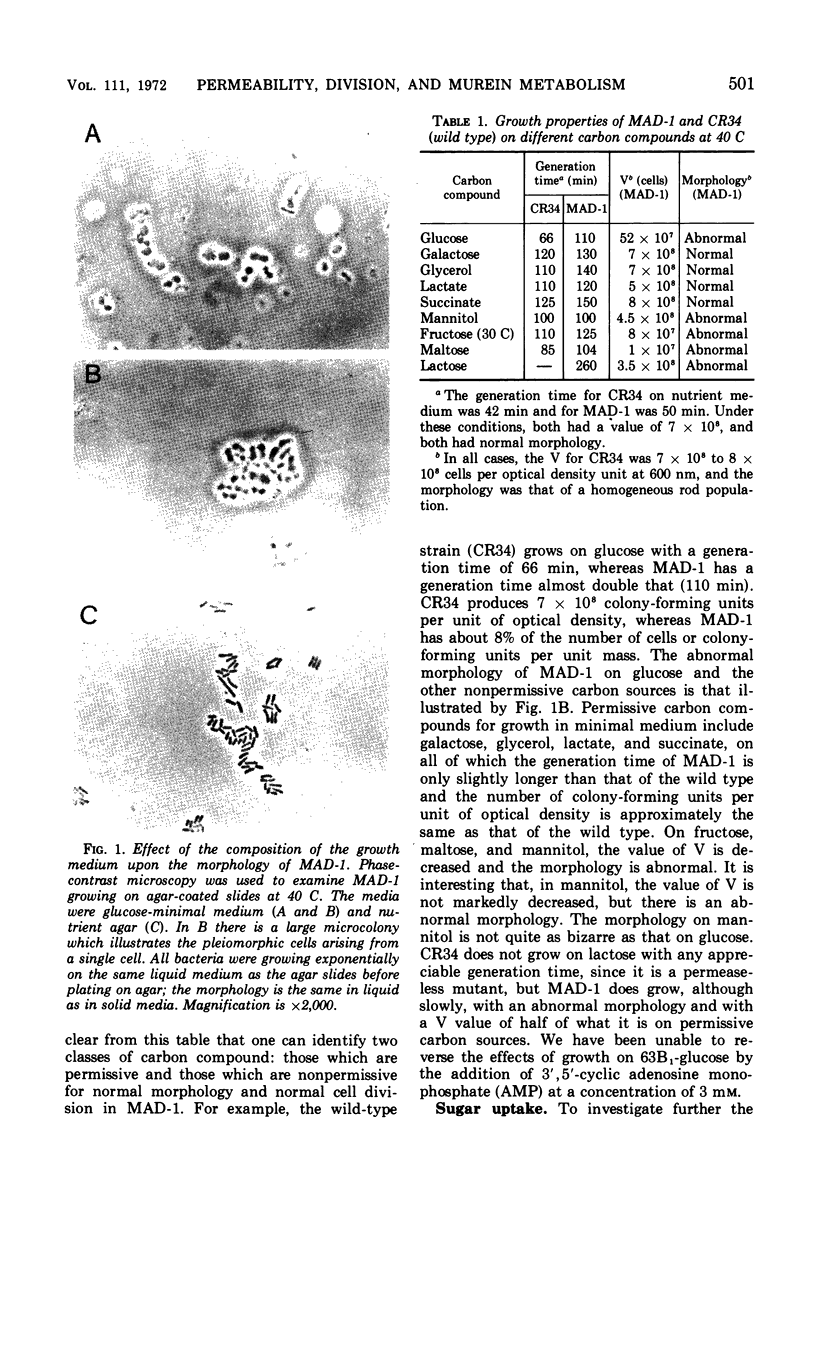
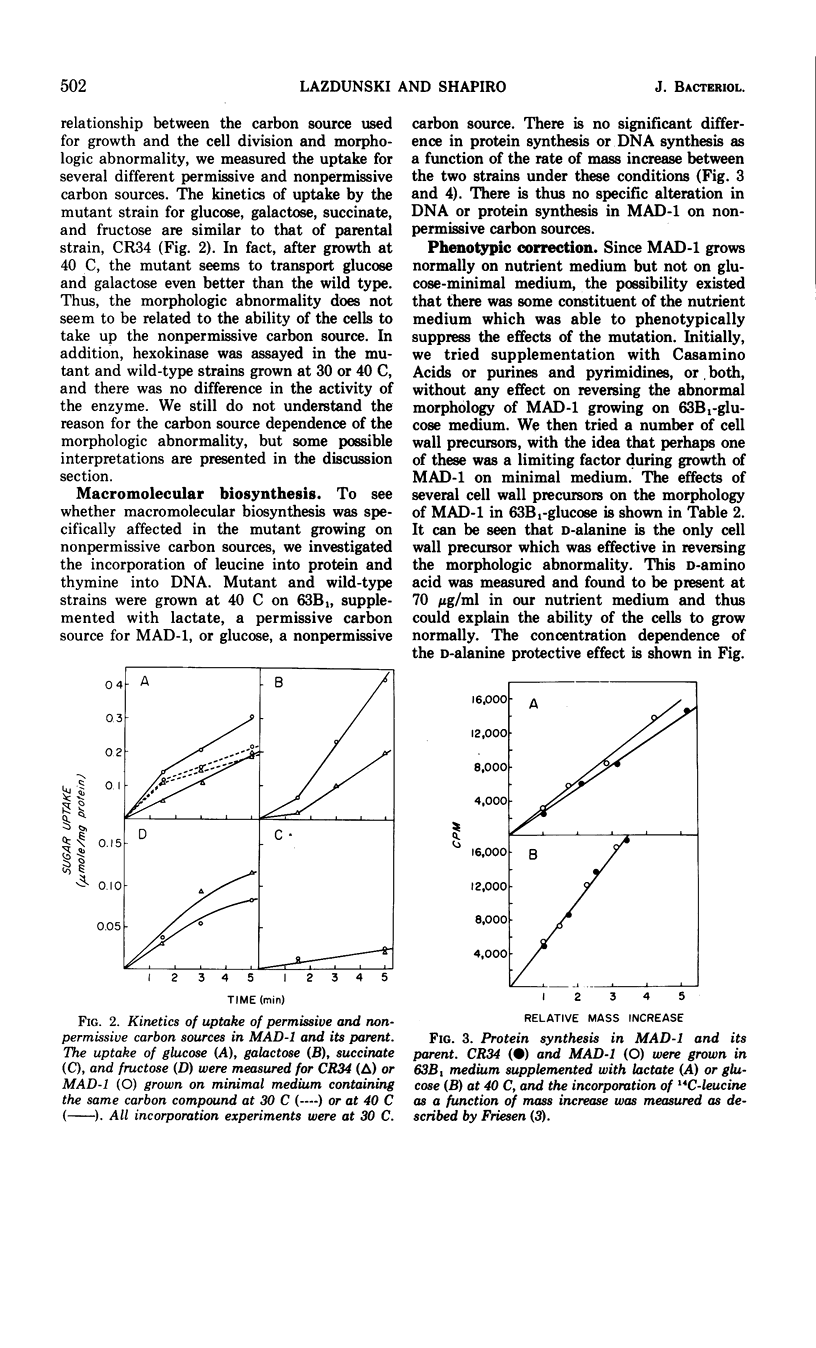
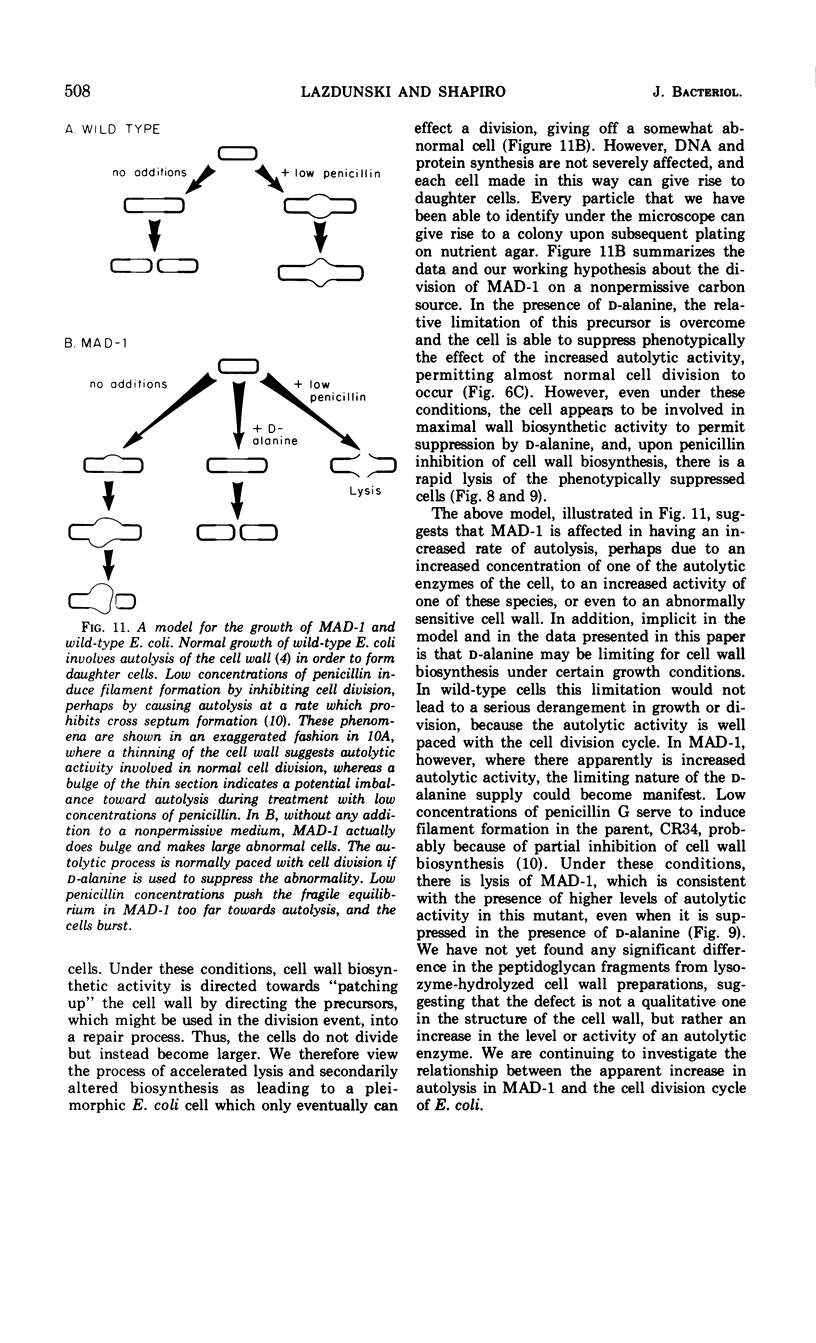
A mutant of Escherichia coli has been found to have an increased sensitivity to actinomycin D and to sodium deoxycholate and an unusual morphology which accompanies an abnormality in cellular division. All of these characteristics are suppressed when the strain is grown in the presence of d-alanine. This strain, called MAD-1, for murein altered division mutant, exhibits its pleiotropic phenotype only when certain carbon compounds are used as energy sources in minimal medium. Nonpermissive carbon sources, which elicit the disturbed phenotype, include glucose, mannitol, fructose, maltose, and lactose; permissive carbon sources include galactose, glycerol, lactate, and succinate. The mutant is able to transport nonpermissive carbon compounds; 3 mM 3′,5′-cyclic adenosine monophosphate included in the medium does not alter the phenotype seen with growth on glucose. Deoxyribonucleic acid and protein synthesis are normal with respect to cellular mass increase. d-Alanine specifically suppresses the pleiotropic phenotype at a concentration six times lower than l-alanine, the only other compound found to be effective. There is no abnormality in the Km or Vmax of l-alanine racemase or d-alanine-d-alanine synthetase of MAD-1 compared to its parent, CR34. MAD-1 is more susceptible to growth inhibition by penicillin or cycloserine than its parent, and is exquisitely sensitive to lysis in the presence of sodium deoxycholate or lysozyme. When cell wall biosynthesis is inhibited, MAD-1 lyses much more rapidly than CR34, even after it has been phenotypically suppressed by growth on d-alanine. The incorporation of l-alanine and diaminopimelic acid into the peptidoglycan of the mutant and wild type is identical; d-alanine is incorporated 1.5 times more rapidly into MAD-1 cells grown under nonpermissive conditions. The peptidoglycan fragments seen after digestion with lysozyme were similar for MAD-1 and the wild type. The results are interpreted as being compatible with an increased autolytic rate in MAD-1, caused either by an increase in the quantity or activity of an autolysin, or by an abnormal cell wall which is especially susceptible to autolysis, but which was not detected by analysis of peptidoglycan fragments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASENSIO C., AVIGAD G., HORECKER B. L. PREFERENTIAL GALACTOSE UTILIZATION IN A MUTANT STRAIN OF E. COLI. Arch Biochem Biophys. 1963 Dec;103:299–309. doi: 10.1016/0003-9861(63)90419-3. [DOI] [PubMed] [Google Scholar]
- DIPIETRO D. L., WEINHOUSE S. Hepatic glucokinase in the fed, fasted, and alloxan-diabetic rat. J Biol Chem. 1960 Sep;235:2542–2545. [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Shapiro B. M. Isolation and some properties of cell envelope altered mutants of Escherichia coli. J Bacteriol. 1972 Aug;111(2):495–498. doi: 10.1128/jb.111.2.495-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb W., Schwarz U. Zur Biosynthese des formgebenden Elements der Bakterienzellwand. I. Abbau des Mureins als erster Schritt beim Wachstum des Sacculus. Z Naturforsch B. 1967 May;22(5):545–549. [PubMed] [Google Scholar]
- NEUHAUS F. C. The enzymatic synthesis of D-alanyl-D-alanine. I. Purification and properties of D-alanyl-D-alanine synthetase. J Biol Chem. 1962 Mar;237:778–786. [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Wargel R. J., Shadur C. A., Neuhaus F. C. Mechanism of D-cycloserine action: transport systems for D-alanine, D-cycloserine, L-alanine, and glycine. J Bacteriol. 1970 Sep;103(3):778–788. doi: 10.1128/jb.103.3.778-788.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]