Abstract
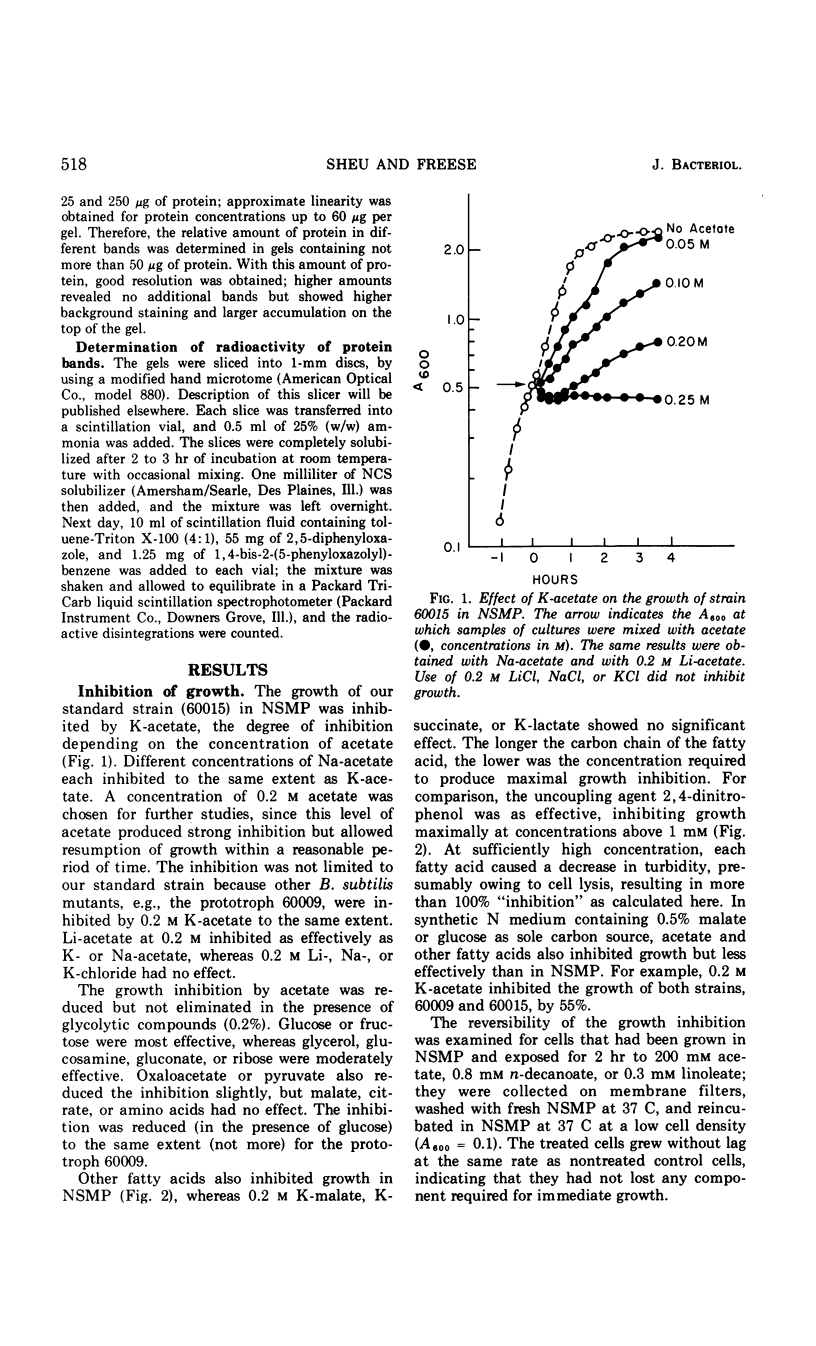
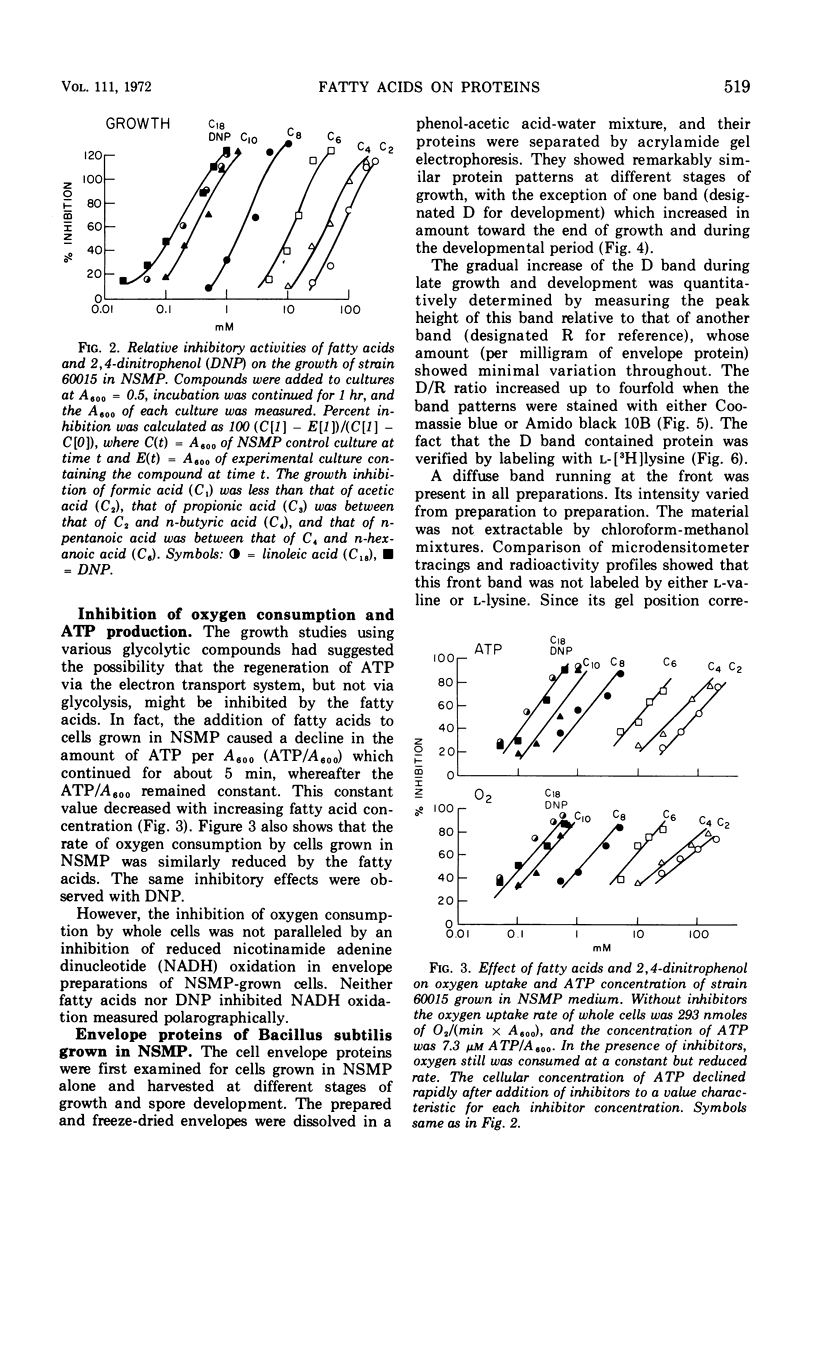
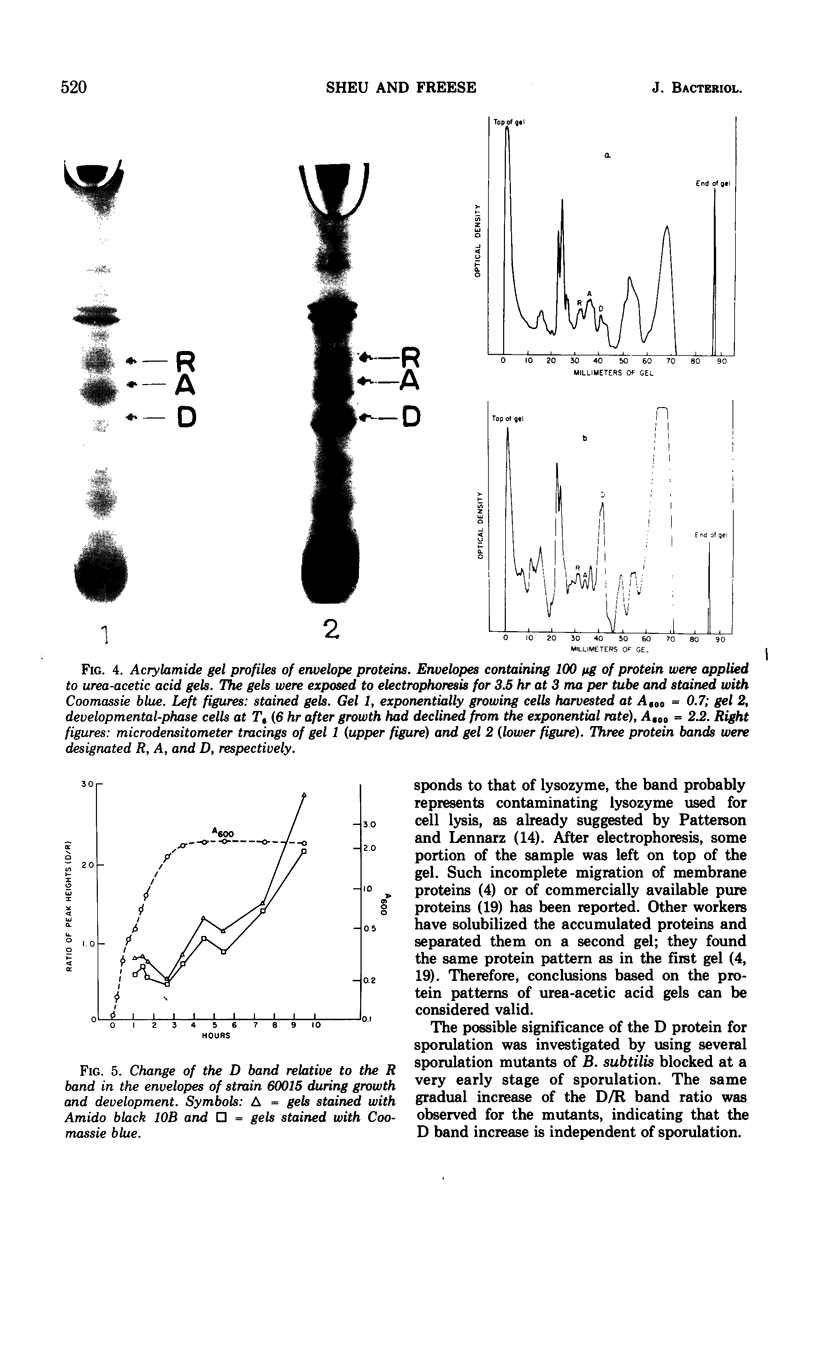
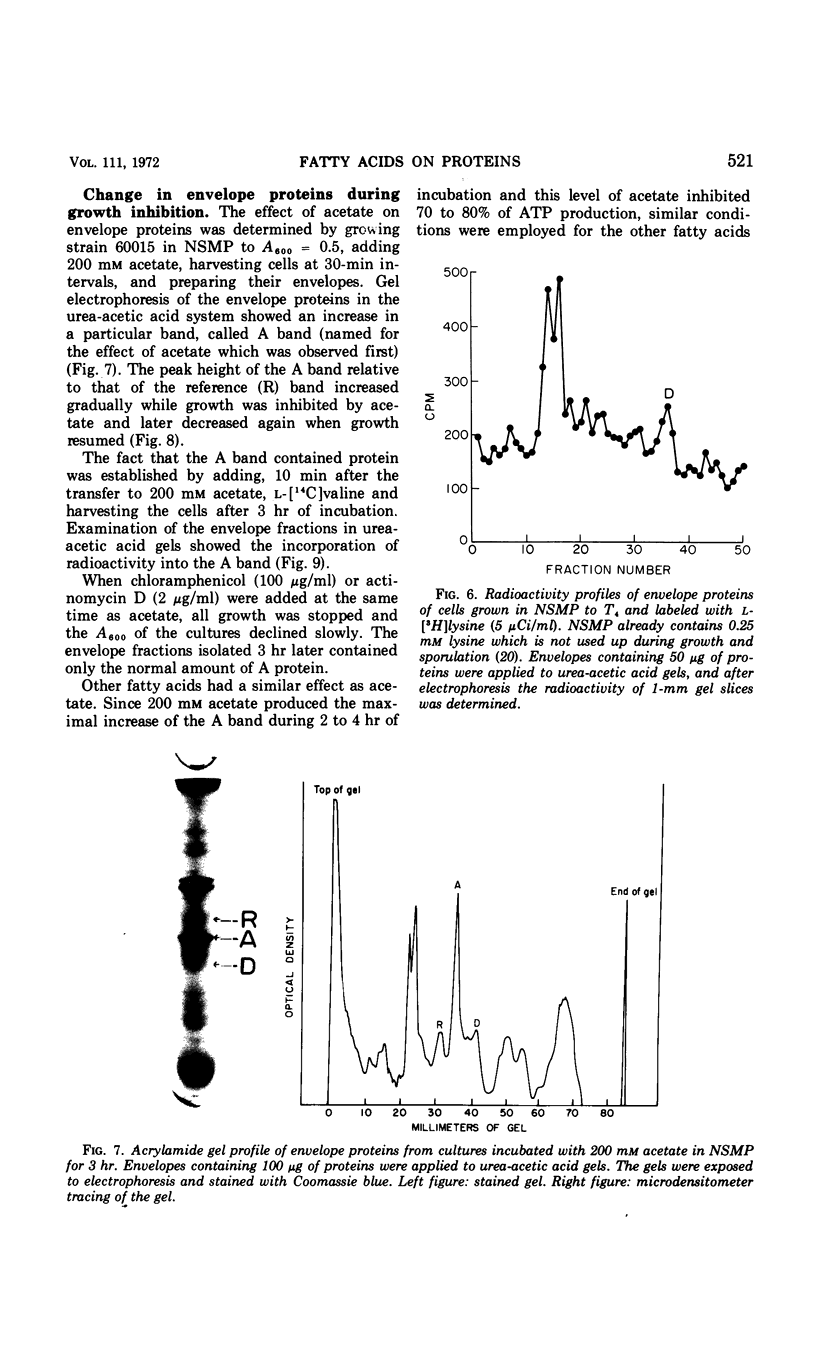
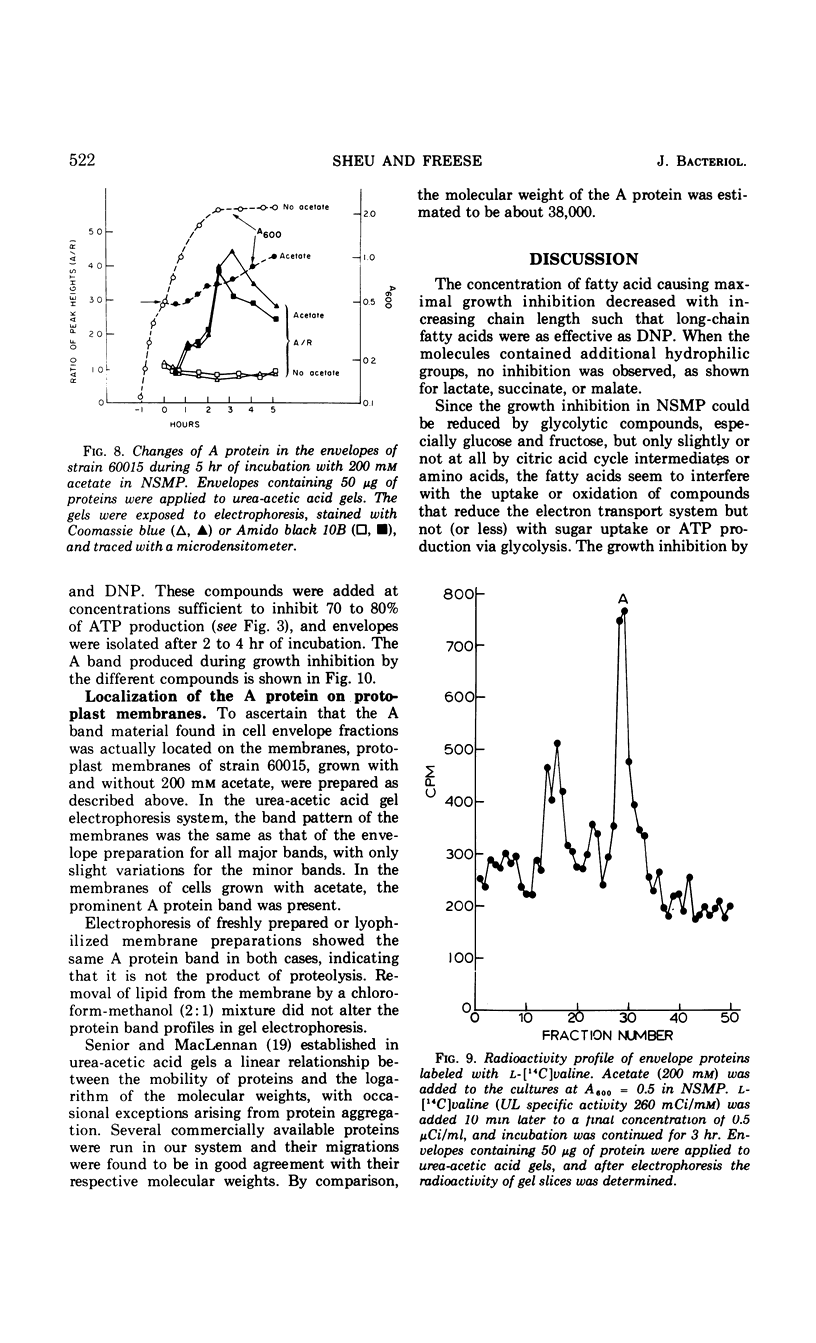
Fatty acids of different chain lengths were added to cultures of Bacillus subtilis growing in nutrient sporulation medium, and the effects of these fatty acids on growth, oxygen uptake, adenosine triphosphate (ATP) concentration, and membrane protein composition were examined. All fatty acids inhibited growth, the effect being reduced in the presence of glycolytic compounds and reversed by transfer to medium without fatty acids. The inhibition of growth was correlated with a reduction in both the rate of oxygen consumption and the concentration of ATP per cell. The concentration required to obtain a certain degree of inhibition increased with decreasing molecular weight of the fatty acid. However, the reduced nicotinamide adenine dinucleotide oxidation system of cell envelope preparations (i.e., the electron transport system) was not inhibited. Submaximal growth inhibition was accompanied by the relative increase of a membrane protein band revealed by urea-acetic acid gel electrophoresis. This increase was blocked by actinomycin or chloramphenicol. All of the above changes could also be produced by 2,4-dinitrophenol. The inhibition results are best explained by assuming that the fatty acids reversibly react with the cell membrane or proteins in it; they could either alter the membrane structure or uncouple the electron transport chain from two types of proteins, those used for ATP regeneration and others needed for the transport of certain compounds into the cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORST P., LOOS J. A., CHRIST E. J., SLATER E. C. Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta. 1962 Aug 27;62:509–518. doi: 10.1016/0006-3002(62)90232-9. [DOI] [PubMed] [Google Scholar]
- Borst-Pauwels G. W., Jager S. Inhibition of phosphate and arsenate uptake in yeast by monoiodoacetate, fluoride, 2,4-dinitrophenol and acetate. Biochim Biophys Acta. 1969 Apr 8;172(3):399–406. doi: 10.1016/0005-2728(69)90136-4. [DOI] [PubMed] [Google Scholar]
- CAMIEN M. N., DUNN M. S. Potassium acetate inhibition of Lactobacillus casei and its reversal by lithium, sodium and fatty acids. Proc Soc Exp Biol Med. 1957 Aug-Sep;95(4):697–700. doi: 10.3181/00379727-95-23334. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Mahler H. R., Hugli T. E. Isolation and characterization of insoluble proteins of the synaptic plasma membrane. Arch Biochem Biophys. 1968 Sep 10;126(3):821–837. doi: 10.1016/0003-9861(68)90476-1. [DOI] [PubMed] [Google Scholar]
- Freese E., Fortnagel P. Analysis of sporulation mutants. I. Response of uracil incorporation to carbon sources, and other mutant properties. J Bacteriol. 1967 Dec;94(6):1957–1969. doi: 10.1128/jb.94.6.1957-1969.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Fortnagel U. Growth and sporulation of Bacillus subtilis mutants blocked in the pyruvate dehydrogenase complex. J Bacteriol. 1969 Sep;99(3):745–756. doi: 10.1128/jb.99.3.745-756.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane I., Razin S. Synthesis and turnover of membrane protein and lipid in Mycoplasma laidlawii. Biochim Biophys Acta. 1969 Jun 3;183(1):79–89. doi: 10.1016/0005-2736(69)90131-x. [DOI] [PubMed] [Google Scholar]
- Klofat W., Picciolo G., Chappelle E. W., Freese E. Production of adenosine triphosphate in normal cells and sporulation mutants of Bacillus subtilis. J Biol Chem. 1969 Jun 25;244(12):3270–3276. [PubMed] [Google Scholar]
- Konings W. N., Freese E. Amino acid transport in membrane vesicles of Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2408–2418. [PubMed] [Google Scholar]
- Konings W. N., Freese E. L-serine transport in membrane vesicles of Bacillus subtilis energized by NADH or reduced phenazine methosulfate. FEBS Lett. 1971 Apr 12;14(1):65–68. doi: 10.1016/0014-5793(71)80276-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leggett J. E. Entry of phosphate into yeast cell. Plant Physiol. 1961 May;36(3):277–284. doi: 10.1104/pp.36.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H., Lennarz W. J. Novel protein composition of a bacterial membrane. Biochem Biophys Res Commun. 1970 Jul 27;40(2):408–415. doi: 10.1016/0006-291x(70)91024-7. [DOI] [PubMed] [Google Scholar]
- SAMSON F. E., KATZ A. M., HARRIS D. L. Effects of acetate and other short-chain fatty acids on yeast metabolism. Arch Biochem Biophys. 1955 Feb;54(2):406–423. doi: 10.1016/0003-9861(55)90054-0. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H. Composition of the membranes isolated from several Gram-positive bacteria. Biochim Biophys Acta. 1965 Oct 18;107(3):531–538. doi: 10.1016/0304-4165(65)90197-2. [DOI] [PubMed] [Google Scholar]
- Schmitt R., Freese E. Curing of a sporulation mutant and antibiotic activity of Bacillus subtilis. J Bacteriol. 1968 Oct;96(4):1255–1265. doi: 10.1128/jb.96.4.1255-1265.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E., MacLennan D. H. Mitochondrial "structural protein." A reassessment. J Biol Chem. 1970 Oct 10;245(19):5086–5095. [PubMed] [Google Scholar]
- Sugae K., Freese E. Requirement for Acetate and Glycine (or Serine) for Sporulation Without Growth of Bacillus subtilis. J Bacteriol. 1970 Dec;104(3):1074–1085. doi: 10.1128/jb.104.3.1074-1085.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., MacLennan D. H., Tzagoloff A., Stoner C. D. Studies on the electron transfer system. LXVII. Polyacrylamide gel electrophoresis of the mitochondrial electron transfer complexes. Arch Biochem Biophys. 1966 Apr;114(1):223–230. doi: 10.1016/0003-9861(66)90324-9. [DOI] [PubMed] [Google Scholar]
- Weeks G., Shapiro M., Burns R. O., Wakil S. J. Control of fatty acid metabolism. I. Induction of the enzymes of fatty acid oxidation in Escherichia coli. J Bacteriol. 1969 Feb;97(2):827–836. doi: 10.1128/jb.97.2.827-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]