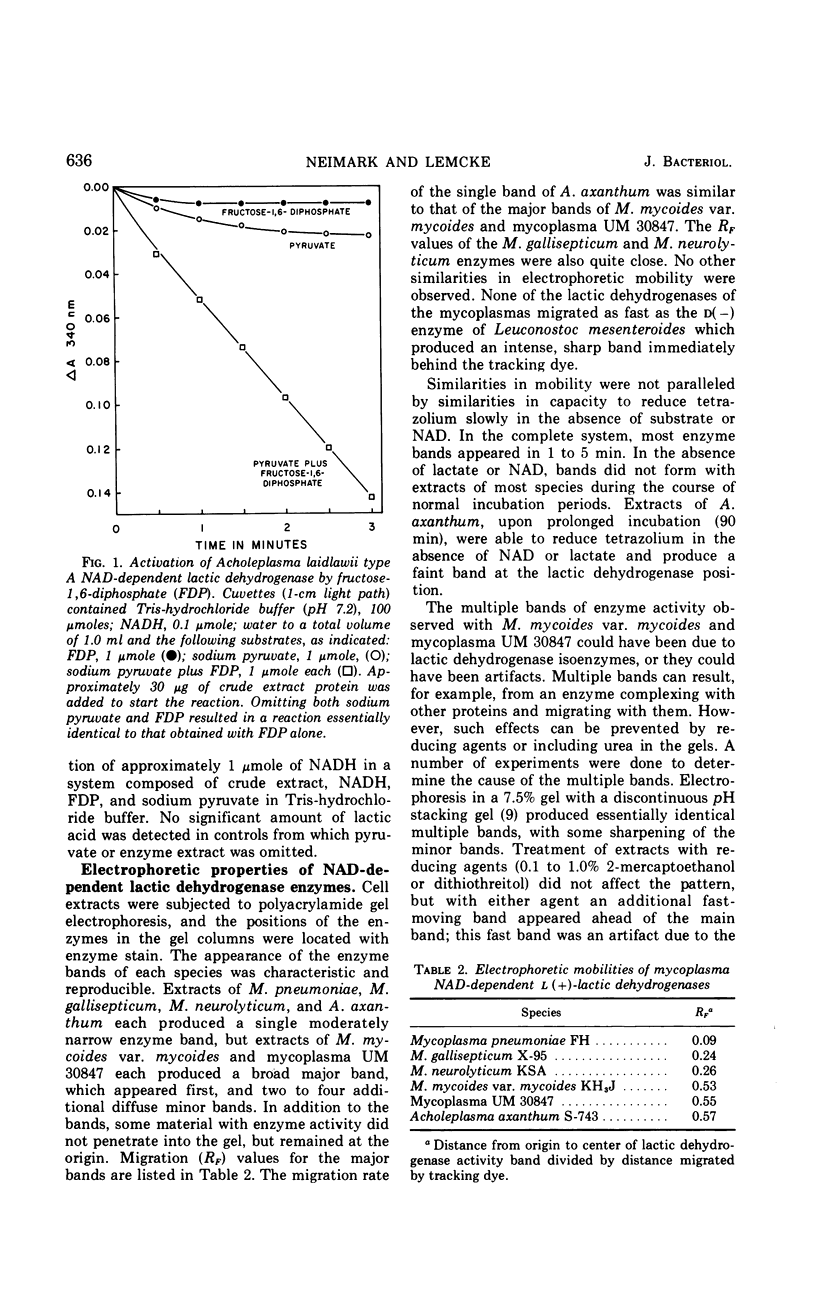
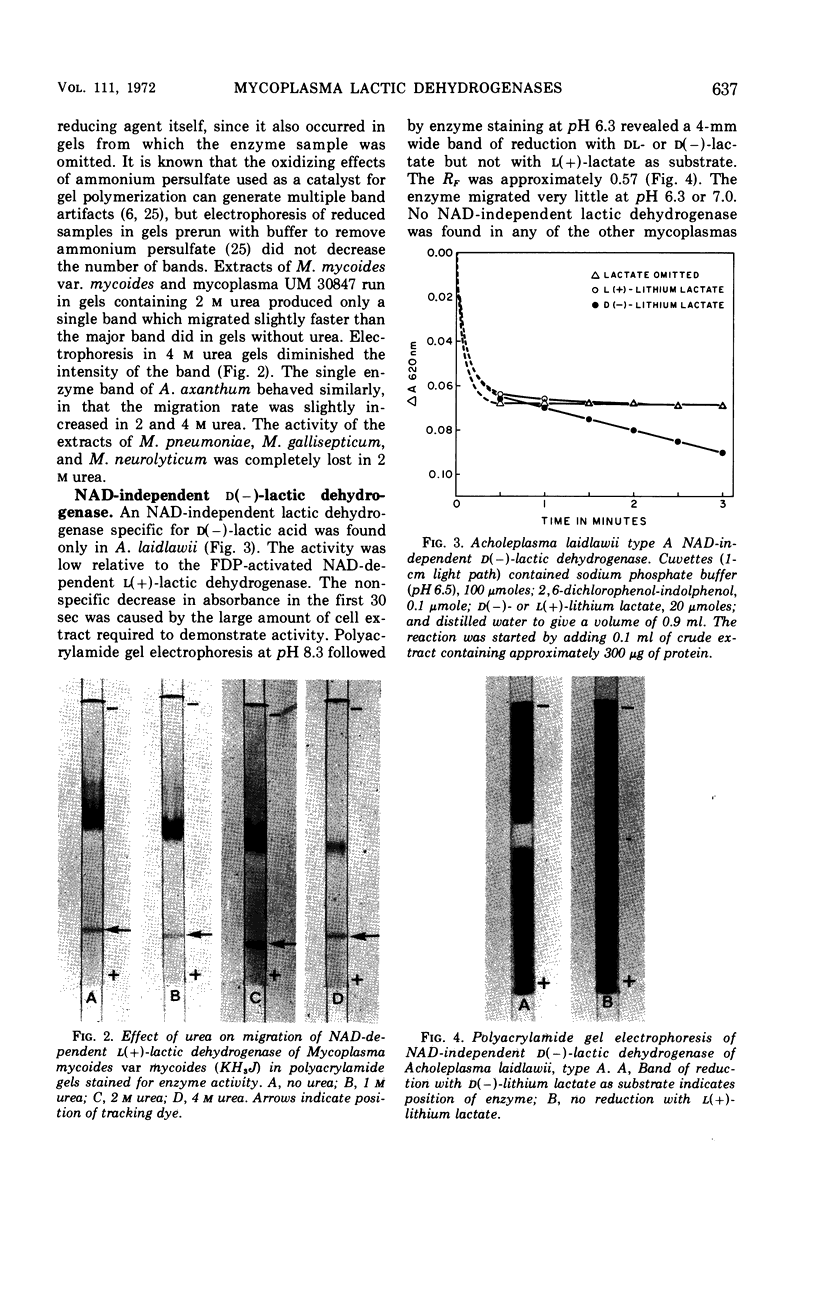
Abstract
Eight fermentative mycoplasmas differing in genome size, deoxyribonucleic acid (DNA) base composition, or sterol dependence were examined for lactic dehydrogenase composition by spectrophotometric assay and polyacrylamide gel electrophoresis. Three completely different patterns of lactic dehydrogenase composition were found. (i) A nicotinamide adenine dinucleotide (NAD)-dependent l(+)-lactic dehydrogenase was found in Mycoplasma pneumoniae, M. gallisepticum, M. mycoides var. mycoides, mycoplasma UM 30847, M. neurolyticum, and Acholeplasma axanthum. Electrophoresis of cell-free extracts of each of these mycoplasmas produced, with the exception of M. mycoides var. mycoides and UM 30847, single, different enzyme bands. M. mycoides var. mycoides and UM 30847 were similar and formed multiple bands of enzyme activity. We were unable to establish whether these multiple bands were due to lactic dehydrogenase isoenzymes or artifacts. (ii) An NAD-dependent d(−)-lactic dehydrogenase which could not be reversed to oxidize lactate was found in M. fermentans. (iii) A. laidlawii A possessed an NAD-independent d(−)-lactic dehydrogenase capable of reducing dichlorophenol-indophenol, and an NAD-dependent l(+)-lactic dehydrogenase which is specifically activated by fructose-1,6-diphosphate. Heretofore, this enzyme regulatory mechanism was known to occur only among the Lactobacillaceae. No yeast-type lactic dehydrogenase activity was found in any of the mycoplasmas examined. The stereoisomer of lactic acid accumulated during growth correlated perfectly with the type of NAD-dependent lactic dehydrogenase found in each mycoplasma. The types of lactic dehydrogenase activity found in these mycoplasmas were not related to genome size, DNA base composition, or sterol dependence.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEBY C. A., MORTON R. K. Lactic dehydrogenase and cytochrome b2 of baker's yeast; purification and crystallization. Biochem J. 1959 Mar;71(3):492–499. doi: 10.1042/bj0710492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak A. L., Black F. T., Christiansen C., Freundt E. A. Genome size of mycoplasmal DNA. Nature. 1969 Dec 20;224(5225):1209–1210. doi: 10.1038/2241209a0. [DOI] [PubMed] [Google Scholar]
- Barile M. F., Schimke R. T., Riggs D. B. Presence of the arginine dihydrolase pathway in Mycoplasma. J Bacteriol. 1966 Jan;91(1):189–192. doi: 10.1128/jb.91.1.189-192.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J. M. Artifact produced in disc electrophoresis by ammonium persulfate. Science. 1967 Apr 14;156(3772):256–257. doi: 10.1126/science.156.3772.256. [DOI] [PubMed] [Google Scholar]
- CASTREJON-DIEZ J., FISHER T. N., FISHER E., Jr GLUCOSE METABOLISM OF TWO STRAINS OF MYCOPLASMA LAIDLAWII. J Bacteriol. 1963 Oct;86:627–636. doi: 10.1128/jb.86.4.627-636.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Glucose-dependent secretion and destruction of hydrogen peroxide by Mycoplasma pneumoniae. J Bacteriol. 1969 May;98(2):547–551. doi: 10.1128/jb.98.2.547-551.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Garvie E. I. Lactic dehydrogenases of strains of the genus Leuconostoc. J Gen Microbiol. 1969 Sep;58(1):85–94. doi: 10.1099/00221287-58-1-85. [DOI] [PubMed] [Google Scholar]
- Gasser F. Electrophoretic characterization of lactic dehydrogenases in the genus Lactobacillus. J Gen Microbiol. 1970 Aug;62(2):223–239. doi: 10.1099/00221287-62-2-223. [DOI] [PubMed] [Google Scholar]
- Gasser F., Gasser C. Immunological relationships among lactic dehydrogenases in the genera Lactobacillus and Leuconostoc. J Bacteriol. 1971 Apr;106(1):113–125. doi: 10.1128/jb.106.1.113-125.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMANN E., DIKSTEIN S. A D-lactic dehydrogenase from Leuconostoc mesenteroides. Nature. 1961 Apr 22;190:346–346. doi: 10.1038/190346a0. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Barnes E. M., Jr Mechanisms of active transport in isolated membrane vesicles. II. The mechanism of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in membrane preparations from Escherichia coli. J Biol Chem. 1971 Sep 10;246(17):5523–5531. [PubMed] [Google Scholar]
- Kline E. S., Mahler H. R. The lactic dehydrogenases of E. coli. Ann N Y Acad Sci. 1965 Jul 31;119(3):905–919. doi: 10.1111/j.1749-6632.1965.tb47451.x. [DOI] [PubMed] [Google Scholar]
- LECCE J. G., MORTON H. E. Metabolic studies on three strains of Pleuropneumonia-like organisms isolated from man. J Bacteriol. 1954 Jan;67(1):62–68. doi: 10.1128/jb.67.1.62-68.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low I. E., Eaton M. D., Proctor P. Relation of catalase to substrate utilization by Mycoplasma pneumoniae. J Bacteriol. 1968 Apr;95(4):1425–1430. doi: 10.1128/jb.95.4.1425-1430.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell W. M. A potential source of electrophoretic artifacts in polyacrylamide gels. Biochim Biophys Acta. 1967 Sep 19;147(1):171–174. doi: 10.1016/0005-2795(67)90101-8. [DOI] [PubMed] [Google Scholar]
- Morowitz H. J., Bode H. R., Kirk R. G. The nucleic acids of mycoplasma. Ann N Y Acad Sci. 1967 Jul 28;143(1):110–114. doi: 10.1111/j.1749-6632.1967.tb27650.x. [DOI] [PubMed] [Google Scholar]
- NEIMARK H. C., PICKETT M. J. Products of glucose metabolism by pleuropneumonialike organisms. Ann N Y Acad Sci. 1960 Jan 15;79:531–537. doi: 10.1111/j.1749-6632.1960.tb42719.x. [DOI] [PubMed] [Google Scholar]
- Neimark H. C. Division of mycoplasmas into subgroups. J Gen Microbiol. 1970 Oct;63(2):249–263. doi: 10.1099/00221287-63-2-249. [DOI] [PubMed] [Google Scholar]
- Neimark H. Heterogeneity among the mycoplasma and relationships to bacteria. Ann N Y Acad Sci. 1967 Jul 28;143(1):31–37. doi: 10.1111/j.1749-6632.1967.tb27640.x. [DOI] [PubMed] [Google Scholar]
- RODWELL A. W., RODWELL E. S. The breakdown of carbohydrates by Asterococcus mycoides, the organism of bovine pleuropneumonia. Aust J Biol Sci. 1954 Feb;7(1):18–30. [PubMed] [Google Scholar]
- SMITH S. L., VANDEMARK P. J., FABRICANT J. RESPIRATORY PATHWAYS IN THE MYCOPLASMA. I. LACTATE OXIDATION BY MYCOPLASMA GALLISEPTICUM. J Bacteriol. 1963 Nov;86:893–897. doi: 10.1128/jb.86.5.893-897.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNOSWELL A. M. OXIDIZED NICOTINAMIDE-ADENINE DINUCLEOTIDE-INDEPENDENT LACTATE DEHYDROGENASES OF LACTOBACILLUS ARABINOSUS 17.5. Biochim Biophys Acta. 1963 Sep 3;77:7–9. doi: 10.1016/0006-3002(63)90464-5. [DOI] [PubMed] [Google Scholar]
- Sobeslavsky O., Chanock R. M. Peroxide formation by mycoplasmas which infect man. Proc Soc Exp Biol Med. 1968 Nov;129(2):531–535. doi: 10.3181/00379727-129-33362. [DOI] [PubMed] [Google Scholar]
- Somerson N. L., Walls B. E., Chanock R. M. Hemolysin of Mycoplasma pneumoniae: tentative identification as a peroxide. Science. 1965 Oct 8;150(3693):226–228. doi: 10.1126/science.150.3693.226. [DOI] [PubMed] [Google Scholar]
- Stockland A. E., San Clemente C. L. Multiple forms of lactate dehydrogenase in Staphylococcus aureus. J Bacteriol. 1969 Oct;100(1):347–353. doi: 10.1128/jb.100.1.347-353.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOURTELLOTTE M. E., JACOBS R. E. Physiological and serologic comparisons of PPLO from various sources. Ann N Y Acad Sci. 1960 Jan 15;79:521–530. doi: 10.1111/j.1749-6632.1960.tb42718.x. [DOI] [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Chemical characterization of D-lactate dehydrogenase from Escherichia coli B. J Biol Chem. 1968 May 25;243(10):2579–2586. [PubMed] [Google Scholar]
- Thomas L., Bitensky M. W. Methaemoglobin formation by Mycoplasma gallisepticum: the role of hydrogen peroxide. Nature. 1966 May 28;210(5039):963–964. doi: 10.1038/210963a0. [DOI] [PubMed] [Google Scholar]
- Van Demark P. J. Respiratory pathways in the mycoplasma. Ann N Y Acad Sci. 1967 Jul 28;143(1):77–84. doi: 10.1111/j.1749-6632.1967.tb27647.x. [DOI] [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]
- Wittenberger C. L., Angelo N. Purificationa and properties of a fructose-1,6-diphosphate-activated lactate dehydrogenase from Streptococcus faecalis. J Bacteriol. 1970 Mar;101(3):717–724. doi: 10.1128/jb.101.3.717-724.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries W., Stouthamer A. H. Fermentation of glucose, lactose, galactose, mannitol, and xylose by bifidobacteria. J Bacteriol. 1968 Aug;96(2):472–478. doi: 10.1128/jb.96.2.472-478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]