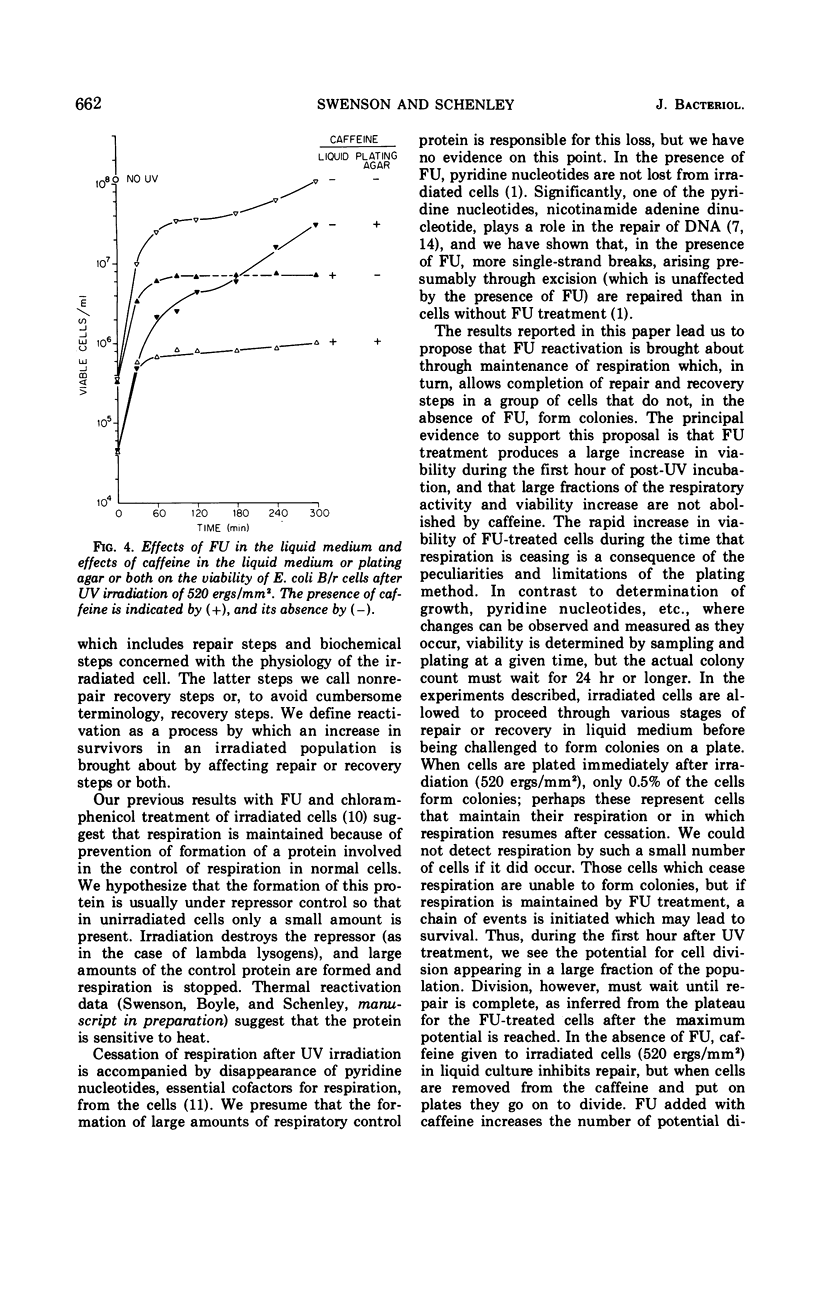
Abstract
Escherichia coli B/r cells grown on a glycerol-containing medium and ultraviolet (UV)-irradiated to about 0.5% survival respire for about 1 hr and then cease for several hours. The cells that have completed repair and recovery processes begin to divide about 120 min after UV treatment, but this division is completely inhibited in liquid medium by caffeine, which delays repair of the irradiated deoxyribonucleic acid (DNA). When 5-fluorouracil (FU) is used to maintain respiration, the number of cells which form colonies when plated increases about 60-fold within 1 hr after irradiation. At least part of this increase does not involve repair while the cells are in the liquid medium because when caffeine is present there is still a 20-fold increase in colony formation. We conclude that many irradiated cells, although capable of carrying out complete and accurate repair of their DNA, die of respiratory failure; only when continuance of respiration is favored by FU treatment is their colony-forming potential realized. After an early increase, the number of cells able to form colonies in medium that contains FU remains constant while the completion of repair and recovery occurs. After these processes are completed, the number of cells able to form colonies increases slowly, except in the presence of caffeine, presumably because the late increase requires that repair steps take place while the cells are in liquid medium prior to cell division.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle J. M., Schenley R. L., Swenson P. A. Role of pyridine nucleotides in 5-fluorouracil-mediated reactivation of ultraviolet radiation damage. J Bacteriol. 1971 Jun;106(3):896–903. doi: 10.1128/jb.106.3.896-903.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domon M., Barton B., Porte A., Rauth A. M. The interaction of caffeine with ultra-violet-light-irradiated DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(4):395–399. doi: 10.1080/09553007014550481. [DOI] [PubMed] [Google Scholar]
- Hamkalo B. A., Swenson P. A. Effects of ultraviolet radiation on respiration and growth in radiation-resistant and radiation-sensitive strains of Escherichia coli B. J Bacteriol. 1969 Sep;99(3):815–823. doi: 10.1128/jb.99.3.815-823.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm W. Analysis of photoenzymatic repair of UV lesions in DNA by single light flashes. V. Determination of the reaction-rate constants in E. coli cells. Mutat Res. 1970 Oct;10(4):277–290. doi: 10.1016/0027-5107(70)90043-6. [DOI] [PubMed] [Google Scholar]
- Harm W. Differential effects of acriflavine and caffeine on various ultraviolet-irradiated Escherichia coli strains and T1 phage. Mutat Res. 1967 Mar-Apr;4(2):93–110. doi: 10.1016/0027-5107(67)90061-9. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Diphosphopyridine nucleotide: a cofactor for the polynucleotide-joining enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1700–1704. doi: 10.1073/pnas.57.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideropoulos A. S., Shankel D. M. Mechanism of caffeine enhancement of mutations induced by sublethal ultraviolet dosages. J Bacteriol. 1968 Jul;96(1):198–204. doi: 10.1128/jb.96.1.198-204.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson P. A., Schenley R. L., Boyle J. M. Effects of 5-fluorouracil and pantoyl lactone on viability and division of ultraviolet-irradiated Escherichia coli FIL + and FIL - . J Gen Microbiol. 1972 Aug;71(3):567–574. doi: 10.1099/00221287-71-3-567. [DOI] [PubMed] [Google Scholar]
- Swenson P. A., Schenley R. L. Evidence for the control of respiration by DNA in ultraviolet-irradiated Escherichia coli B-r cells. Mutat Res. 1970 May;9(5):443–453. doi: 10.1016/0027-5107(70)90028-x. [DOI] [PubMed] [Google Scholar]
- Swenson P. A., Schenley R. L. Role of Pyridine Nucleotides in the Control of Respiration in Ultraviolet-Irradiated Escherichia coli B/r Cells. J Bacteriol. 1970 Dec;104(3):1230–1235. doi: 10.1128/jb.104.3.1230-1235.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSO P. O., LU P. INTERACTION OF NUCLEIC ACIDS, I. PHYSICAL BINDING OF THYMINE, ADENINE, STEROIDS, AND AROMATIC HYDROCARBONS TO NUCLEIC ACIDS. Proc Natl Acad Sci U S A. 1964 Jan;51:17–24. doi: 10.1073/pnas.51.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B., Little J. W., Oshinsky C. K., Gellert M. Enzymatic joining of DNA strands: a novel reaction of diphosphopyridine nucleotide. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1841–1848. doi: 10.1073/pnas.57.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]


