Abstract
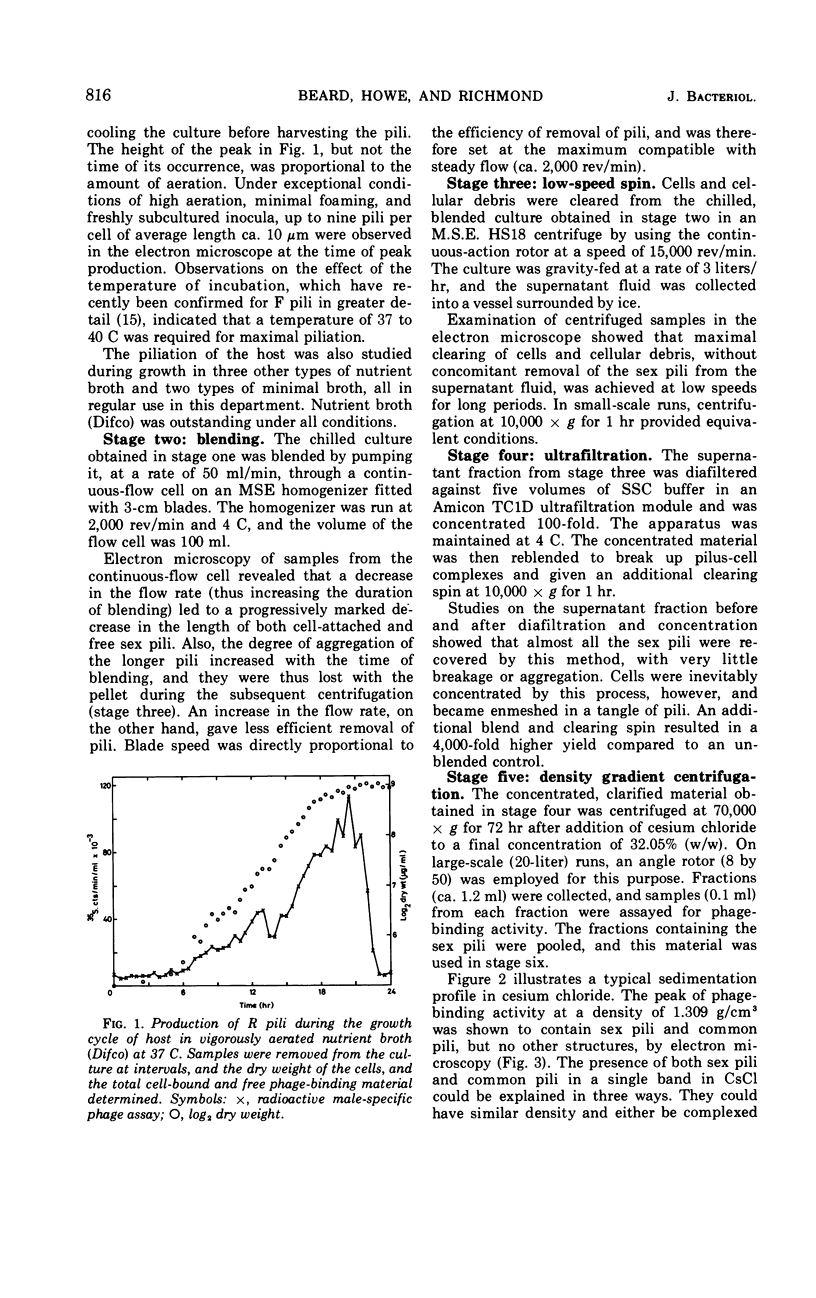
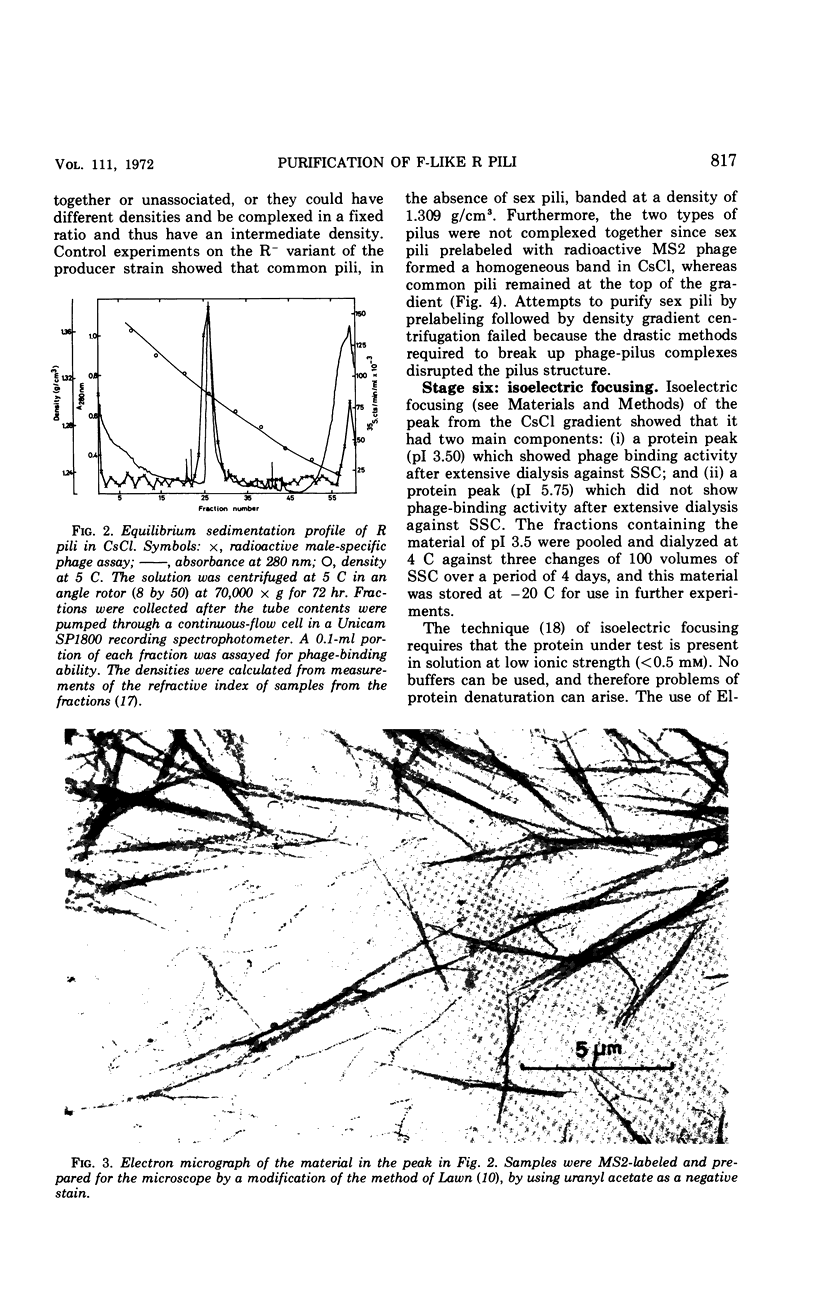
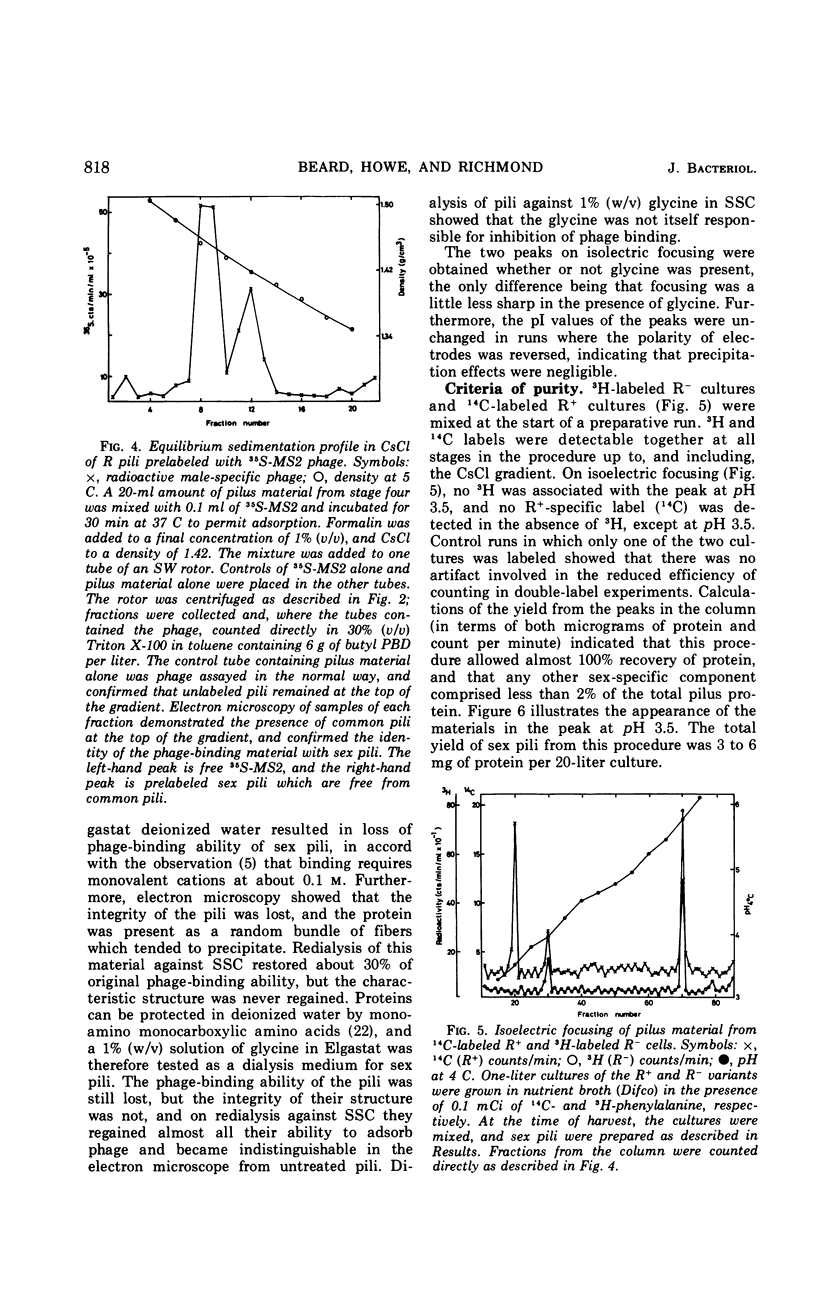
A procedure for the purification of sex pili is described. Escherichia coli K-12 carrying Rldrd19 was grown in nutrient broth and blended at the time of peak sex pilus production. The cells were removed by centrifugation, and the supernatant fraction was concentrated, dialyzed, and clarified in an ultrafiltration system. After an additional blend and a clearing spin, the material was centrifuged in a CsCl gradient, and the fractions containing the sex pili were subjected to isoelectric focusing. About 5 mg of intact pili of approximately 98% purity were obtained by this method from about 100 g (wet weight) of cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard J. P., Howe T. G., Richmond M. H. Centrifugation properties of sex pili. J Mol Biol. 1972 May 14;66(2):311–313. doi: 10.1016/0022-2836(72)90483-4. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The properties of sex pili, the viral nature of "conjugal" genetic transfer systems, and some possible approaches to the control of bacterial drug resistance. CRC Crit Rev Microbiol. 1971 May;1(1):105–160. doi: 10.3109/10408417109104479. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Danziger R. E., Paranchych W. Stages in phage R17 infection. II. Ionic requirements for phage R17 attachment to F-pili. Virology. 1970 Mar;40(3):547–553. doi: 10.1016/0042-6822(70)90198-4. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Compatibility groups among fi - R factors. Nature. 1971 Nov 26;234(5326):222–223. doi: 10.1038/234222a0. [DOI] [PubMed] [Google Scholar]
- Ippen K. A., Valentine R. C. An assay for the male substance (F-pili) of Escherichia coli K-12. Biochem Biophys Res Commun. 1965 Oct 8;21(1):21–27. doi: 10.1016/0006-291x(65)90420-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawn A. M., Meynell E., Cooke M. Mixed infections with bacterial sex factors: sex pili of pure and mixed phenotype. Ann Inst Pasteur (Paris) 1971 Jan;120(1):3–8. [PubMed] [Google Scholar]
- Lawn A. M. Morphological features of the pili associated with Escherichia coli K 12 carrying R factors or the F factor. J Gen Microbiol. 1966 Nov;45(2):377–383. doi: 10.1099/00221287-45-2-377. [DOI] [PubMed] [Google Scholar]
- Meynell E., Cooke M. Repressor-minus and operator-constitutive de-repressed mutants of F-like R factors: their effect on chromosomal transfer by HfrC. Genet Res. 1969 Dec;14(3):309–313. doi: 10.1017/s0016672300002123. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C. P., Lavin K. Some effects of temperature on the growth of F pili. J Bacteriol. 1971 Sep;107(3):671–682. doi: 10.1128/jb.107.3.671-682.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C., Raizen E., Knight W. S., Brinton C. C., Jr Functions of F pili in mating-pair formation and male bacteriophage infection studies by blending spectra and reappearance kinetics. J Bacteriol. 1969 Jun;98(3):1307–1319. doi: 10.1128/jb.98.3.1307-1319.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Wadström T. Studies on extracellular proteins from Staphylococcus aureus. IV. Separation of alpha-toxin by isoelectric focusing. Biochim Biophys Acta. 1968 Oct 21;168(2):228–242. doi: 10.1016/0005-2795(68)90146-3. [DOI] [PubMed] [Google Scholar]
- Wendt L. W., Ippen K. A., Valentine R. General properties of F-pili. Biochem Biophys Res Commun. 1966 May 25;23(4):375–380. doi: 10.1016/0006-291x(66)90736-4. [DOI] [PubMed] [Google Scholar]




