Abstract
The activity of l-type Ca2+ channels is increased by dihydropyridine (DHP) agonists and inhibited by DHP antagonists, which are widely used in the therapy of cardiovascular disease. These drugs bind to the pore-forming α1 subunits of l-type Ca2+ channels. To define the minimal requirements for DHP binding and action, we constructed a high-affinity DHP receptor site by substituting a total of nine amino acid residues from DHP-sensitive l-type α1 subunits into the S5 and S6 transmembrane segments of domain III and the S6 transmembrane segment of domain IV of the DHP-insensitive P/Q-type α1A subunit. The resulting chimeric α1A/DHPS subunit bound DHP antagonists with high affinity in radioligand binding assays and was inhibited by DHP antagonists with high affinity in voltage clamp experiments. Substitution of these nine amino acid residues yielded 86% of the binding energy of the l-type α1C subunit and 92% of the binding energy of the l-type α1S subunit for the high-affinity DHP antagonist PN200–110. The activity of chimeric Ca2+ channels containing α1A/DHPS was increased 3.5 ± 0.7-fold by the DHP agonist (−)Bay K8644. The effect of this agonist was stereoselective as in l-type Ca2+ channels since (+) Bay K8644 inhibited the activity of α1A/DHPS. The results show conclusively that DHP agonists and antagonists bind to a single receptor site at which they have opposite effects on Ca2+ channel activity. This site contains essential components from both domains III and IV, consistent with a domain interface model for binding and allosteric modulation of Ca2+ channel activity by DHPs.
Voltage-gated Ca2+ channels mediate Ca2+ influx in response to membrane depolarization and thereby initiate cellular activities such as secretion, contraction, and gene expression. Several types of voltage-gated Ca2+ channels have been distinguished by their physiological and pharmacological properties and have been designated L, N, P/Q, R, and T (reviewed in refs. 1 and 2). l-Type Ca2+ channels are the molecular targets for the dihydropyridine (DHP) Ca2+ channel blockers that are widely used in the therapy of cardiovascular diseases; DHP modulation is the hallmark used to characterize these channels (reviewed in refs. 3 and 4).
The l-type Ca2+ channels consist of pore-forming α1 subunits of 190 to 250 kDa in association with disulfide-linked α2δ subunits of approximately 140 kDa, intracellular β subunits of 55 to 72 kDa, and, for the skeletal muscle l-type channel, an additional transmembrane γ subunit of 33 kDa (5). The α1 subunits confer the characteristic pharmacologic and functional properties of Ca2+ channels, but their function is modulated by association with the auxiliary subunits. The pore-forming α1 subunits can be divided into two distinct families, l-type and non-l-type, that share less than 40% amino acid identity. The l-type α1 subunit family includes α1S, which is expressed in skeletal muscle (6), α1C, which is expressed in cardiac and smooth muscle, neurons, and many other cell types (7–9), and α1D, which is expressed in endocrine and neuronal cells (10, 11). The non-l-type α1 subunit family consists of at least three distinct gene products that are expressed primarily in neurons: α1B (N-type; refs. 12 and 13), α1A (P/Q-type; refs. 14 and 15), and α1E (R-type; ref. 16). The α1 subunits contain four homologous domains (I through IV) that each contain six transmembrane segments (S1 through S6) (6–16).
The DHPs are allosteric modulators that act on l-type Ca2+ channels as either agonists or antagonists (reviewed in refs. 3, 4, and 17). Charged DHPs are thought to traverse an extracellular pathway to gain access to the DHP receptor site located within the lipid bilayer 11–14 Å from the extracellular surface of the cell membrane (18–21). Photoreactive DHPs specifically label the α1 subunit of the Ca2+ channel (5, 22–27). The predominant sites of labeling correspond to transmembrane segment S6 in domain III (IIIS6) and transmembrane segment S6 in domain IV (IVS6; refs. 28–31). Analysis of chimeric Ca2+ channels implicated transmembrane segments IIIS5, IIIS6, and IVS6 (32–34) in DHP binding. Site-directed mutagenesis of single amino acid residues in segments IIIS6 and IVS6 that are conserved in all Ca2+ channel subtypes had large effects on DHP affinity (35, 36). In addition, mutations of residues that differ between l-type and non-l-type Ca2+ channels revealed multiple amino acids in transmembrane segments IIIS5, IIIS6, and IVS6 that are important determinants of high-affinity binding of DHP agonists and antagonists to l-type Ca2+ channels (34–38). In the experiments reported here, we have substituted nine key amino acid residues that are present in all l-type α1 subunits into the non-l-type α1A subunit and measured both activation by DHP agonists and inhibition by DHP antagonists. The results show that these nine amino acid residues are sufficient to constitute a high-affinity receptor site for DHPs that responds appropriately to both DHP agonists and antagonists and is stereoselective like the native DHP receptor of l-type Ca2+ channels.
EXPERIMENTAL PROCEDURES
Construction of Mutant Ca2+ Channels.
For the construction of α1A/DHPS, the AscI–BsrGI fragment (nucleotides 2582–5408) from α1A (14) was ligated into pNEB193 (New England Biolabs) and used as template for mutagenic PCR reactions. The mutations in transmembrane segment IIIS5 were made in a single PCR reaction using primers that overlapped the BstXI (nucleotide 2950) and ApoI (nucleotide 4057) sites. The mutagenic mismatches were incorporated by the primer overlapping the ApoI site. The mutations in IIIS6 were made in a single PCR reaction using primers that overlapped the XcmI (nucleotide 4316) and BspEI (nucleotide 5102) sites. The mutagenic mismatches were incorporated by the primer overlapping the XcmI site. The mutations in IVS6 were made using the splice overlap extension method (39). In the first reactions, mutagenic primers annealing within the cDNA encoding IVS6 were paired with primers that overlapped either the BspEI or the NotI (nucleotides 5102 and 5384, respectively) sites that flank IVS6. These overlapping fragments were coupled and amplified in a subsequent PCR reaction to give the full-length BspEI–NotI fragment containing the mutations in IVS6. The PCR products containing the desired mutations were assembled in AscI–BsrGI/pNEB193, and the AscI–BsrGI fragment was ligated into full-length α1A in the expression vector pMT2. The desired mutations were verified, and the integrity of the clone was confirmed by cDNA sequencing and extensive restriction digest analysis.
Expression of Ca2+ Channels.
Human tsA-201 cells, a simian virus 40 (SV40) T-antigen expressing derivative of the human embryonic kidney cell line HEK293 (a gift of Robert DuBridge, Cell Genesis, Foster City, CA), were maintained in DMEM/F-12 (GIBCO/BRL) enriched with 10% fetal bovine serum. Human tsA-201 cells were cotransfected with α1A, α1A/DHPS, or α1CII (9); β1b (40); α2δ (41); and CD8 antigen (EBO-pCD-Leu2, American Type Culture Collection) such that the molar ratio of the plasmids was 1:1:1:0.8. Cells were transfected by Ca2+ phosphate precipitation (42), and cells were replated at low density for electrophysiological recording 20–24 hours later. The α1CII cDNA was in the expression plasmid ZEM 229 (a gift of Eileen Mulvihill, Zymogenetics, Seattle). The α2δ cDNA was in the expression plasmid ZEM 228 (a gift of Eileen Mulvihill). The α1A, α1A/DHPS, and β1b cDNA were in the expression plasmid pMT2 (Genetics Institute, Boston).
Electrophysiology.
Transfectants were recognized by labeling with anti-CD8 antibody-coated beads (M450 CD8 Dynabeads, Dynal). Barium currents through Ca2+ channels were recorded using the whole-cell configuration of the patch clamp technique. Patch electrodes were pulled from VWR micropipettes and fire-polished to produce an inner tip diameter of 4–6 μm. Currents were recorded using an Axon Instruments Axopatch 200B patch clamp amplifier and filtered at 1 or 2 kHz (8-pole Bessel filter, −3 dB). Voltage pulses were applied and data were acquired using pClamp6 software (Axon Instruments). Linear leak and capacitance currents have been subtracted using an on-line P/−4 subtraction paradigm. (±)PN200–110 was applied to cells using a fast perfusion system with background perfusion. (−)Bay K 8644 was added to the bath, without background perfusion, as a 10× stock. The bath saline contained 150 mM of Tris, 2 mM of MgCl2, and 10 mM of BaCl2. The intracellular saline contained 130 mM of N-methyl-d-glucamine, 10 mM of EGTA, 60 mM of Hepes, 2 mM of MgATP, and 1 mM of MgCl2. The pH of both solutions was adjusted to 7.3 with methanesulfonic acid. All experiments were performed at room temperature (20–23°C).
Preparation of Membranes.
Transfected tsA-201 cells were washed twice, scraped from the cell culture dish, and homogenized using a glass-teflon homogenizer in Buffer A (50 mM of Tris/100 μM of phenylmethylsulfonyl fluoride/100 μM of benzamidine/1.0 μM of pepstatin A/1.0 μg/ml of leupeptin/2.0 μg/ml of aprotinin, pH 8.0). The homogenate was centrifuged at 700 × g for 5 min. The resulting pellet was discarded and the supernatant was centrifuged 30 min at 100,000 × g. The supernatant was discarded and the membrane pellet was washed and homogenized in Buffer A. The resulting membrane homogenate was divided into aliquots and stored at −80°C for up to 3 months with no detectable loss of (+)-[3H]PN200–110 binding activity.
Radioligand Binding.
Equilibrium binding assays were performed in Buffer A with 20–200 μg of membrane protein, 0.01–5 nM of (+)-[3H]PN200–110, and 1 mM of Ca2+ at 32°C for 180–210 min. Nonspecific binding was determined in the presence of 1 μM (±)-PN200–110, and bound and free ligands were separated by vacuum filtration over GF/C glass fiber filters. Filters were washed using ice-cold wash buffer (10 mM of Tris/1% polyethylene glycol 8000/0.1% BSA/0.01% Triton X-100, pH 8.0), and bound radioactivity was detected by liquid scintillation counting. Dissociation constants (KD) were determined using the radioligand data analysis program ligand (Biosoft, Cambridge, U.K.). All data are means ± SEM.
RESULTS
Construction of a High Affinity DHP Site in α1A.
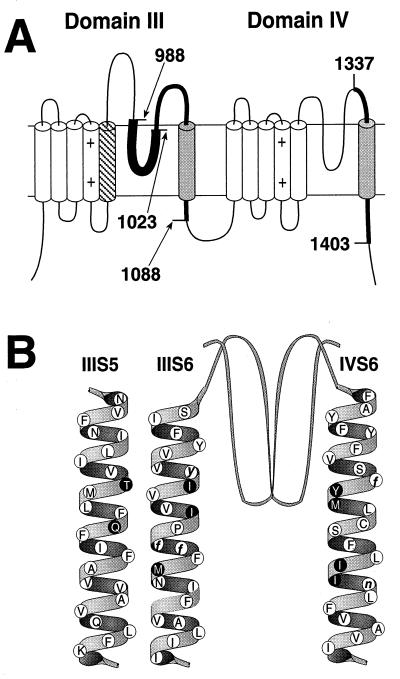
The key amino acid residues required for high affinity DHP binding as determined in previous studies are illustrated in Fig. 1. Photoaffinity labeling followed by antibody mapping of the labeled peptide fragments showed that transmembrane segments IIIS6 and IVS6 are components of the DHP receptor site in l-type Ca2+ channels (refs. 28 and 29; Fig. 1A, shaded segments). Construction and analysis of chimeric Ca2+ channels confirmed the importance of transmembrane segments IIIS6 (32) and IVS6 (32–34) and further demonstrated an important role of transmembrane segment IIIS5 (ref. 32; Fig. 1A, diagonally hatched segment). Mutation of single amino acid residues identified three conserved amino acid residues in transmembrane segment IIIS6 and two in IVS6 that are important for DHP binding and are present in all Ca2+ channels (refs. 35 and 36; Fig. 1B, white circles, lowercase letters). In addition, two l-type-specific residues in segment IIIS5, three in segment IIIS6, and four in segment IVS6 were found to be important for DHP binding (refs. 34–38; Fig. 1B, dark circles). The success of experiments with chimeric Ca2+ channels in which segments of l-type Ca2+ channels are transferred into non-l-type Ca2+ channels suggests that the basic structure of non-l-type channels is appropriate to support DHP binding, even though the amino acid sequence is less than 40% identical. Similarly, the requirement for amino acid residues that are conserved between l-type and non-l-type Ca2+ channels for high-affinity DHP binding suggests that the DHP binding site contains structural features that are common to all Ca2+ channels. If all of the l-type-specific amino acid residues that are required for high affinity DHP binding have been identified, substitution of the nine amino acid residues highlighted as dark circles in Fig. 1B into a non-l-type Ca2+ channel should be sufficient to construct a high-affinity DHP binding site. To test this idea, we substituted these nine amino acid residues for their counterparts in the DHP-insensitive rbA isoform of the α1A subunit of P/Q-type Ca2+ channels using site-directed mutagenesis methods as described in Experimental Procedures.
Figure 1.
Localization of the DHP binding site in l-type channels. (A) Studies utilizing DHP photoaffinity labels and site-directed antibodies (28, 29) identified transmembrane segments IIIS6 and IVS6 of l-type Ca2+ channels as components of the DHP binding site. Boundaries of the labeled peptides (shaded cylinders and thick lines) are shown as amino acid sequence numbers from α1S (6). Subsequent studies utilizing chimeric Ca2+ channels further demonstrated the role of IIIS6 and IVS6 as well as transmembrane domain IIIS5 in DHP moduation of l-type channels (32–34). (B) Structure of α1A/DHPS. Studies of single amino acid substitutions in l-type channels revealed nine l-type-specific (black circles with white letters) and five conserved (white circles with black lower case letters) amino acid residues to be critical for DHP binding and block of l-type channels (34–38). The nine l-type-specific amino acid residues critical for DHP action were inserted into the DHP-insensitive α1A subunit amino acid sequence (14) (white circles with black letters) to a construct a mutant α1A subunit that is modulated by DHPs (α1A/DHPS).
Functional Characteristics of the DHP-Sensitive α1A Chimera.
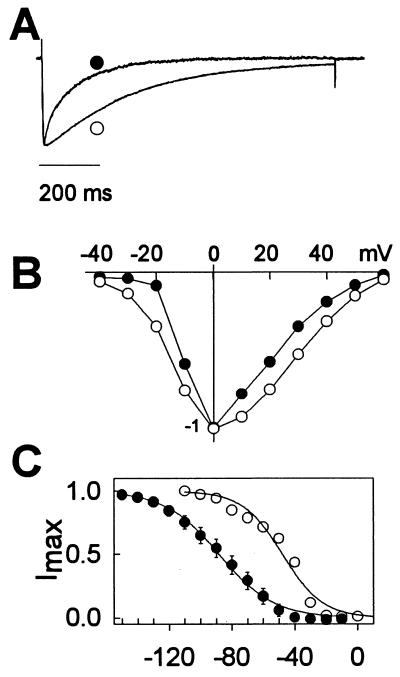
The resulting α1A/DHPS subunit was expressed in tsA-201 cells with α2δ and β1b subunits and analyzed by whole cell voltage clamp as described in Experimental Procedures. Barium currents through the α1A/DHPS subunit activated normally but inactivated more rapidly than wild-type α1A (Fig. 2A; α1A, τh = 279 ms; α1A/DHPS, τh = 123 ms). The two channels had similar current-voltage relationships with peak barium current near 0 mV (Fig. 2B). However, the voltage dependence of inactivation of α1A/DHPS was shifted 40 mV toward more negative membrane potentials compared with wild-type α1A (Fig. 2C; α1A, Vh1/2 = −48 mV; α1A/DHPS, Vh1/2 = −88 mV). Thus, the α1A/DHPS chimera is functional as a Ca2+ channel, but has altered inactivation properties.
Figure 2.
Biophysical properties of Ca2+ channels containing α1A and α1A/DHPS. (A) Ca2+ currents were evoked from cells expressing α1A (open circle) or α1A/DHPS (solid circle) subunits along with the β1b and α2δ subunits by a 1s depolarization to 0 mV from a holding potential of −120 mV (α1A/DHPS) or −80 mV (α1A). Current amplitudes were normalized to facilitate comparison of inactivation kinetics. The time courses of inactivation were fit to a single exponential function. The time constant of inactivation (τ) was 279 ± 28 ms (n = 6) for wild type and 123 ± 6 ms (n = 6) for the mutant. (B) Current-voltage relationships for Ca2+ channels containing α1A or α1A/DHPS. From a holding potential of −120 mV (α1A/DHPR; solid circles) or −80 mV (α1A, open circles), the membrane potential was depolarized in 10 mV increments for 100 ms to test pulse potentials from −40 mV to +60 mV and barium currents were recorded. (C) Voltage-dependent inactivation of Ca2+ channels containing α1A (open circles) and α1A/DHPS (solid circles). Cells expressing α1A or α1A/DHPS were held at −80 mV or −120 mV, respectively. From these holding potentials, cells were depolarized to the indicated conditioning pulse potentials for 10 seconds, followed immediately by a 100 ms test pulse to 0 mV. The amplitude of the Ca2+ current during the test pulse is plotted against the voltage of the preceding 10 s conditioning pulse. Smooth lines are fits to a Boltzman function with slope factors of 12.7 and 17.3 for α1A and α1A/DHPS, respectively. For α1A, Vh1/2 = −47.7 ± 1.8 mV (n = 6), and for α1A/DHPs, Vh1/2 = −88.1 ± 1.0 mV (n = 4).
Inhibition of Barium Currents Through α1A/DHPS by a DHP Antagonist.
DHP antagonists bind with higher affinity to the inactivated state of l-type Ca2+ channels (43–45). To compare the DHP sensitivity of wild-type α1A and α1A/DHPS, we compensated for the shift in the voltage dependence of inactivation by measuring the block of barium currents from holding potentials that differed by 40 mV: −80 mV for α1A and −120 mV for α1A/DHPS. These conditions yield the same ratio of resting and inactivated channels for each α1 subunit. The more negative membrane potential used for the α1A/DHPS chimera would reduce inhibition of l-type Ca2+ channels by DHPs, so it is unlikely that this membrane potential per se would increase inhibition of the α1A/DHPS chimera.
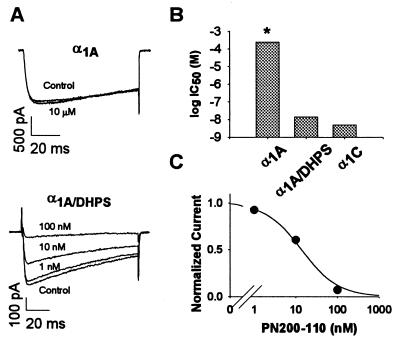
The high-affinity DHP antagonist PN200–110 (isradipine) had no effect on P/Q-type Ca2+ channels containing α1A, even at the high concentration of 10 μM (Fig. 3A). In contrast, chimeric Ca2+ channels containing α1A/DHPS were substantially inhibited by 10 nM of PN200–110 and completely inhibited by 100 nM of PN200–110 (Fig. 3A). The IC50 value estimated from titration experiments was 13.8 nM ± 3 nM (Fig. 3C, n = 9). This compares favorably with the IC50 of 6.8 nM ± 1 nM for block of l-type Ca2+ channels containing α1C expressed with α2δ and β1b in the same cell line (Fig. 3B). Ca2+ channels containing α1A are unaffected by PN200–110 up to at least 10 μM. Based on this, the minimum estimate of the IC50 for inhibition of P/Q-type Ca2+ channels containing α1A is 250 μM, more than four orders of magnitude higher than for α1A/DHPS. Thus, substitution of nine amino acid residues in α1A is sufficient to constitute a high affinity receptor site for a DHP antagonist with more than 10,000-fold higher affinity than wild-type α1A.
Figure 3.
Inhibition of Ca2+ channels containing α1A, α1C, and α1A/DHPS subunits by the DHP antagonist PN200–110. (A) Representative current traces from cells expressing α1A or α1A/DHPS in the presence and absence of the indicated concentrations of PN200–110. Cells expressing α1A or α1A/DHPS were held at −80 or −120, respectively, and depolarized to 0 mV for 100 ms every 20 seconds. After a control baseline had been established, PN200–110 was perfused onto the cells at the concentrations indicated. (B) IC50 values for inhibition of α1A, α1A/DHPS, and α1C by PN200–110. The IC50 for the α1A/DHPS channel was 13.8 ± 3.0 nM (n = 9); for the α1C channel, IC50 was 6.8 ± 1.2 nM (36). The asterisk over the IC50 value for the α1A channel (250 μM) is to indicate that this value is a lower limit since no block was detected at 10 μM PN200–110, the highest concentration studied. (C) Concentration-response curve of α1A/DHPS for PN200–110. The mean data were fit to the equation 1 − 1/(1+([IC50]/[PN200–110])). Standard error (n = 9) was smaller than the diameter of the symbols in the figure. Current amplitudes at each drug concentration were expressed as a fraction of the current amplitude in the absence of drug.
Increase of Barium Currents Through α1A/DHPS by a DHP Agonist.
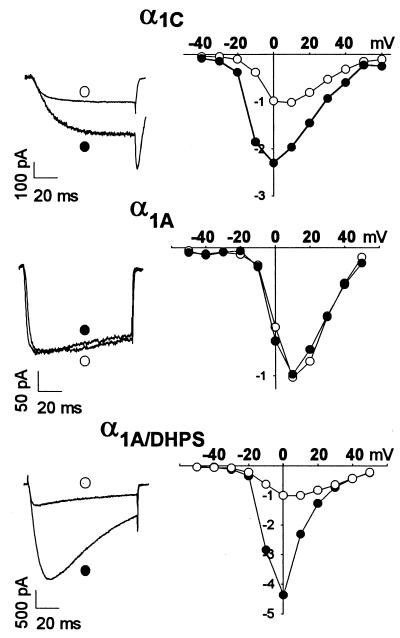
DHPs are allosteric modulators of l-type Ca2+ channels and can either inhibit or enhance channel activity (3, 4, 17). Activation of l-type Ca2+ channels is increased by DHP agonists like (−)Bay K8644 (17). Our previous results on mutations in the α1S subunit of l-type Ca2+ channels indicated that many of the same amino acid residues are required for binding of both DHP agonists and antagonists (35). To test whether DHP agonists can also bind and act at the minimal DHP receptor site constructed in α1A/DHPS, we measured barium currents from a holding potential of −100 mV in the presence and absence of 10 μM (−) Bay K8644 (Fig. 4). Bay K8644 increased Ca2+ currents through l-type Ca2+ channels containing α1C, but had no effect on P/Q-type channels containing α1A (Fig. 4 Left). In contrast, (−) Bay K8644 increased barium currents through chimeric Ca2+ channels containing α1A/DHPS by 3.53 ± 0.66-fold (Fig. 4 Left; n = 3). The increased barium current was observed over a wide range of test pulse potentials, as for l-type Ca2+ channels containing α1C (Fig. 4 Right). In contrast, (−) Bay K8644 had no effect on barium currents through P/Q-type Ca2+ channels containing α1A at any test pulse potential (Fig. 4 Right). Thus, the minimal DHP receptor site of α1A/DHPS is sufficient for both binding and functional effect of a DHP agonist.
Figure 4.
Modulation of Ca2+ channels containing α1C, α1A, or α1A/DHPS subunits by the DHP agonist (−) Bay K 8644. (Left) Current traces from cells expressing the indicated channel types in the absence and presence of 10 μM of (−) Bay K 8644. Cells expressing α1A, or α1A/DHPS were depolarized from a holding potential of −100 mV to 0 mV for 100 ms. Cells expressing α1C were held at −60 mV and depolarized to +10 mV for 100 ms. For Ca2+ channels containing α1A or α 1A/DHPS, a series of ten 1 s depolarizations to 0 mV was used to bring the agonist effect to equilibrium. (Right) Current-voltage relationship before and after addition of 10 μM of (−) Bay K 8644 is shown for each channel type. The peak current amplitude of each I/V curve in the absence of (−) Bay K 8644 was set to 1, and the current amplitudes in the presence of (−) Bay K 8644 were expressed relative to the current amplitudes in the absence of drug. Cells expressing α1A or α1A/DHPS were held at −80 mV or −120 mV, respectively. From these holding potentials, cells were depolarized to the indicated test pulse potentials from −50 mV to +50 mV in 10 mV increments for 100 ms, and barium currents were recorded.
The functional effects of many DHP agonists are stereospecific. For Bay K8644, the (−) enantiomer is an agonist while the (+) isomer is an antagonist (17). As for l-type Ca2+ channels containing α1C, we found that chimeric Ca2+ channels containing α1A/DHPS are inhibited by (+) Bay K8644 (IC50 = 303 ± 102 nM, n = 8; data not shown), and no activating effect of (+) Bay K8644 was observed at any concentration tested. Thus, the stereoselectivity of DHP binding and action is also present in the minimal DHP receptor site constructed in α1A/DHPS.
High Affinity Binding of PN200–110 to α1A/DHPS in Cell Membrane Preparations.
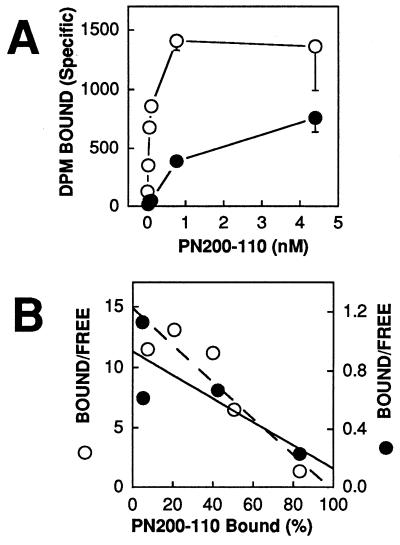
Inhibition of barium currents by PN200–110 measures primarily drug binding to the resting state of Ca2+ channels that is predominant at the negative holding potential. Because DHP antagonists have higher affinity for the inactivated state of Ca2+ channels (43–45), their binding affinity is higher in radioligand binding assays using membrane preparations whose membrane potential is near 0 mV where inactivation is complete. We first compared binding of [3H]PN200–110 at a fixed concentration of 4.4 nM. Ca2+ channels containing α1C bound [3H]PN200–110 specifically (63.7 fmol/mg cell protein) while those containing α1A did not have significant specific binding. As expected from the electrophysiological results, Ca2+ channels containing α1A/DHPS also bound [3H]PN200–110 specifically (36.9 fmol/mg cell protein), but not as well as channels containing α1C. To compare the binding affinity of α1C and α1A/DHPS quantitatively, we carried out a saturation binding study (Fig. 5A) and analyzed the data by Scatchard plot (Fig. 5B, note the different axes for α1C and α1A/DHPS). The results indicate a KD for binding to α1C of 50 pM compared with a KD for α1A/DHPS of 1.48 nM, 27-fold higher. Thus, the α1A/DHPS chimera has high affinity for PN200–110 in a radioligand binding assay as well as in electrophysiological experiments, but the difference in binding between α1C and α1A/DHPS is greater in the ligand binding assay.
Figure 5.
Binding of [3H]PN200–110 to membrane preparations from cells expressing α1C or α1A/DHPS channels. (A) Equilibrium binding of [3H]PN200–110 to membrane preparations from cells expressing α1C (open circles) or α1A/DHPR (solid circles) subunits. Specific binding was determined by subtracting nonspecific binding of [3H]PN200–110 in the presence of 1 μM of unlabeled PN200–110 from total binding in the absence of the unlabeled drug at each [3H] PN200–110 concentration. Means of the data at each concentration are plotted ± SEM (n = 3). (B) Scatchard analysis of equilibrium binding of [3H]PN200–110 to α1C (open circles; dashed line) and α1A/DHPS channels (solid circles; solid line). The ordinate axes are different for each subunit type to normalize the data. The KD values in this experiment were 79 pM for α1C and 1.48 nM for α1A/DHPS.
DISCUSSION
Molecular Requirements for a High Affinity DHP Receptor Site.
Substitution of nine amino acid residues previously shown to be required for DHP binding (34–38) into the sequence of the non-l-type α1A subunit is sufficient to construct a high-affinity DHP receptor site. This site was constructed in the structural context of the α1A subunit that contains at least five conserved residues that are necessary for high-affinity DHP binding (35, 36). The resulting chimera α1A/DHPS has many of the pharmacological properties of an l-type Ca2+ channel with respect to DHPs. The IC50 for inhibition of Ca2+ current by PN200–110 is 13.8 nM, only twofold higher than the IC50 value for inhibition of l-type Ca2+ channels containing α1C expressed under the same conditions. Inhibition of barium currents measures primarily the affinity of the resting state for DHPs because of the negative holding potential used in these experiments. In contrast, the binding experiments on membranes from transfected cells measure primarily the higher affinity of the inactivated state because of the prolonged depolarization of the membranes in the binding assay. The KD for binding of [3H]PN200–110 to Ca2+ channels containing α1A/DHPS is 1.48 nM, compared with 55 pM for l-type Ca2+ channels containing α1C (36) and 250 pM l-type Ca2+ channels containing α1S (35) expressed under the same conditions. Thus, the amino acid substitutions in α1A/DHPS are more effective in establishing binding and block of resting Ca2+ channels than inactivated Ca2+ channels. Nevertheless, of the −14.3 kcal/mol and −13.4 kcal/mol binding energy calculated from the KD values for PN200–110 binding to α1C and α1S, respectively, −12.3 kcal/mol (86% of α1C or 92% of α1S) is observed in α1A/DHPS. The remaining binding energy of α1C and α1S may be contributed by several amino acid residues whose mutation had measurable, but less than twofold, effects on DHP binding affinity (35, 36) or by the overall structural context of α1A versus α1C and α1S.
A Single Receptor Site for DHP Agonists and Antagonists.
DHP agonists inhibit high-affinity binding of DHP antagonists, consistent with the idea that they share the same receptor site (45–47). However, binding studies on intact cells reveal complex interactions between binding of agonist and antagonist DHPs, and it has been hypothesized that agonists and antagonists occupy different sites when causing their opposite effects on Ca2+ channel function (48). In contrast to this hypothesis, our results show conclusively that DHP agonists and antagonists occupy the same receptor site and cause opposite effects on Ca2+ channel activity when bound there. Each of the nine single amino acids substituted in α1A/DHPS is required for high-affinity antagonist binding in l-type Ca2+ channels, and mutations of many of these residues also reduce affinity for binding of agonists to l-type channels. Construction of a minimal DHP receptor site for high-affinity antagonist binding also is sufficient to confer stereoselective agonist binding and action. Molecular differences in the interactions of these two classes of drugs with the same receptor site must be responsible for the dramatic differences in their pharmacological effects.
A Domain-Interface Model for DHP Binding and Action.
Based on the original photoaffinity labeling of the DHP receptor site, it was proposed that DHPs bind to a single site at the interface of domains III and IV (30). This model is confirmed by the results presented here, which show that the minimal receptor site sufficient for high-affinity binding of DHP antagonists and for stereoselective binding and action of DHP agonists includes amino acid residues in transmembrane segments S5 and S6 in domain III and transmembrane S6 in domain IV. Allosteric effectors of enzymes most often bind to sites at the interfaces between subunits or domains (e.g., refs. 49 and 50), and current evidence indicates that the agonist binding site of nicotinic acetylcholine receptors is at a subunit interface (51). Evidently, these sites are particularly sensitive to binding of small ligands, which can alter interactions between domains or subunits when bound at their interface. Based on these analogies, it is likely that DHPs act as allosteric effectors that alter domain-domain interactions within the α1 subunits of Ca2+ channels. Determination of the structural basis for the opposite, stereoselective effects of DHP agonists and antagonists on Ca2+ channel function when bound at the DHP receptor site between domains III and IV will provide further information on the mechanism of action of these drugs and on the interactions between these Ca2+ channel domains in channel gating.
ABBREVIATION
- DHP
dihydropyridine
References
- 1.Tsien R W, Lipscombe D, Madison D, Bley K, Fox A. Trends Neurosci. 1995;18:52–54. [PubMed] [Google Scholar]
- 2.Dunlap K, Luebke J I, Turner T J. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 3.McDonald T F, Pelzer S, Trautwein W, Pelzer D J. Physiol Rev. 1994;74:365–707. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 4.Hockerman G H, Peterson B Z, Johnson B D, Catterall W A. Annu Rev Pharmacol Toxicol. 1997;37:361–397. doi: 10.1146/annurev.pharmtox.37.1.361. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi M, Seagar M J, Jones J F, Reber B F, Catterall W A. Proc Natl Acad Sci USA. 1987;84:5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S. Nature (London) 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- 7.Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Nature (London) 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- 8.Koch W J, Ellinor P T, Schwartz A. J Biol Chem. 1990;265:17786–17791. [PubMed] [Google Scholar]
- 9.Snutch T P, Tomlinson W J, Leonard J P, Gilbert M M. Neuron. 1991;7:45–47. doi: 10.1016/0896-6273(91)90073-9. [DOI] [PubMed] [Google Scholar]
- 10.Williams M E, Feldman D H, McCue A F, Brenner R, Velicelebi G, Ellis S B, Harpold M M. Neuron. 1992;8:71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- 11.Seino S, Chen L, Seino M, Blondel O, Takeda J, Johnson J H, Bell G I. Proc Natl Acad Sci USA. 1992;89:584–588. doi: 10.1073/pnas.89.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubel S J, Starr V B, Hell J, Ahlijanian M K, Enyeart J J, Catterall W A, Snutch T P. Proc Natl Acad Sci USA. 1992;89:5058–5062. doi: 10.1073/pnas.89.11.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams M E, Brust P F, Feldman D H, Patthi S, Simerson S, Maroufi A, McCue A F, Velicelebi G, Ellis S B, Harpold M M. Science. 1992;257:389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- 14.Starr V B, Prystay W, Snutch T P. Proc Natl Acad Sci USA. 1991;88:5621–5625. doi: 10.1073/pnas.88.13.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori Y, Friedrich T, Kim M-S, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, Mikoshiba K, Imoto K, Tanabe T, Numa S. Nature (London) 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- 16.Soong T W, Stea A, Hodson C D, Dubel S J, Vincent S R, Snutch T P. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- 17.Bechem M, Schramm M. J Mol Cell Cardiol. 1987;19:63–75. doi: 10.1016/s0022-2828(87)80005-6. [DOI] [PubMed] [Google Scholar]
- 18.Kass R S, Arena J P. J Gen Physiol. 1989;93:1109–1127. doi: 10.1085/jgp.93.6.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kass R S, Arena J P, Chin S. J Gen Physiol. 1991;98:63–75. doi: 10.1085/jgp.98.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strubing C, Hering S, Glossman H. Br J Pharmacol. 1993;108:884–891. doi: 10.1111/j.1476-5381.1993.tb13482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bangalore R, Baindur N, Rutledge A, Triggle D J, Kass R S. Mol Pharmacol. 1994;46:660–666. [PubMed] [Google Scholar]
- 22.Ferry D R, Rombusch M, Goll A, Glossman H. FEBS Lett. 1984;169:112–167. doi: 10.1016/0014-5793(84)80299-9. [DOI] [PubMed] [Google Scholar]
- 23.Galizzi J P, Borsotto M, Barhanin J, Fosset M, Lazdunski M. J Biol Chem. 1986;261:1393–1397. [PubMed] [Google Scholar]
- 24.Sharp A H, Imagawa T, Leung A T, Campbell K P. J Biol Chem. 1987;262:12309–12315. [PubMed] [Google Scholar]
- 25.Vaghy P L, Striessnig J, Miwa K, Knause H-G, Itagaki K, McKenna E, Glossmann H, Striessnig J. J Biol Chem. 1987;262:14337–14342. [PubMed] [Google Scholar]
- 26.Sieber M, Nastainczyk W, Zubor V, Werner W, Hofmann F. Eur J Biochem. 1987;167:117–122. doi: 10.1111/j.1432-1033.1987.tb13311.x. [DOI] [PubMed] [Google Scholar]
- 27.Hosey M M, Barhanin J, Schmid A, Vandaele S, Ptasienski J, O’Callahan C, Cooper C, Lazdunski M. Biochem Biophys Res Commun. 1987;147:1137–1145. doi: 10.1016/s0006-291x(87)80188-2. [DOI] [PubMed] [Google Scholar]
- 28.Striessnig J, Murphy B J, Catterall W A. Proc Natl Acad Sci USA. 1991;88:10769–10773. doi: 10.1073/pnas.88.23.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama H, Taki M, Striessnig J, Catterall W A, Kanaoka Y. Proc Natl Acad Sci USA. 1991;88:9203–9207. doi: 10.1073/pnas.88.20.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catterall W A, Striessnig J. Trends Pharmacol Sci. 1992;13:256–262. doi: 10.1016/0165-6147(92)90079-l. [DOI] [PubMed] [Google Scholar]
- 31.Kalasz H, Watanabe T, Yabana H, Itagaki K, Naito K, Nakayama H, Schwartz A, Vaghy P L. FEBS Lett. 1993;331:177–181. doi: 10.1016/0014-5793(93)80321-k. [DOI] [PubMed] [Google Scholar]
- 32.Grabner M, Wang Z Y, Hering S, Striessnig J, Glossmann H. Neuron. 1996;16:207–218. doi: 10.1016/s0896-6273(00)80037-9. [DOI] [PubMed] [Google Scholar]
- 33.Tang S, Yatani A, Bahinski A, Mori Y, Schwartz A. Neuron. 1993;11:1013–1021. doi: 10.1016/0896-6273(93)90215-d. [DOI] [PubMed] [Google Scholar]
- 34.Schuster A, Lacinova’ L, Klugbauer N, Ito H, Birnbaumer L, Hofmann F. EMBO J. 1996;15:2365–2370. [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson B Z, Tanada T N, Catterall W A. J Biol Chem. 1996;271:5293–5296. doi: 10.1074/jbc.271.10.5293. [DOI] [PubMed] [Google Scholar]
- 36.Peterson B Z, Johnson B D, Hockerman G H, Acheson M, Scheuer T, Catterall W A. J Biol Chem. 1997;272:18752–18758. doi: 10.1074/jbc.272.30.18752. [DOI] [PubMed] [Google Scholar]
- 37.Mitterdorfer J, Wang Z, Sinnegger M J, Hering S, Striessnig J, Grabner M, Glossmann H. J Biol Chem. 1996;271:30330–30335. doi: 10.1074/jbc.271.48.30330. [DOI] [PubMed] [Google Scholar]
- 38.He M, Bodi I, Mikala G, Schwartz A. J Biol Chem. 1997;272:2629–2633. doi: 10.1074/jbc.272.5.2629. [DOI] [PubMed] [Google Scholar]
- 39.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 40.Pragnell M, Leveille C F, Jay S D, Campbell K P. FEBS Lett. 1991;291:253–257. doi: 10.1016/0014-5793(91)81296-k. [DOI] [PubMed] [Google Scholar]
- 41.Ellis S B, Williams M E, Ways N R, Brenner R, Sharp A H, Leung A T, Campbell K P, McKenna E, Koch W J, Hui A, Schwartz A, Harpold M M. Science. 1988;241:1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- 42.Margolskee R F, McHendry-Rinde B, Horne R. Biotechniques. 1994;15:906–911. [PubMed] [Google Scholar]
- 43.Kokubun S, Reuter H. Proc Natl Acad Sci USA. 1984;81:4824–4827. doi: 10.1073/pnas.81.15.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bean B P. Proc Natl Acad Sci USA. 1984;81:6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanguinetti M, Kass R. Circ Res. 1984;55:336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- 46.Janis R A, Sarmiento J G, Maurer S C, Bolger G T, Triggle D J. J Pharmacol Exp Ther. 1984;231:8–15. [PubMed] [Google Scholar]
- 47.Greenberg D A, Cooper E C, Carpenter C L. Brain Res. 1984;305:365–368. doi: 10.1016/0006-8993(84)90444-x. [DOI] [PubMed] [Google Scholar]
- 48.Porzig H, Kokubun S, Prod’hom B, Becker C, Reuter H. Biochim Biophys Acta. 1987;46:S370–374. [PubMed] [Google Scholar]
- 49.Barford D, Johnson L. Nature (London) 1989;340:609–616. doi: 10.1038/340609a0. [DOI] [PubMed] [Google Scholar]
- 50.Kantrowitz E, Lipscomb W N. Trends Biochem Sci. 1990;15:53–59. doi: 10.1016/0968-0004(90)90176-c. [DOI] [PubMed] [Google Scholar]
- 51.Karlin A, Akabas M H. Neuron. 1995;15:1231–1244. doi: 10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]