Abstract
The depletion of inositol trisphosphate-sensitive intracellular pools of calcium causes activation of store-operated calcium (SOC) channels. Loperamide at 10–30 μM has no effect on intracellular calcium levels alone, but augments calcium levels in cultured cells when SOC channels have been activated. In HL-60 leukemic cells, the apparent positive modulatory effect of loperamide on SOC channels occurs when these channels have been activated after ATP, thapsigargin, or ionomycin-elicited depletion of calcium from intracellular storage sites. Loperamide has no effect when levels of intracellular calcium are elevated through a mechanism not involving SOC channels by using sphingosine. Loperamide caused augmentation of intracellular calcium levels after activation of SOC channels in NIH 3T3 fibroblasts, astrocytoma 1321N cells, smooth muscle DDT-MF2 cells, RBL-2H3 mast cells, and pituitary GH4C1 cells. Only in astrocytoma cells did loperamide cause an elevation in intracellular calcium in the absence of activation of SOC channels. The augmentation of intracellular calcium elicited by loperamide in cultured cells was dependent on extracellular calcium and was somewhat resistant to agents (SKF 96365, miconazole, clotrimazole, nitrendipine, and trifluoperazine) that in the absence of loperamide effectively blocked SOC channels. It appears that loperamide augments influx of calcium through activated SOC channels.
Keywords: thapsigargin, ATP, inositol trisphosphate, trifluoperazine, phorbol ester
The depletion of intracellular stores of calcium can result in the opening of calcium channels in the plasma membranes of cells (1). Such channels have been referred to as receptor-operated calcium channels, calcium-release-activated calcium channels, capacitative calcium entry channels, and store-operated calcium (SOC) channels. The mechanism(s) whereby depletion of inositol trisphosphate (IP3)-sensitive stores of calcium causes opening of SOC channels remains uncertain although several hypotheses have been advanced (2). SOC channels activate after receptor-mediated generation of IP3, which releases calcium from intracellular stores, or after treatment of cells with either the Ca2+-ATPase inhibitor thapsigargin, which blocks re-uptake of calcium into storage sites, or with the calcium ionophore ionomycin, which directly mobilizes calcium from storage sites. SOC channels can be blocked by imidazoles such as SKF 96365, clotrimazole, and miconazole and a small selection of other agents (3–6). Recently, loperamide was found to augment levels of intracellular calcium in HL-60 cells in which SOC channels were activated after P2Y-receptor-mediated formation of IP3 and release of intracellular calcium (6). The augmentation by loperamide of SOC channel-mediated elevation of intracellular calcium levels now has been shown to be a general phenomenon, occurring in several cell types after receptor-, thapsigargin-, or ionomycin-induced activation of SOC channels. Loperamide appears to be a novel agent for the study of SOC channels and their functional role in cells.
MATERIALS AND METHODS
Loperamide, econazole, nifedipine, nitrendipine, trifluoperazine, chlorpromazine, diphenoxylate, and trifluperidol were from Research Biochemicals (Natick, MA). Miconazole, clotrimazole, N-formyl-Met-Leu-Phe, histamine, N-ethylcarboxamidoadenosine, phorbol 12-myristate 13-acetate (PMA), geneticin sulfate, and dibutyryl cyclic AMP (sodium salt) were from Sigma. SKF 96365 and sphingosine were from Biomol (Plymouth Meeting, PA). Ionomycin and thapsigargin were from Calbiochem, and ATP was from Fluka. Naloxone was provided by A. Jacobson (National Institutes of Health, Bethesda, MD). Antigen consisting of dinitrophenol conjugated with human serum albumin and a dinitrophenol-specific Ig (IgE) were provided by O. Choi (National Institutes of Health). DMEM, RPMI 1640 medium, fetal bovine serum, l-glutamine (200 mM), trypsin-EDTA (0.5% trypsin and 0.53 mM EDTA), and penicillin-streptomycin (10,000 units/ml penicillin G sodium and 10,000 μg/ml streptomycin sulfate) were from GIBCO/BRL.
Cell Culture.
The HL-60 leukocytes, NIH 3T3 fibroblasts, astrocytoma 1321N cells, smooth muscle DDT-MF2 cells, and RBL-2H3 mast cells were from the American Type Culture Collection. The α7-transfected rat pituitary GH4C1 cells (7) were provided by M. Quik (Parkinson’s Institute, Sunnyvale, CA).
The HL-60 cells were grown in suspension in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin, 100 units/ml penicillin G, and 2 mM l-glutamine. The RBL-2H3 cells were grown in DMEM supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin, and 100 units/ml penicillin G. The astrocytoma 1321N cells were grown in high glucose DMEM supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin, 100 units/ml penicillin G, and 900 μg/ml geneticin sulfate. The DDT-MF2, α7-GH4C1, and NIH 3T3 cells were grown in DMEM supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin, and 100 units/ml penicillin G.
Cell Preparation.
The HL-60 cells were differentiated in the supplemented RPMI medium containing 500 μM dibutyryl cyclic AMP for 48 hr before each experiment. The RBL-2H3 cells were incubated overnight with 0.5 μg/ml dinitrophenol-IgE before each experiment. The RBL-2H3, astrocytoma 1321N, DDT-MF2, and NIH 3T3 cells were detached from the cell culture surface by using 2 ml of trypsin-EDTA and resuspended in the same culture media for RBL-2H3 cells in Hepes-buffered salt solution containing 2.5 mM probenecid. The α7-GH4C1 cells were detached from the culture surface by using 2 ml of Hepes-buffered salt solution (118 mM NaCl/4.6 mM KCl/10 mM d-glucose/20 mM Hepes, pH 7.4) containing 5 mM EDTA and resuspended in the cell culture medium. The resuspended cells were incubated with gentle agitation at 37°C for 1–2 hr before each experiment.
Ca2+ Measurements.
Aliquots (10–20 ml) of differentiated HL-60 cells were centrifuged at 1,000 rpm for 10 min at 20–25°C, and the supernatant was removed. Aliquots (10–20 ml) of the NIH 3T3, astrocytoma 1321N, DDT-MF2, RBL-2H3, and α7-GH4C1 cells were centrifuged at 800 rpm for 3 min at 20–25°C. The packed cells were resuspended in 10–20 ml of buffered media consisting of one part of the cell culture media and one part of Krebs–Ringer–Hepes buffer (KRH: 125 mM NaCl/1.2 mM KH2PO4/1.2 mM MgSO4/2 mM CaCl2/6 mM glucose/25 mM Hepes, pH 7.4). The GH4C1 cells were resuspended in 10–20 ml of buffered media consisting of one part of the DMEM media and one part of Hepes-buffered salt solution. In all cases, the cell count was adjusted to approximately 106 cells/ml. Fluo3-AM or fura-2 (10 μg in 10 μl of dimethyl sulfoxide) was added to the cell suspension. After 30–40 min at 20–25°C in the dark, the cell suspension was centrifuged, as described above, and the supernatant was removed. The packed cells were resuspended in either KRH, calcium-free KRH buffer (127 mM NaCl/1.2 mM KH2PO4/1.2 mM MgSO4/2 mM CaCl2/6 mM glucose/25 mM Hepes/100 μM EGTA, pH 7.4), or Hepes-buffered salt solution to give a final cell count of approximately 106 cells/ml.
For each experiment a 2-ml aliquot of the dye-loaded cell suspension was transferred into a cuvette and stirred with a magnetic stirrer bar throughout the experiment. Agents were added to the cells in 1 to 20 μl volumes. Intracellular calcium levels were monitored by fluorescence spectroscopy by using a PTI Deltascan Fluorescence Spectrophotometer (Photon Technology International, Princeton) set at 506 nm (excitation) and 526 nm (emission) for fluo3-AM, and 340 nm (excitation) and 380 nm (excitation) for fura-2. Results are expressed in counts/sec. The basal levels of calcium were calculated as described (8, 9) and were found to be in the range of 80–156 nM. Thus, the figures depict the fold increase above such levels.
RESULTS
HL-60 Cells.
The dose dependency of the effects of loperamide after activation of SOC channels after depletion by ATP of IP3-sensitive calcium stores indicated a threshold value of about 10 μM and a maximal effect at 30 μM (ref. 6 and data not shown). A concentration of 30 μM loperamide was used in further experiments.
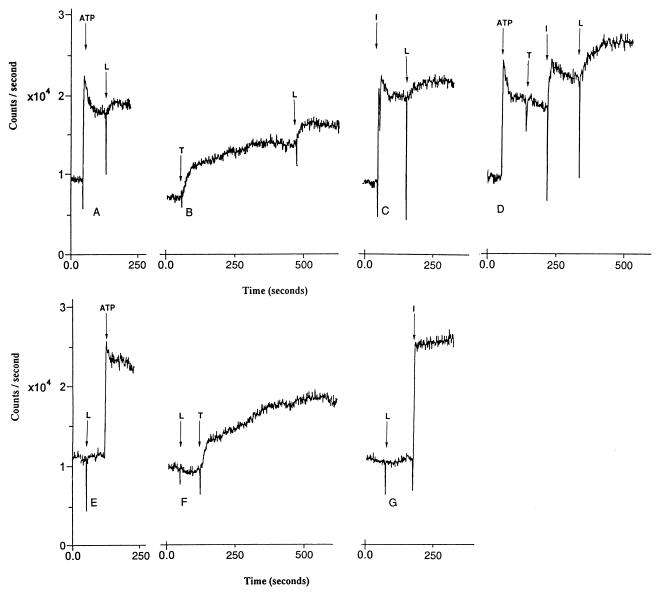
Loperamide caused an increase in intracellular calcium levels in HL-60 cells when added after release of calcium from intracellular stores by using 10 μM ATP, 15 nM thapsigargin or 1 μM ionomycin (Fig. 1 A–C). Loperamide added after mobilization of calcium stores by a combination of ATP, thapsigargin, and ionomycin caused a larger further increase in calcium levels than it did when added after ATP, thapsigargin, or ionomycin alone (Fig. 1D). Loperamide added initially did not elevate intracellular calcium levels, but did augment the prolonged increase in intracellular calcium levels that follows addition of ATP, thapsigargin, or ionomycin (Fig. 1 E–G).
Figure 1.
Effect of loperamide (L; 30 μM) on levels of intracellular calcium before and after release of intracellular calcium and subsequent activation of SOC channels in HL-60 cells. Elevation of intracellular calcium levels before the addition of loperamide was by (A) 10 μM ATP, (B) 15 nM thapsigargin (T), (C) 10 μM ionomycin (I), and (D) a combination of 10 μM ATP, 15 nM thapsigargin, and 1 μM ionomycin. Elevation of intracellular calcium levels after the addition of loperamide was by (E) 10 μM ATP, (F) 15 nM thapsigargin (T), and (G) 10 μM ionomycin (I). Cells were loaded with fluo3-AM.
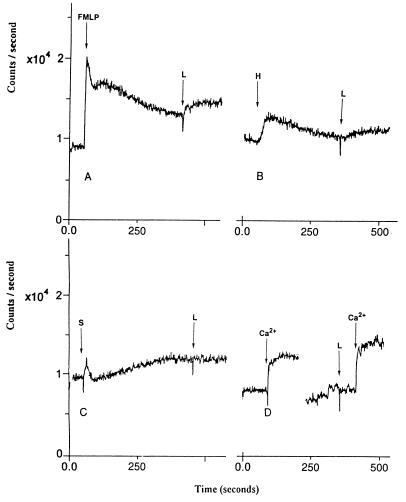
Loperamide elicited an increase in intracellular calcium after activation of SOC channels by using 10 μM N-formyl-met-leu-phe or 10 μM histamine, but not after 10 μM sphingosine (Fig. 2 A–C). After exposure of HL-60 cells to low-calcium media, loperamide caused an augmentation of the increase in intracellular calcium levels that follows reintroduction of external calcium (Fig. 2D).
Figure 2.
Effect of loperamide (L; 30 μM) on levels of intracellular calcium after release of intracellular calcium and subsequent activation of SOC channels in HL-60 cells. Elevation of intracellular calcium levels was by (A) 10 μM N-formyl-met-leu-phe (FMLP), (B) 10 μM histamine (H), and (C) 10 μM sphingosine (S). (D) Effect of loperamide (L) on levels of intracellular calcium in low-calcium buffer followed by reintroduction of 2 mM Ca2+. Cells were loaded with fluo3-AM.
Other Cells.
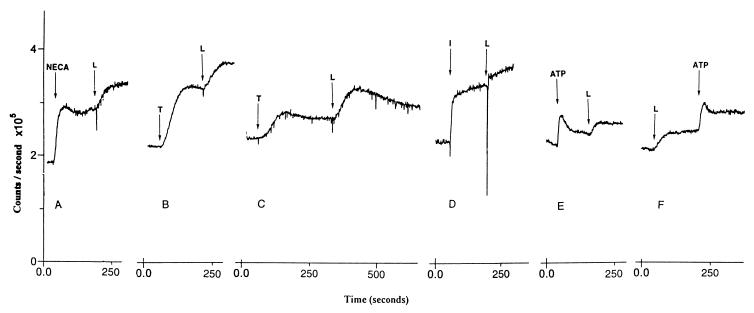
In RBL-2H3 cells loperamide caused an additional increase in intracellular calcium when added after 10 μM N-ethylcarboxamidoadenosine (Fig. 3A). In NIH 3T3 and DDT-MF2 cells, loperamide caused an increase in intracellular calcium after depletion of calcium stores by thapsigargin (Fig. 3 B and C). In GH4C1 cells, loperamide caused an additional increase in intracellular calcium when added after ionomycin (Fig. 3D). In astrocytoma 1321 cells, loperamide caused an additional increase in intracellular calcium when added after ATP (Fig. 3E). In NIH 3T3, DDT-MF2, RBL-2H3, and GH4C1 cells, loperamide alone did not elicit an increase in intracellular calcium. However, subsequent addition of receptor agonists, thapsigargin, or ionomycin led to an increase in higher calcium levels than those observed for the calcium-mobilizing agents in the absence of loperamide (data not shown). In astrocytoma cells, loperamide alone caused an elevation in intracellular calcium (Fig. 3F).
Figure 3.
Effect of loperamide (L; 30 μM) on levels of intracellular calcium after release of intracellular calcium and subsequent activation of SOC channels in (A) RBL-2H3 cells, (B) NIH 3T3 cells, (C) DDT-MF2 cells, (D) GH4C1 cells, and (E) astrocytoma 1321N cells. Intracellular calcium levels were elevated by using (A) 10 μM N-ethylcarboxamidoadenosine (NECA) + 20 μg/ml dinitrophenol conjugated with human serum albumin, (B and C) 15 nM thapsigargin (T), (D) 1 μM ionomycin (I), and (E) 10 μM ATP. (F) Effect of loperamide added before ATP in astrocytoma 1321N cells. Cells were loaded with fura-2AM.
Inhibitors.
The ability of loperamide to augment SOC channel-mediated elevation of intracellular calcium was investigated in the presence of a series of agents that can effectively block SOC channels in HL-60 cells. The agents were the imidazoles SKF 96365 (30 μM), miconazole (3 μM), econazole (3 μM), and clotrimazole (10 μM), the dihydropyridine nitrendipine (10 μM), and the phenothiazine trifluoperazine (10 μM). The effect of the protein kinase C activator PMA (10 μM) also was studied. The concentrations of the inhibitors were selected based on their efficacy as SOC channel inhibitors for HL-60 cells (6).
In an earlier study (6) trifluoperazine was cited as having no inhibitory effects, but it has since been found that the compound tested was actually trifluperidol. Trifluoperazine is, in fact, an effective SOC channel blocker (see below). Nitrendipine also was cited as having no significant effect on SOC channels (6), but in subsequent studies 10 μM nitrendipine was found to cause inhibition of SOC channels.
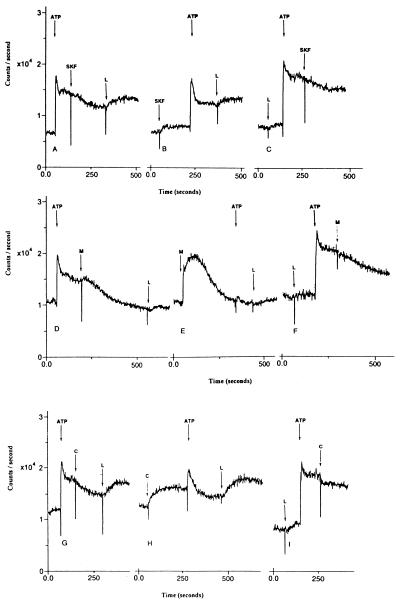
SKF 96365, miconazole, econazole (data not shown), and clotrimazole appeared to have two effects on intracellular calcium levels. When added before a calcium-mobilizing agent, these compounds caused an increase in intracellular calcium levels (Fig. 4 B, E, and H), but caused a decrease in intracellular calcium levels after activation of SOC channels (Fig. 4 A, D, and G). Miconazole was the most potent inhibitor of SOC channels, whereas SKF 96365 was the weakest. Miconazole alone, however, caused the greatest increase in intracellular calcium levels.
Figure 4.
Effect of loperamide (L; 30 μM) on levels of intracellular calcium before and after inhibition of SOC channels in HL-60 cells. The imidazole inhibitors of SOC channels were as follows: (A–C) 30 μM SKF 96365 (SKF), (D–F) 3 μM miconazole (M), and (G–I) 10 μM clotrimazole (C). Cells were loaded with fluo3-AM.
Loperamide caused an additional increase in intracellular calcium levels after inhibition by 30 μM SKF 96365 of SOC channels activated by ATP in HL-60 cells (Fig. 4A). SKF 96365 added before ATP caused a small elevation in intracellular calcium levels and a transitory increase in intracellular calcium was observed at addition of ATP (Fig. 4B). Subsequent addition of loperamide elicited an increase in intracellular calcium (Fig. 4B). SKF 96365 had a somewhat weaker inhibitory effect when added after loperamide and ATP (Fig. 4C) compared with addition after ATP alone (Fig. 4A).
Loperamide caused a minimal increase in intracellular calcium levels after inhibition by 3 μM miconazole of SOC channels activated by ATP in HL-60 cells (Fig. 4D). The addition of miconazole before ATP elicited a large increase in intracellular calcium, and the subsequent ATP response was almost completely blocked (Fig. 4E). Subsequent addition of loperamide elicited a minimal increase in intracellular calcium (Fig. 4E). Miconazole had a somewhat weaker inhibitory effect when added after loperamide and ATP (Fig. 4F) compared with addition after ATP alone (Fig. 4D). Similar results were obtained when 3 μM miconazole was replaced with 3 μM econazole (data not shown). In contrast, loperamide had more pronounced effects on levels of intracellular calcium in experiments where 3 μM miconazole was replaced with 10 μM clotrimazole (Fig. 4 G–I). Thus, it appears that miconazole and econazole are more effective SOC channel blockers compared with SKF 96365 and clotrimazole, even in the presence of loperamide.
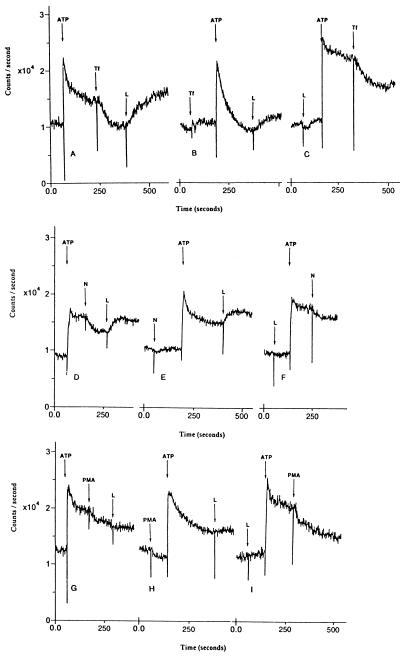
Loperamide caused an additional increase in intracellular calcium levels after inhibition by 10 μM trifluoperazine of SOC channels activated by ATP in HL-60 cells (Fig. 5A). The addition of trifluoperazine before ATP completely blocked the SOC channel-mediated sustained increase in intracellular calcium levels that normally follows ATP (Fig. 5B). However, subsequent addition of loperamide still caused an increase in the levels of intracellular calcium. Trifluoperazine had a slightly weaker inhibitory effect when added after loperamide and ATP (Fig. 5C). At 30 μM concentrations, trifluoperazine caused complete blockade of SOC channels even in the presence of loperamide (data not shown). The phenothiazine chlorpromazine was much weaker than trifluoperazine in blocking SOC channels in HL-60 cells (data not shown).
Figure 5.
Effect of loperamide (L; 30 μM) on levels of intracellular calcium before and after inhibition of SOC channels in HL-60 cells. The inhibitors of SOC channels were as follows: (A–C) 10 μM trifluoperazine (Tf), (D–F) 10 μM nitrendipine (N), and (G–I) 10 μM PMA. Cells were loaded with fluo3-AM.
Loperamide caused a large increase in intracellular calcium levels after inhibition by 10 μM nitrendipine of SOC channels activated by ATP in HL-60 cells (Fig. 5D). The addition of loperamide, after nitrendipine and ATP, caused a large increase in intracellular calcium levels (Fig. 5E). Nitrendipine had a weaker inhibitory effect when added after loperamide and ATP (Fig. 5F). Nifedipine, a close structural analog of nitrendipine, had no inhibitory effect on SOC channels at concentrations up to 30 μM (data not shown).
Loperamide had little or no effect on intracellular calcium levels after inhibition by 10 μM PMA of SOC channels activated by ATP in HL-60 cells (Fig. 5G). The addition of loperamide after PMA and ATP caused only a very small increase in intracellular calcium (Fig. 5H). PMA had a marginally weaker inhibitory effect when added after loperamide and ATP (Fig. 5I).
DISCUSSION
Loperamide is a commonly used antidiarrheal agent whose effects are attributed in part to its agonist activity at opioid receptors (10–12). Non-opioid effects of loperamide include functional inhibition of calmodulin (12–14) and calcium channel blockade (6, 12, 15, 16). Recently, loperamide was reported to augment the levels of intracellular calcium in HL-60 cells when SOC channels were activated by depletion of IP3-sensitive calcium stores by ATP (6).
To investigate this phenomenon further, a number of cell types were used and SOC channels were activated by depletion of IP3-sensitive stores of calcium by using the receptor agonists ATP, N-formyl-met-leu-phe, histamine, and N-ethylcarboxamidoadenosine, the calcium ATP-ase inhibitor thapsigargin, the calcium ionophore ionomycin, or low-calcium media. All of these treatments cause depletion of intracellular stores of calcium in cells and activation of SOC channels (6, 17–20). The effect of loperamide on cells in which the levels of intracellular calcium were raised without activation of SOC channels also was studied by using sphingosine, which causes depletion of intracellular stores of calcium by pathways that do not result in the activation of SOC channels (21, 22).
The HL-60 cells proved particularly well suited to the study of SOC channel function by using fluorescent probes. The HL-60 cells grow in suspension, and the SOC channels continue to function for extended periods after activation by receptor agonists. In some cell types SOC channels rapidly cease to function after depletion of intracellular stores, perhaps because of facile restoration of IP3-sensitive intracellular stores of calcium.
In HL-60 cells, the enhancement by loperamide of intracellular calcium levels occurred regardless of whether receptor agonists, thapsigargin, ionomycin, or low-calcium media were used to activate SOC channels (Fig. 1 A–G). Loperamide did not enhance intracellular calcium levels when intracellular calcium was elevated through activation of calcium channels by sphingosine. In addition, loperamide had no effect on the magnitude of the initial release of calcium by IP3 after a receptor agonist (Figs. 1A, 4 C, F, and I, and 5 C, F, and I). Therefore, it seems unlikely that the elevation of calcium levels by loperamide is simply because of blockade of pathways for calcium storage in cells. It is more likely that loperamide has a modulatory effect on SOC channel function. That loperamide had the greatest effect after a maximal increase in SOC channel elevation of intracellular calcium in HL-60 cells appears to support this hypothesis (Fig. 1D). Intracellular calcium has been linked to inhibition of SOC (23), and it is possible that loperamide in some way disrupts this feedback mechanism by causing further elevation of intracellular calcium levels. SOC channels have been proposed to be activated by an intracellular messenger generated in calcium-depleted endoplasmic reticulum (2), and it is possible that loperamide might enhance formation or inhibit degradation of the putative messenger. Opioid mechanisms do not appear to be involved because 1 μM naloxone does not affect the loperamide response in HL-60 cells (data not shown).
The augmentation of intracellular calcium levels by loperamide appears to be a general effect and, as such, is observed in a number of other cell types that exhibit prolonged activation of SOC channels after depletion of intracellular stores of calcium (Fig. 3 A–D). The astrocytoma 1321N cells were the only cells tested in which loperamide alone caused elevation of intracellular calcium (Fig. 3 E and F). It is possible that in these cells loperamide alone causes activation of SOC channels or that there exists a small basal population of activated SOC channels.
The imidazoles SKF 96365, miconazole, econazole, and clotrimazole are classical inhibitors of SOC channels (3, 4, 6, 18). SKF 96365 and clotrimazole were less effective in the presence of loperamide (Fig. 4 C and I), whereas miconazole and econazole were only marginally less effective as inhibitors in the presence of loperamide (Fig. 4F and data not shown). Miconazole and econazole alone caused a large increase in intracellular calcium levels and prevented the ATP-elicited increase in intracellular calcium, perhaps by depletion of the IP3-sensitive calcium pool (Fig. 4E and data not shown). Neither imidazole blocks ATP-elicited breakdown of phosphoinositide in HL-60 cells (6). The mechanism by which loperamide blunts the inhibitory effects of the imidazoles is unknown. It could be an allosteric effect, lowering the efficacy or potency of the imidazole inhibitors, or it could be the result of direct competition for the imidazole binding site, or it could be recruitment by loperamide of SOC channels that are refractory to inhibition by the imidazoles. Loperamide also appears to reduce the inhibitory effects of trifluoperazine (Fig. 5C) and nitrendipine (Fig. 5F) on SOC channels in HL-60 cells.
The phenothiazine trifluoperazine is a very effective inhibitor of SOC channels and at 30 μM appears to inhibit all SOC channels even in the presence of loperamide (data not shown). However, at 10 μM trifluoperazine the loperamide response was still apparent despite complete blockade of the SOC channels activated by ATP (Fig. 5 A and B).
The dihydropyridine nitrendipine at 10 and 30 μM blocked SOC channels in HL-60 cells (Fig. 5 D–F). In earlier studies no significant inhibition was detected (6). It should be noted that the threshold for inhibition and extent of inhibition by different agents can vary in HL-60 cells depending on passage number and other possible variables. The inhibitory effect of nitrendipine was strongly blunted in the presence of loperamide (Fig. 5F). There was no effect of 10–30 μM nifedipine on the SOC channel-mediated increase in intracellular calcium (data not shown).
The regulation of capacitative calcium entry by phorbol esters, through the activation of protein kinase C, appears to vary in different cell types (24, 25). In HL-60 cells, PMA caused inhibition of SOC channels (Fig. 5 G–I). The effects of loperamide were undetectable after treatment with PMA even though some SOC channels appeared to be functioning (Fig. 5 G–I).
Loperamide is the first compound known to augment levels of intracellular calcium when SOC channels are activated. The effect appears to be general as it is observed in a variety of cell types. Loperamide also alters the sensitivity of SOC channels to various inhibitors. The mechanism of action remains unknown, but appears to require the activation of SOC channels. Loperamide has no effect when the levels of intracellular calcium are elevated by a sphingosine-elicited pathway that does not involve SOC channels, nor on the initial elevation of intracellular calcium levels by receptor agonists. Structural analogs of loperamide, such as diphenoxylate and trifluperidol, did not elicit an increase in intracellular calcium levels in the presence of activated SOC channels (ref. 6 and data not shown). In view of the unique effect of loperamide, further investigation into the activity of compounds with similar chemical structures is needed to ascertain the specific structural requirements for this effect and perhaps find more potent modulators. Further studies on loperamide itself and the development of more potent analogs as potential probe molecules should provide additional understanding of the nature of the loperamide effect and of SOC channel function.
Acknowledgments
We thank Dr. J. Putney for discussion and comments on this research.
ABBREVIATIONS
- SOC
store-operated calcium
- IP3
inositol trisphosphate
- PMA
phorbol 12-myristate 13-acetate
References
- 1.Putney J W., Jr Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 2.Berridge M J. Biochem J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clementi E, Meldolesi J. Cell Calcium. 1996;19:269–279. doi: 10.1016/s0143-4160(96)90068-8. [DOI] [PubMed] [Google Scholar]
- 4.Mason M J, Mayer B, Hymel L J. Am J Physiol. 1993;264:654–662. doi: 10.1152/ajpcell.1993.264.3.C654. [DOI] [PubMed] [Google Scholar]
- 5.Gericke M, Oike M, Droogmans G, Nilius B. Eur J Pharmacol. 1994;269:381–384. doi: 10.1016/0922-4106(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 6.Daly J W, Lueders J, Padgett W L, Shin Y, Gusovsky F. Biochem Pharmacol. 1995;50:1187–1197. doi: 10.1016/0006-2952(95)00257-z. [DOI] [PubMed] [Google Scholar]
- 7.Quik M, Choremis J, Komourian J, Lukas R J, Puchacz E. J Neurochem. 1996;67:145–154. doi: 10.1046/j.1471-4159.1996.67010145.x. [DOI] [PubMed] [Google Scholar]
- 8.Mogensen H S, Jorgensen O S. Neurochem Int. 1996;29:497–506. doi: 10.1016/0197-0186(96)00018-6. [DOI] [PubMed] [Google Scholar]
- 9.Grynkiewitz G, Poenie M, Tsien R Y. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 10.Awouters F, Niemegeers J E, Janssen P A J. Annu Rev Pharmacol Toxicol. 1983;23:279–301. doi: 10.1146/annurev.pa.23.040183.001431. [DOI] [PubMed] [Google Scholar]
- 11.Ooms L A A, Degryse A, Janssen P A J. Scand J Gastroenterol Suppl. 1984;96:145–155. [PubMed] [Google Scholar]
- 12.Burleigh D E. Eur J Pharmacol. 1988;152:39–46. doi: 10.1016/0014-2999(88)90833-3. [DOI] [PubMed] [Google Scholar]
- 13.Zavez J H, Jackson T E, Limp G L, Yellin T O. Eur J Pharmacol. 1982;78:375–377. doi: 10.1016/0014-2999(82)90042-5. [DOI] [PubMed] [Google Scholar]
- 14.Diener M, Knobloch S F, Rummel W. Eur J Pharmacol. 1988;152:217–225. doi: 10.1016/0014-2999(88)90716-9. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds I J, Gould R J, Snyder S H. J Pharmacol Exp Ther. 1984;231:626–631. [PubMed] [Google Scholar]
- 16.Church J, Fletcher E J, Abdel-Hamid K, MacDonald J F. Mol Pharmacol. 1994;45:747–757. [PubMed] [Google Scholar]
- 17.Inesi G, Sagara Y. Arch Biochem Biophys. 1992;298:313–317. doi: 10.1016/0003-9861(92)90416-t. [DOI] [PubMed] [Google Scholar]
- 18.Montero M, Alvarez J, Garcia-Sancho J. Biochem J. 1991;277:73–79. doi: 10.1042/bj2770073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin Y, Daly J W, Jacobson K A. Drug Dev Res. 1996;39:36–46. doi: 10.1002/(sici)1098-2299(19960901)39:1<36::aid-ddr5>3.0.co;2-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Encabo A, Romanin C, Birke F W, Kukovetz W R, Groschner K. Br J Pharmacol. 1996;119:702–706. doi: 10.1111/j.1476-5381.1996.tb15729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh T K, Bian J, Gill D L. Science. 1990;248:1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh T K, Bian J, Gill D L. J Biol Chem. 1994;269:22628–22635. [PubMed] [Google Scholar]
- 23.Zweifach A, Lewis R S. J Biol Chem. 1995;270:14445–14451. doi: 10.1074/jbc.270.24.14445. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro C M P, Putney J W., Jr J Biol Chem. 1996;271:21522–21528. doi: 10.1074/jbc.271.35.21522. [DOI] [PubMed] [Google Scholar]
- 25.Marriott I, Mason M J. J Biol Chem. 1996;271:26732–26738. doi: 10.1074/jbc.271.43.26732. [DOI] [PubMed] [Google Scholar]