Abstract
To determine the mechanisms responsible for the termination of Ca2+-activated Cl− currents (ICl(Ca)), simultaneous measurements of whole cell currents and intracellular Ca2+ concentration ([Ca2+]i) were made in equine tracheal myocytes. In nondialyzed cells, or cells dialyzed with 1 mM ATP, ICl(Ca) decayed before the [Ca2+]i decline, whereas the calcium-activated potassium current decayed at the same rate as [Ca2+]i. Substitution of AMP-PNP or ADP for ATP markedly prolonged the decay of ICl(Ca), resulting in a rate of current decay similar to that of the fall in [Ca2+]i. In the presence of ATP, dialysis of the calmodulin antagonist W7, the Ca2+/calmodulin-dependent kinase II (CaMKII) inhibitor KN93, or a CaMKII-specific peptide inhibitor the rate of ICl(Ca) decay was slowed and matched the [Ca2+]i decline, whereas H7, a nonspecific kinase inhibitor with low affinity for CaMKII, was without effect. When a sustained increase in [Ca2+]i was produced in ATP dialyzed cells, the current decayed completely, whereas in cells loaded with 5′-adenylylimidodiphosphate (AMP-PNP), KN93, or the CaMKII inhibitory peptide, ICl(Ca) did not decay. Slowly decaying currents were repeatedly evoked in ADP- or AMP-PNP-loaded cells, but dialysis of adenosine 5′-O-(3-thiotriphosphate) or okadaic acid resulted in a smaller initial ICl(Ca), and little or no current (despite a normal [Ca2+]i transient) with a second stimulation. These data indicate that CaMKII phosphorylation results in the inactivation of calcium-activated chloride channels, and that transition from the inactivated state to the closed state requires protein dephosphorylation.
Calcium-activated chloride currents (ICl(Ca)) have been identified in numerous cell types including neurons (1, 2), secretory cells (3), and smooth and striated muscle (4–7). Although the function of these currents in the regulation of cell excitability remains uncertain, evidence suggests that ICl(Ca) prolongs the action potential and calcium entry (1, 5, 8–10) and mediates fast postsynaptic potentials in smooth muscle (4). Moreover, in smooth muscle, ICl(Ca) is associated with the sporadic release of calcium from intracellular stores, resulting in spontaneous transient inward currents (STICs) at resting membrane potentials (11), which may act to couple intracellular calcium release to spontaneous phasic electrical activity (12).
Following a rise in intracellular calcium ([Ca2+]i), ICl(Ca) activates and decays rapidly (2–5 s) in muscle cells, Xenopus oocytes, and cultured spinal neurons (4, 13, 14). It has been suggested that the gating of calcium-activated chloride channels is controlled by [Ca2+]i alone, and that the rapid current decay observed after cell stimulation is because of the decline in [Ca2+]i, rather than channel inactivation (15). We have previously observed a marked difference in the rate of decay of ICl(Ca) and [Ca2+]i after release of intracellular calcium—ICl(Ca) begins to decline before the peak [Ca2+]i is achieved and decays completely before [Ca2+]i falls below the threshold required for current activation (16). This finding, and evidence that single channel currents rapidly run down in excised patches (17), suggested that calcium-activated chloride channels might undergo an inactivation process independent of calcium removal. We describe experiments demonstrating that the termination of ICl(Ca) in smooth muscle is a result of channel inactivation, resulting from phosphorylation of the channel or an associated protein by calcium/calmodulin-dependent protein kinase II (CaMKII). We show that calcium-activated chloride channels rapidly inactivate despite a sustained rise in [Ca2+]i, and that subsequent channel availability requires protein dephosphorylation. These results indicate that ICl(Ca) is terminated by a negative feedback mechanism that effectively uncouples channel activity from cellular calcium.
MATERIALS AND METHODS
Single smooth muscle cells were isolated from equine trachealis by using a pressure-perfusion technique as described previously (18, 19). Membrane currents were recorded by the nystatin-perforated or standard patch clamp method by using an EPC9 system (HEKA Electronics, Lambrecht/Pfalz, Germany). For perforated patch experiments cells were voltage-clamped at −60 mV by using electrode pipettes of 2–4 MΩ resistance; in dialysis experiments the resistance of patch pipettes was 1–3 MΩ. For simultaneous measurements of [Ca2+]i, cells were loaded with Fura-2/AM (2 μM) for 10 min or dialyzed with Fura-2 free acid (75 μM) through a patch pipette. A single-excitation wavelength fluorescence method was used for experiments after dual wavelength observations (20, 21). Photo-bleaching was minimized by using quartz neutral density filters in the optical pathway and blocking the fluorescence excitation by an electrical shutter between sampling periods. Background fluorescence was determined by either removing the cell from the field after the measurements for perforated patch clamp experiments or after establishing a GΩ seal, but before “breaking in” to the cell in standard patch clamp experiments. Current and fluorescence signals were filtered at 100 Hz and digitized at 250 Hz. The bath solution was 125 mM NaCl, 5 mM KCl, 1 mM MgSO4, 10 mM Hepes, 1.8 mM CaCl2, and 10 mM glucose (pH 7.4). In the perforated patch clamp experiments, the patch pipette solution was 130 mM CsCl, 5 mM MgCl2, 3 mM EGTA, 1 mM CaCl2, and 10 mM Hepes (pH 7.3). The pipette solution contained 130 mM CsCl, 1.2 mM MgCl2, 1 mM ATP-Mg, and 10 mM Hepes (pH 7.3) for the standard patch clamp experiments. All experiments were performed at 35°C.
Caffeine, nystatin, adenosine 5′-O-(3-thiotriphosphate) (ATPγs), 5′-adenylylimidodiphosphate (AMP-PNP), and ATP were obtained from Sigma; Fura-2, ionomycin, W7, KN93, and the peptide inhibitor of calcium/calmodulin-dependent kinase II (amino acid residues 281–301) were from Calbiochem; and H7 was from Research Biochemicals. Statistical analysis was performed by one-way ANOVA by using the Dunnett’s test for significance.
RESULTS AND DISCUSSION
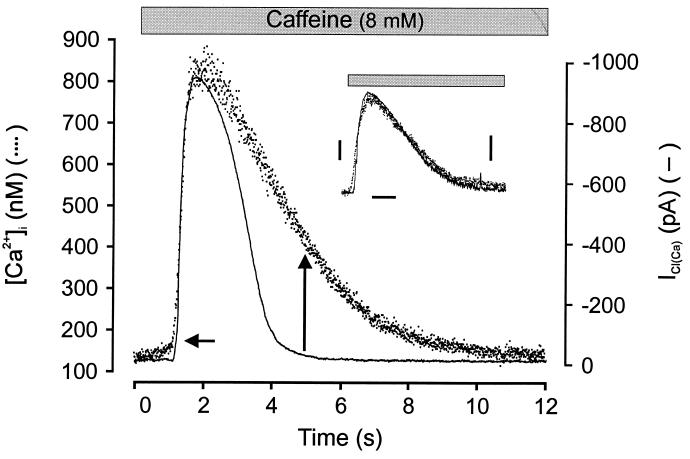
The application of 8 mM caffeine to nystatin voltage-clamped cells (−60 mV, Cs+ pipette solution to block potassium currents) induced an increase in the spatially averaged [Ca2+]i signal and a large ICl(Ca) (Fig. 1). We have previously shown that the caffeine-activated current in these cells is chloride-selective and requires intracellular calcium release (16, 22). In 11 cells, [Ca2+]i increased from a resting level of 151 ± 14 to 748 ± 49 nM, and the current had a mean amplitude of 1,087 ± 47 pA. As shown in Fig. 1, the time course of the increase in [Ca2+] was quite similar to that of ICl(Ca), whereas the decay of ICl(Ca) occurred substantially before the decline in spatially averaged [Ca2+]i. ICl(Ca) began to decay before [Ca2+]i reached a peak value and had declined to 50% of peak and completely decayed when [Ca2+]i had decreased by only 19% and 63% (from 748 ± 49 to 632 ± 38 and to 372 ± 26 nM), respectively. In some experiments the activation rate of the current was slightly greater than that of the [Ca2+]i signal (e.g., Fig. 2B); however, there was always a dramatic discrepancy between decay rates of the two signals. The mean time required for the current to decay to 50% of peak (t1/2) was 1.36 ± 0.11 s, whereas the t1/2 for the fall in [Ca2+]i was 2.78 ± 0.19 s. By contrast, similar experiments indicated that, after activation by caffeine, the calcium-activated potassium current (IK(Ca)) decayed with kinetics that matched the cellular [Ca2+]i signal. Fig. 1 (Inset) shows an example of four similar experiments in which cells were held at the chloride equilibrium potential and recorded with potassium-containing pipette solution to isolate the calcium-activated potassium current. These results are consistent with our previous findings on the activation of ICl(Ca) by muscarinic receptor stimulation (16) and suggest that ICl(Ca) inactivates by a mechanism independent of the removal of calcium. It was considered unlikely that the accelerated decay of ICl(Ca) was because of a decline in near membrane [Ca2+]i not reflected in our spatially averaged [Ca2+]i measurement, because the decay of IK(Ca) closely matched this signal. Moreover, ICl(Ca) but not IK(Ca) decayed in experiments in which ionomycin was used to achieve a sustained increase in [Ca2+]i (see below).
Figure 1.
Time course of ICl(Ca) and [Ca2+]i in a single smooth muscle cell. The time course of [Ca2+]i increase (dots) and ICl(Ca) (line) induced by caffeine (8 mM) in a cell voltage-clamped at −60 mV by using the perforated patch clamp technique. The macroscopic ICl(Ca) activates after [Ca2+]i increases to a threshold level (horizontal arrow), but the current completely decays while [Ca2+]i remains well above the activation threshold (vertical arrow). Note that (unlike the rate of current decay) the rate of current activation is very similar to the rate of rise of [Ca2+]i. VH = −60 mV; cell loaded with Fura-2/AM. The current has been inverted and the two signals have been scaled to the same peak amplitude. Inset shows caffeine activation of [Ca2+]i transient and calcium-activated potassium current. The cell was held at −20 mV (calculated ECl) and recorded with potassium-containing pipette solution. Exposure to caffeine (8 mM) resulted in an outward current with similar activation and decay kinetics as the [Ca2+]i signal. Dots show [Ca2+]i transient (left bar = 100 nM), solid line shows current (right bar = 100 pA), and time calibration bar is 2 s.
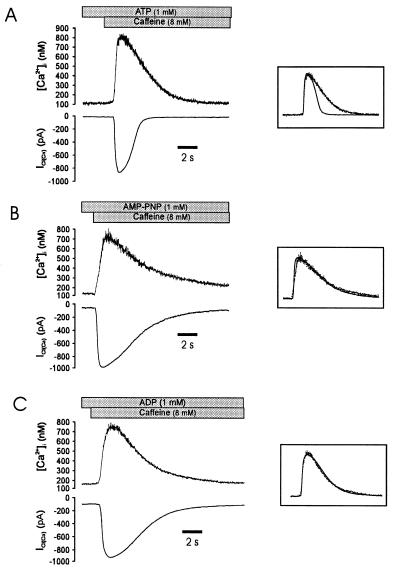
Figure 2.
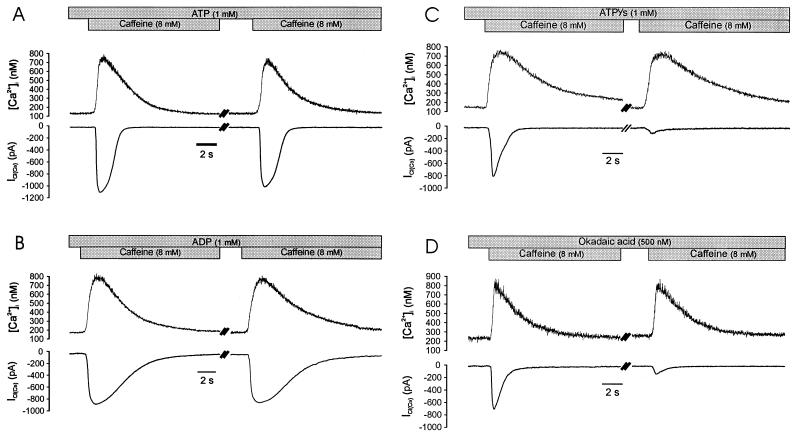
The rapid decay of ICl(Ca) requires ATP. (A) Caffeine evokes a typical [Ca2+]i (Upper) and ICl(Ca) (Lower) response in a cell dialyzed for 5 min with pipette solution containing ATP and Fura-2-free acid in the patch pipette. There is a marked difference in the rate of decay of the [Ca2+]i and ICl(Ca) signals. The Inset demonstrates the different kinetics by showing the two signals scaled to the same peak amplitude and superimposed. (B) Substitution of AMP-PNP for ATP (different cell) in the patch pipette resulted in a markedly slowed decay of ICl(Ca), such that the rate of decay of [Ca2+]i and ICl(Ca) were similar (Inset). Note that the current activation rate was not affected. (C) Substitution of ADP for ATP (different cell) also slowed the rate of decay of ICl(Ca) such that the current decayed with the decline in [Ca2+]i (Inset). VH was −60 mV for all cells.
These experiments suggested that factors other than a decline in [Ca2+]i were associated with the decay of ICl(Ca). Previous studies have shown that in the absence of intracellular ATP ICl(Ca) persists in smooth muscle cells (15), and that attenuation of glycosis or application of the metabolic inhibitor cyanide leads to a significant increase in the duration of ICl(Ca) in rat dorsal root ganglia neurons (23, 24). To determine whether the inactivation of ICl(Ca) was related to ATP hydrolysis, currents were examined in cells dialyzed with ATP, ADP, and the nonhydrolyzable analogue AMP-PNP. In cells dialyzed with 1 mM ATP for 5 min by using the standard whole-cell method, caffeine (8 mM) induced a similar calcium and current response as observed in nondialyzed cells (Fig. 2A). The mean amplitudes of [Ca2+]i and ICl(Ca) were 715 ± 44 nM and 956 ± 59 pA, respectively (n = 7), and t1/2 for the current decayed was 1.22 ± 0.08 s, whereas the mean time required for the [Ca2+]i decay was 2.68 ± 0.23 s (n = 7). As shown in Fig. 2B (Left), application of caffeine to cells dialyzed with 1 mM AMP-PNP for 5 min induced a current with a dramatically slowed rate of inactivation; moreover, the rate of current inactivation was quite similar to the rate of intracellular calcium decay. In eight experiments, the t1/2 for the decay of ICl(Ca) and [Ca2+]i was 3.06 ± 0.27 s and 3.49 ± 0.17 s, respectively. The half time of decay of ICl(Ca) was significantly greater than in cells dialyzed with ATP (P < 0.05, see Table 1). As with AMP-PNP, dialysis of ADP prolonged the decay of the caffeine-activated currents when compared with ATP (Fig. 2B Right; P < 0.05, n = 6) and resulted in a time course of current decay that closely matched the fall in [Ca2+]i (Fig. 2B Inset). No attempt was made to adjust for different calcium-binding affinities of the nucleoside phosphates, but the current decay kinetics were not markedly affected by the resting level of [Ca2+]i or the peak [Ca2+]i achieved after exposure to caffeine. Therefore, we concluded that protein phosphorylation plays a role in the rapid inactivation of ICl(Ca) observed under more physiological conditions (nondialyzed cells).
Table 1.
Inhibition of inactivation of Ca2+-activated Cl− currents by the nonhydrolyzable ATP analogues and the blockers of CaM kinase in equine tracheal smooth muscle cells
| n | ICl(Ca), pA | t½, s | [Ca2+]i, nM | t½, s | |
|---|---|---|---|---|---|
| ATP | 7 | 956 ± 59 | 1.22 ± 0.08 | 584 ± 46 | 2.68 ± 0.23 |
| AMP-PNP | 8 | 843 ± 82 | 3.06 ± 0.27* | 601 ± 28 | 3.49 ± 0.17 |
| ADP | 6 | 822 ± 74 | 2.56 ± 0.36* | 594 ± 57 | 2.77 ± 0.09 |
| W7 | 7 | 944 ± 57 | 2.79 ± 0.34* | 616 ± 31 | 3.53 ± 0.36 |
| KN93 | 6 | 975 ± 78 | 2.85 ± 0.37* | 591 ± 39 | 3.39 ± 0.35 |
| H7 | 5 | 1,012 ± 68 | 1.64 ± 0.19 | 563 ± 45 | 2.95 ± 0.09 |
| Peptide inhibitor | 6 | 959 ± 59 | 2.99 ± 0.36* | 574 ± 26 | 3.43 ± 0.39 |
t½ refers to the half time of current (left) and [Ca2+]i (right) decay.
P < 0.05 compared with control (ATP).
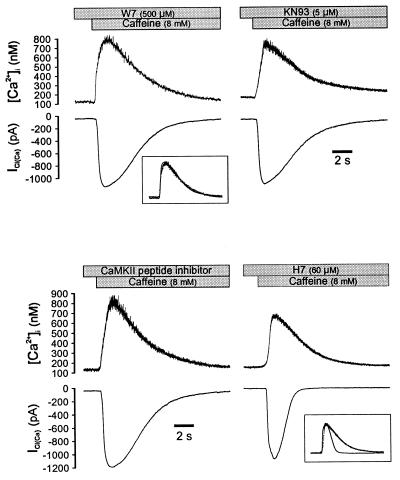
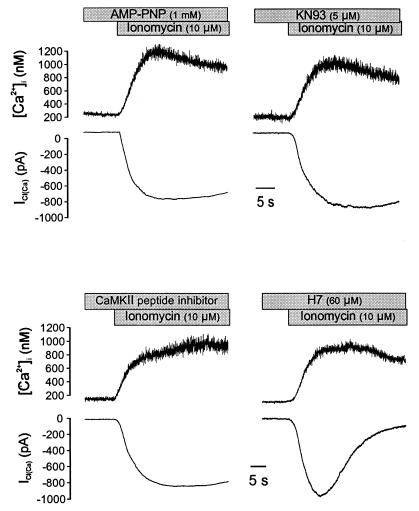
We reasoned that the increase in [Ca2+]i initiating ICl(Ca) might activate calcium/calmodulin-dependent protein kinase II (CaMKII), which is highly expressed in smooth muscle and has been implicated in the regulation of membrane ion channels (25, 26), and that CaMKII-mediated phosphorylation promoted channel closing, thereby constituting an efficient negative feedback system to ensure rapid termination of the postsynaptic response (4). To test this hypothesis, we first dialyzed myocytes with 1 mM ATP plus either the calmodulin antagonist, W7, or the CaMKII inhibitor, KN93. As shown in Fig. 3, the decay of ICl(Ca) was slowed in cells dialyzed with either W7 (500 μM) or KN93 (5 μM), similar to the results obtained with AMP-PNP or ADP, and the t1/2 for the current was significantly increased (Table 1). We next dialyzed the CaMKII inhibitory peptide (residues 281–301) (27), which slowed the rate of current inactivation similar to W7 and KN93 (Fig. 3D; Table 1). Conversely, H7, which has a relatively low affinity for CaMKII but inhibits other cellular kinases such as protein kinase C, cAMP-dependent protein kinase, and cGMP-dependent protein kinase, did not produce an appreciable effect on ICl(Ca) (Fig. 3); the t1/2 for current decay was not different from control cells dialyzed with ATP alone and was significantly shorter than the calcium decay (Table 1). These data further demonstrate that the inactivation of ICl(Ca) is mediated by protein phosphorylation and that the signaling pathway involves CaMKII in smooth muscle. CaMKII does not appear to play a role in the opening of Ca2+-activated Cl− channels, however, because the amplitude of ICl(Ca) was not affected by intracellular dialysis of W7, KN93, or the regulatory peptide (Table 1).
Figure 3.
Inhibition of calmodulin or CaMKII slows the decay of ICl(Ca). Dialysis of the calmodulin inhibitor W7 (Upper Left) slowed the rate of ICl(Ca) decay, resulting in a similar rate of current and [Ca2+]i decay (Inset). Dialysis of the CaMKII inhibitor KN93 (Upper Right) or the CaMKII substrate antagonist (50 μM, Lower Left) had an equivalent effect. In contrast, dialysis of H7 (Lower Right) at a concentration predicted to substantially inhibit PKC, PKA, and PKG activity did not slow the rate of ICl(Ca) decay. All solutions contained 1 mM ATP, and cells were dialyzed for 5 min before exposure to caffeine; cells were clamped at −60 mV.
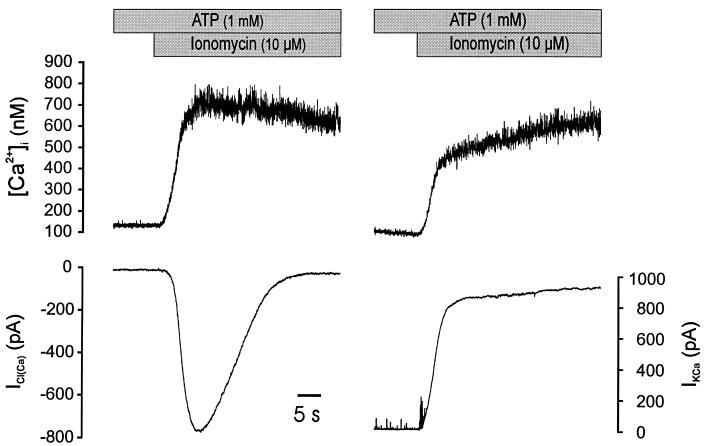
We next wondered whether CaMKII -mediated phosphorylation was sufficient to terminate ICl(Ca) in the absence of a fall in [Ca2+]i. To answer this question a series of experiments were performed using ionomycin to induce a sustained increase in [Ca2+]i in cells dialyzed with ATP. An example of this experiment is shown in Fig. 4. Exposure to 10 μM ionomycin resulted in a sustained increase in [Ca2+]i; however, ICl(Ca) still inactivated. Similar results were obtained from five other cells. In parallel experiments employing conditions designed to isolate the calcium-activated potassium current (130 mM K+ in pipette and Vh = 0 mV, the chloride equilibration potential), that current was sustained following application of ionomycin (Fig. 4; n = 4). This experiment provides further evidence that the termination of ICl(Ca) is not merely a result of a more rapid decrease in near-membrane [Ca2+]i than indicated by the spatially averaged calcium fluorescence signal, because the sustained elevation of the IK(Ca), indicates that the near-membrane calcium is maintained at near-maximal levels.
Figure 4.
ICl(Ca) decays in the presence of a sustained rise in [Ca2+]i. Exposure to ionomycin resulted in a sustained elevation of [Ca2+]i and activation of ICl(Ca) in a cell voltage-clamped at −60 mV (Left). The current completely inactivated, despite the sustained rise in [Ca2+]i. Equivalent experiments were performed in potassium-containing solution, holding at ECl (0 mV) to isolate the calcium-activated potassium current (Right). Unlike ICl(Ca), the calcium-activated potassium current was sustained as long as [Ca2+]i was elevated.
These results further demonstrated dual mechanisms for closing calcium-activated chloride channels: (i) in the absence of CaMKII phosphorylation, channels close as [Ca2+]i falls, indicating that channel open-state probability is a function of [Ca2+]i, and (ii) in the absence of a decline in [Ca2+]i channel closing is effected by CaMKII phosphorylation. Therefore, we anticipated that a sustained increase in [Ca2+]i together with inhibition of channel phosphorylation by CaMKII would result in a sustained opening of Ca2+-activated Cl− channels. To test this hypothesis, myocytes were exposed to ionomycin under conditions that would effectively inhibit phosphorylation. Typical current and calcium traces are depicted in Fig. 5. As predicted, inhibition of phosphorylation by AMP-PNP (n = 5), KN93 (n = 4), or the inhibitory peptide (n = 4) resulted in a sustained ICl(Ca), whereas when cells were dialyzed with H7 the current was not sustained (n = 4).
Figure 5.
ICl(Ca) is sustained in the presence of a sustained rise in [Ca2+]i if CaMKII phosphorylation is inhibited. Ionomycin was used to produce a sustained rise in [Ca2+]i as in Fig. 4. In cells dialyzed with AMP-PNP (Upper Left), with ATP and the CaMKII antagonist KN93 (Upper Right), or with ATP and the CaMKII inhibitory peptide fragment (Upper Left), ICl(Ca) achieved a peak value and was sustained. Conversely, dialysis of cells with ATP and H7, which has a low affinity for CaMKII, did not prevent the decay of ICl(Ca). All cells were dialyzed for 5 min and were voltage-clamped at −60 mV.
Because phosphorylation of the channel or a related protein closed calcium-activated chloride channels, we sought to determine whether dephosphorylation was required for subsequent channel availability (i.e., whether phosphorylation results in channel inactivation). As shown in Fig. 6, when cells were dialyzed with ATP, a second application of caffeine after 5 min reliably evoked calcium and current transients, although there was an approximately 10% decrease in the amplitude of the second ICl(Ca) (from 956 ± 59 pA for the first application, to 861 ± 44 pA for the second application), whereas the mean net increase in [Ca2+]i was not significantly changed. Inhibition of phosphorylation by dialyzing cells with ADP (Fig. 6) or AMP-PNP (data not shown) also resulted in currents that could be evoked repeatedly with little decrease in net current, although the time course of current decay was slowed as previously shown, indicating that the calcium-dependent current decay does not reflect channel inactivation. In marked contrast to these results, however, when channel dephosphorylation was prevented either by thiophosphorylation or by inhibition of endogenous phosphatase activity, currents could not be subsequently activated. As shown in Fig. 6, caffeine induced an ICl(Ca) of smaller amplitude and faster decay in cells dialyzed for 5 min with 1 mM ATPγs substituted for ATP, and a second application of caffeine (5 min after the first application) resulted in a dramatically decreased ICl(Ca) (97 ± 26 pA), which was reduced to 14 ± 3% of the first response (n = 7, P < 0.05). Similarly, in cells dialyzed with the protein phosphatase-1 and -2A inhibitor okadaic acid (500 nM), ICl(Ca) was of smaller amplitude and a second application of caffeine almost failed to evoke ICl(Ca). In a total of six cells tested, the second response was 22 ± 4% of the first response (P < 0.05). In both experimental groups the magnitude or time course of the rise in [Ca2+]i was not different from control (ATP) for the first or second caffeine exposure. These results indicate that phosphorylation of the channel protein or a related protein induces the transition of calcium-activated chloride channels to an inactivated state and that dephosphorylation is required for subsequent channel availability. It is likely that the smaller initial currents observed with ATPγS and okadaic acid reflect an accumulation of channels in the inactivated state during the initial 5-min dialysis.
Figure 6.
The phosphorylation-dependent decay of ICl(Ca) results from channel inactivation. (A) Consecutive exposure to caffeine (cells were dialyzed for 5 min before first application and exposed a second time after 5 min) results in equivalent increases in [Ca2+]i and only a slight decline in the magnitude of ICl(Ca). Note that the current decays before the decline in [Ca2+]i. (B) Substitution of ADP for ATP in the patch pipette results in the typical slowed rate of ICl(Ca) decay but does not alter the current amplitude following a second exposure to caffeine, indicating that channels are available to open. (C) Conversely, intracellular dialysis of ATPγs results in a smaller initial ICl(Ca) and virtually no response on the second application (despite the fact that the [Ca2+]i response was normal), indicating that calcium-activated chloride channels were not available to open. (D) Dialysis of okadaic acid also inhibited the initial caffeine response and almost abolished the subsequent response. All cells were clamped at −60 mV.
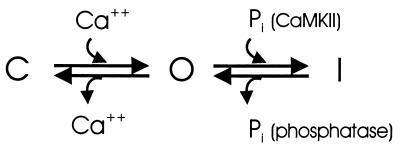
Based on these findings we present the following simplified model of the gating behavior of Ca2+-activated chloride channels (Fig. 7). Channels undergo a calcium-dependent transition from the closed to the open state, as indicated by the simple relationship between current and [Ca2+]i that is observed during the activation of ICl(Ca) (16) and during its decay under conditions in which phosphorylation is inhibited (Figs. 2 and 3). Under physiological conditions open channels inactivate rapidly (before the fall in [Ca2+]i associated with a calcium-release transient) in a process that is mediated by CaMKII, and transition from the inactivated state to the closed state requires protein dephosphorylation (Fig. 6). These dual-gating mechanisms represent a novel ion channel regulatory system. Inactivation of a calcium-dependent ion channel by a calcium-dependent kinase comprises an efficient negative feedback mechanism that uncouples current from the [Ca2+]i signal, ensuring rapid termination of the depolarizing stimulus.
Figure 7.
A simplified model of channel gating. An increase in [Ca2+]i results in a gating transition of calcium-activated chloride channels from the closed (C) to the open (O) state. In the absence of phosphorylation, channels will close when calcium declines, but are available to open (Fig. 6B). Under physiological conditions open channels are rapidly phosphorylated by CaMKII, resulting in transition to the inactivated (I) state before [Ca2+]i falls (Fig. 1), and channels are subsequently dephosphorylated after termination of the calcium signal and a decrease in CaMKII activity, such that they are again available (Fig. 6A). Channels can be trapped in I (unavailable to open) by thiophosphorylation or phosphatase inhibition (Fig. 6 C and D). In this state channels are effectively uncoupled from [Ca2+]i.
Acknowledgments
We thank Ms. Laura Lynch for technical assistance and Ms. Ellen Davison for manuscript preparation. Supported by HL 41084 and HL 45239.
ABBREVIATIONS
- ICl(Ca)
Ca2+-activated Cl− current
- [Ca2+]i
intracellular Ca2+ concentration
- t1/2
the half time of decay
- AMP-PNP
5′-adenylylimidodiphosphate
- ATPgs
adenosine 5′-O-(3-thiotriphosphate)
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
References
- 1.Owen D G, Segal M, Barker J L. Nature (London) 1984;311:567–570. doi: 10.1038/311567a0. [DOI] [PubMed] [Google Scholar]
- 2.Scott R H, Sutton K G, Griffin A, Stapleton S R, Currie K P M. Pharmacol Ther. 1995;66:535–565. doi: 10.1016/0163-7258(95)00018-c. [DOI] [PubMed] [Google Scholar]
- 3.Evans M G, Marty A. J Physiol. 1986;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne N G, Large W A. Br J Pharmacol. 1985;84:711–721. doi: 10.1111/j.1476-5381.1985.tb08950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hume R I, Thomas S A. J Physiol. 1989;417:241–261. doi: 10.1113/jphysiol.1989.sp017799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zygmunt A C, Gibbons W R. J Gen Physiol. 1992;99:391–414. doi: 10.1085/jgp.99.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Large W A, Wang Q. Am J Physiol. 1996;271:C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- 8.Mayer M L. J Physiol. 1985;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthew G, Neher E, Penner R. J Physiol. 1989;418:131–144. doi: 10.1113/jphysiol.1989.sp017831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korn S J, Bolden A, Horn R. J Physiol. 1991;439:423–437. doi: 10.1113/jphysiol.1991.sp018674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Hogg R C, Large W A. J Physiol. 1992;451:535–537. doi: 10.1113/jphysiol.1992.sp019177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson M T, Chang H, Rubart M, Santana L F, Boner A D, Knot H J, Lederer W J. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 13.Bolton R, Singer D, Dascal N. Pflügers Arch. 1990;416:1–6. doi: 10.1007/BF00370214. [DOI] [PubMed] [Google Scholar]
- 14.Hussy N. J Neurophysiol. 1992;68:2042–2050. doi: 10.1152/jn.1992.68.6.2042. [DOI] [PubMed] [Google Scholar]
- 15.Pacaud P, Loirand G, Gregoire G, Mironneau C, Mironneau J. Pflügers Arch. 1992;421:125–130. doi: 10.1007/BF00374818. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y-X, Kotlikoff M I. Am J Physiol. 1997;273:C509–C519. doi: 10.1152/ajpcell.1997.273.2.C509. [DOI] [PubMed] [Google Scholar]
- 17.Klockner U. Pflügers Arch. 1993;424:231–237. doi: 10.1007/BF00384347. [DOI] [PubMed] [Google Scholar]
- 18.Kume H, Graziano M P, Kotlikoff M I. Proc Natl Acad Sci USA. 1992;89:11051–11055. doi: 10.1073/pnas.89.22.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y-X, Fleischmann B K, Kotlikoff M I. Am J Physiol. 1997;272:C1151–C1159. doi: 10.1152/ajpcell.1997.272.4.C1151. [DOI] [PubMed] [Google Scholar]
- 20.Neher E, Augustine G J. J Physiol. 1992;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleischmann B K, Wang Y-X, Pring M, Kotlikoff M I. J Physiol. 1996;492:347–358. doi: 10.1113/jphysiol.1996.sp021313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleischmann B K, Wang Y-X, Kotlikoff M I. Am J Physiol. 1997;272:C341–C349. doi: 10.1152/ajpcell.1997.272.1.C341. [DOI] [PubMed] [Google Scholar]
- 23.Duchen M R. J Physiol. 1990;424:387–409. doi: 10.1113/jphysiol.1990.sp018073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stapleton S R, Scott R H, Bell B. Br J Pharmacol. 1994;111:57–64. doi: 10.1111/j.1476-5381.1994.tb14023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MaCarron J G, McGeown J G, Reardon S, Ikebe M, Fay F S, Walsh J V. Nature (London) 1992;357:74–77. doi: 10.1038/357074a0. [DOI] [PubMed] [Google Scholar]
- 26.Braun A P, Schulman H. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 27.Smith M K, Colbran R J, Brickey D A, Solderling T R. J Biol Chem. 1992;267:1761–1768. [PubMed] [Google Scholar]