Abstract
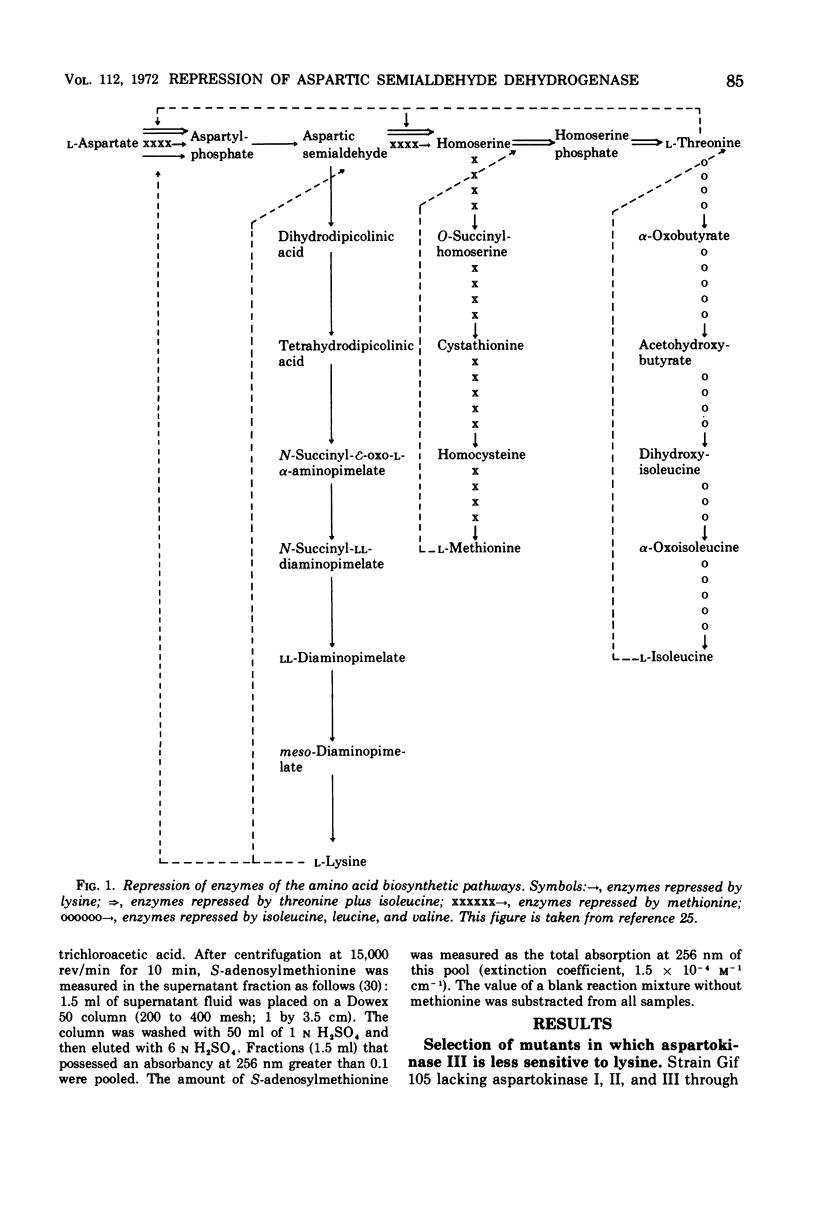
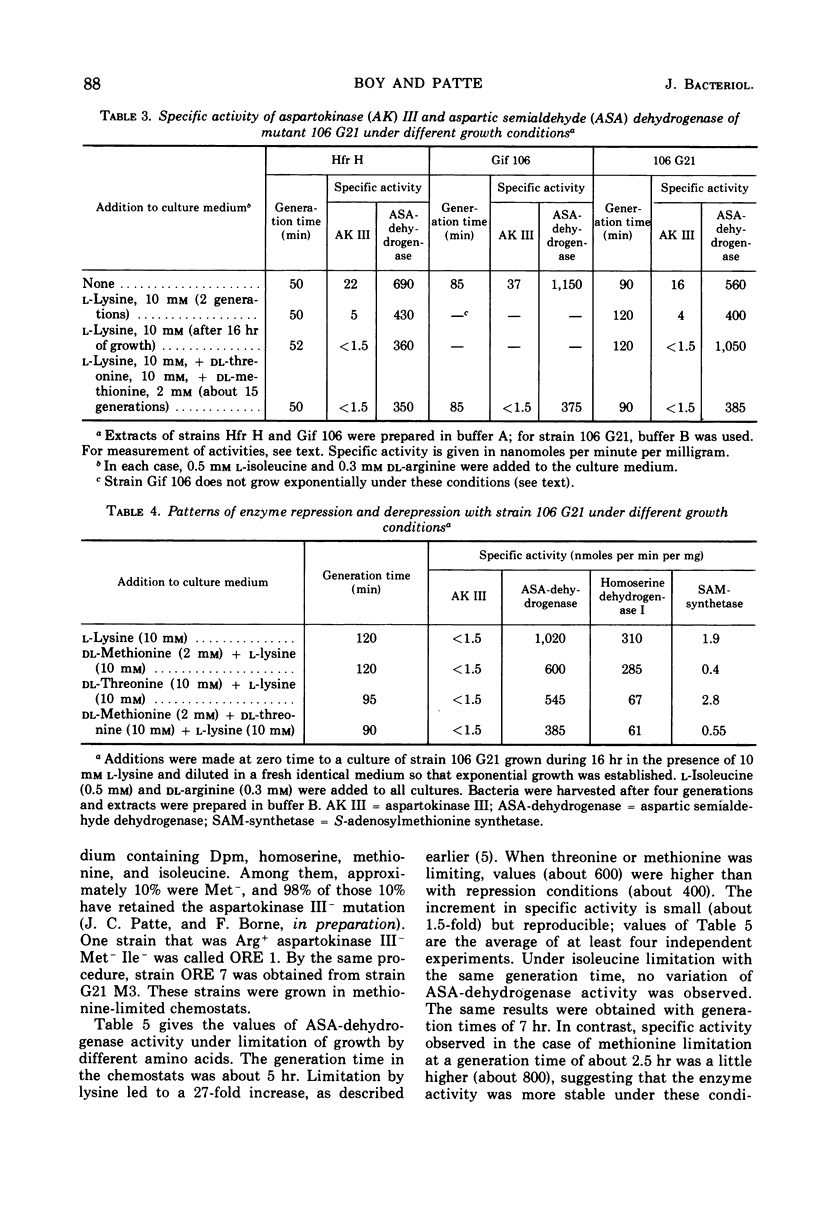
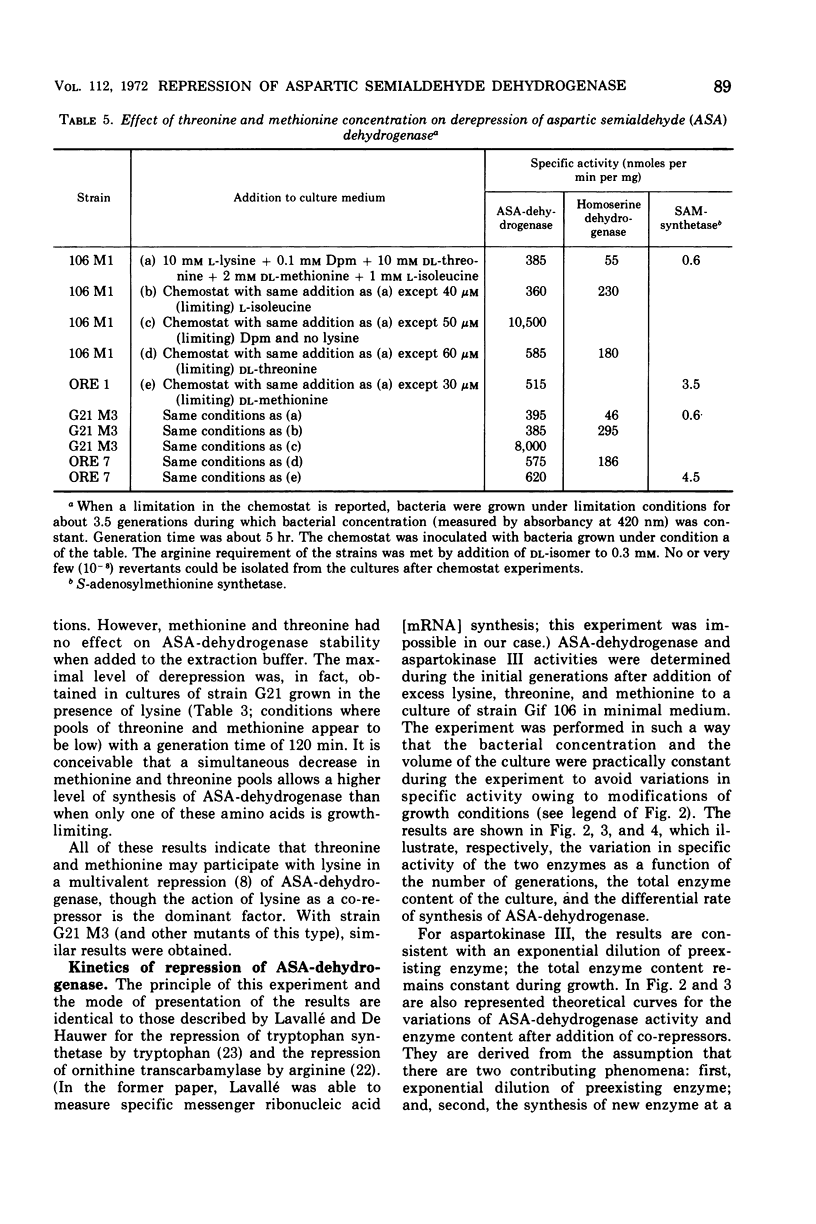
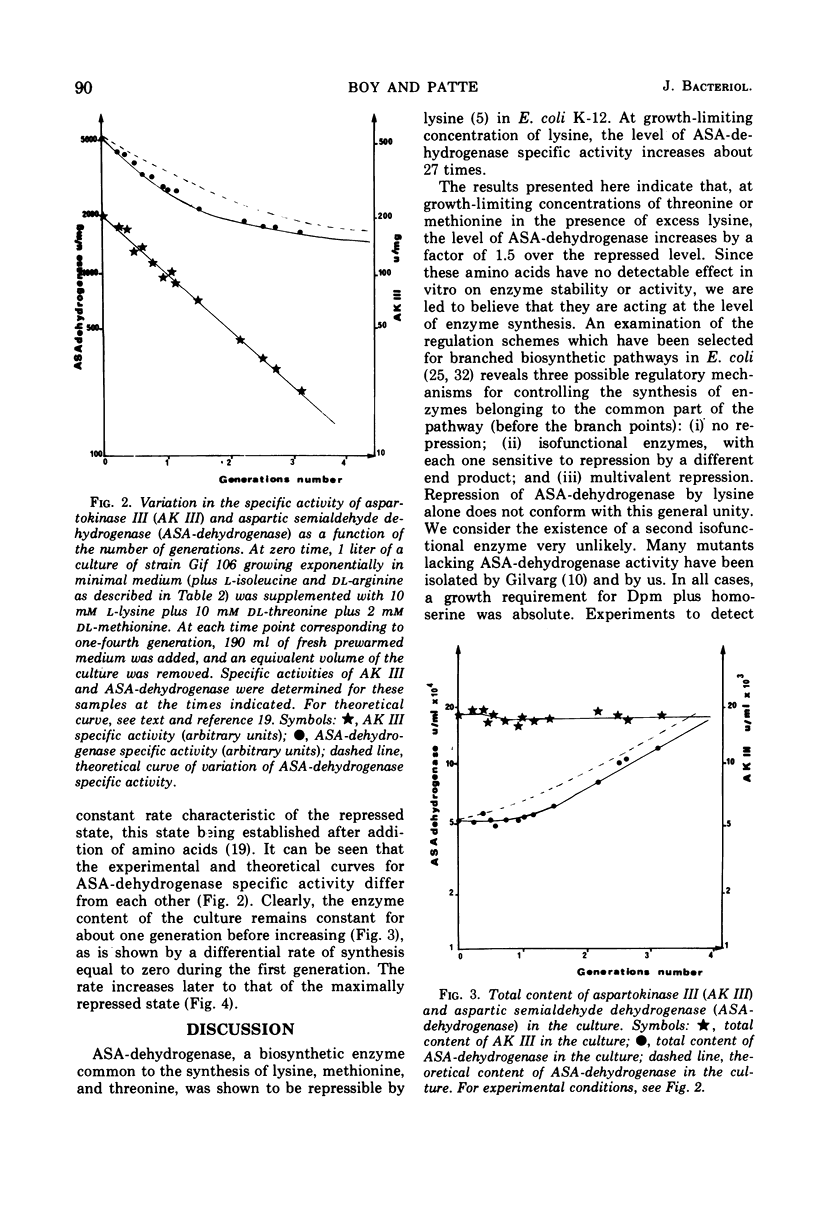
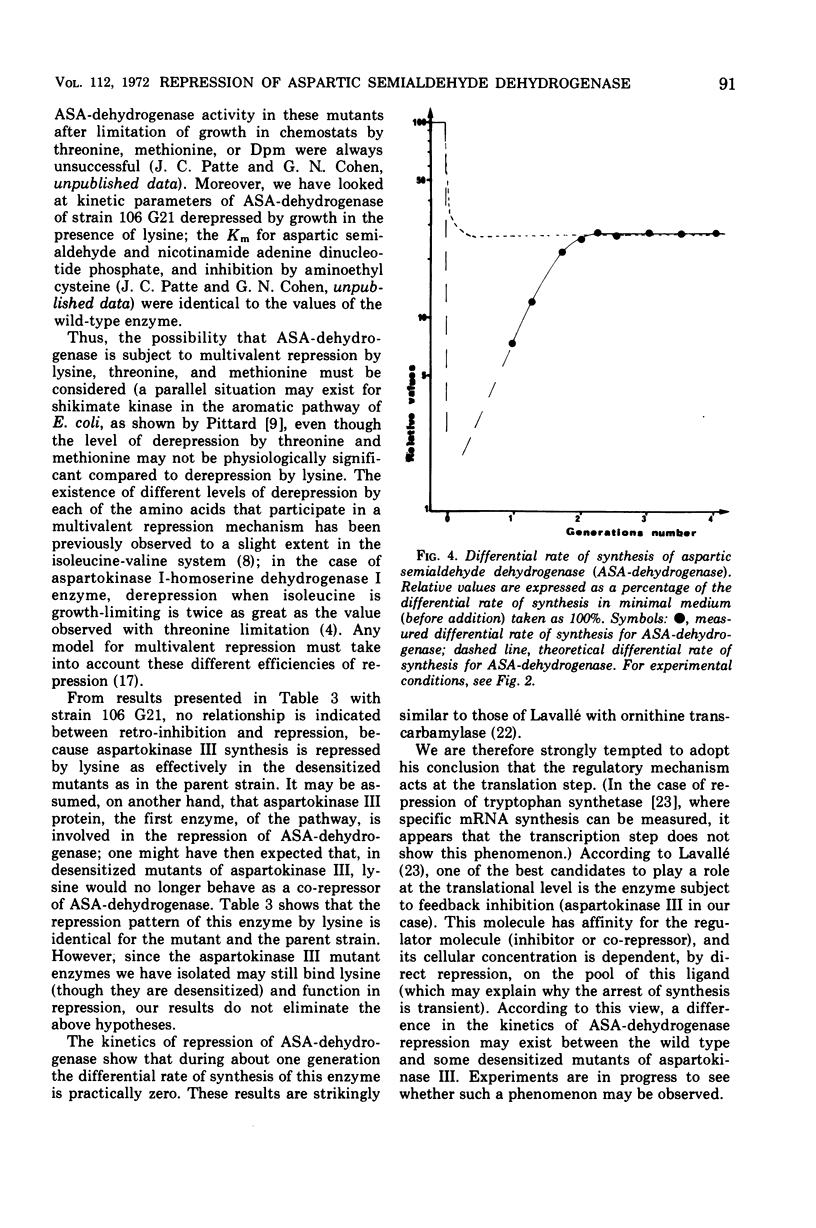
Mutants of Escherichia coli in which the lysine-sensitive aspartokinase is feedback-resistant are described. In these strains, as well as in the wild type, aspartic semialdehyde dehydrogenase is subject to multivalent repression by lysine, threonine, and methionine. When these amino acids were added to a culture in minimal medium, the differential rate of synthesis of the enzyme dropped to zero and remained there for about one generation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollon A. P., Magee P. T. Involvement of threonine deaminase in multivalent repression of the isoleucine-valine pathway in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2169–2172. doi: 10.1073/pnas.68.9.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., PATTE J. C., BOEZI J. [Coordinated derepression of lysine-aspartokinase and of semi-aspartaldehyde dehydrogenase in Escherichia coli]. C R Hebd Seances Acad Sci. 1963 Mar 25;256:2939–2941. [PubMed] [Google Scholar]
- Cline A. L., Bock R. M. Translational control of gene expression. Cold Spring Harb Symp Quant Biol. 1966;31:321–333. doi: 10.1101/sqb.1966.031.01.042. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D. Biosynthetic interrelations of lysine, diaminopimelic acid, and threonine in mutants of Escherichia coli. Nature. 1952 Mar 29;169(4300):534–536. doi: 10.1038/169534a0. [DOI] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILVARG C. The branching point in diaminopimelic acid synthesis. J Biol Chem. 1962 Feb;237:482–484. [PubMed] [Google Scholar]
- Gibson F., Pittard J. Pathways of biosynthesis of aromatic amino acids and vitamins and their control in microorganisms. Bacteriol Rev. 1968 Dec;32(4 Pt 2):465–492. [PMC free article] [PubMed] [Google Scholar]
- Greene R. C., Su C. H., Holloway C. T. S-Adenosylmethionine synthetase deficient mutants of Escherichia coli K-12 with impaired control of methionine biosynthesis. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1120–1126. doi: 10.1016/0006-291x(70)90355-4. [DOI] [PubMed] [Google Scholar]
- Gruber M., Campagne R. N. Regulation of protein synthesis: an alternative to the repressor-operator hypothesis. Proc K Ned Akad Wet C. 1965;68(4):270–276. [PubMed] [Google Scholar]
- Holloway C. T., Greene R. C., Su C. H. Regulation of S-adenosylmethionine synthetase in Escherichia coli. J Bacteriol. 1970 Nov;104(2):734–747. doi: 10.1128/jb.104.2.734-747.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- JACOB F. Transduction of lysogeny in Escherichia coli. Virology. 1955 Jul;1(2):207–220. doi: 10.1016/0042-6822(55)90017-9. [DOI] [PubMed] [Google Scholar]
- Kovach J. S., Berberich M. A., Venetianer P., Goldberger R. F. Repression of the histidine operon: effect of the first enzyme on the kinetics of repression. J Bacteriol. 1969 Mar;97(3):1283–1290. doi: 10.1128/jb.97.3.1283-1290.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach J. S., Phang J. M., Ference M., Goldberger R. F. Studies on repression of the histidine operon. II. The role of the first enzyme in control of the histidine system. Proc Natl Acad Sci U S A. 1969 Jun;63(2):481–488. doi: 10.1073/pnas.63.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuma C., Gros C., Patte J. C. Regulation of the lysine biosynthetic pathway of Escherichia coli K12. Isolation, molecular weight and amino acid analysis of the lysine sensitive aspartokinase. Eur J Biochem. 1970 Jul;15(1):111–115. doi: 10.1111/j.1432-1033.1970.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Lavallé R., De Hauwer G. Tryptophan messenger translation in Escherichia coli. J Mol Biol. 1970 Jul 28;51(2):435–447. doi: 10.1016/0022-2836(70)90153-1. [DOI] [PubMed] [Google Scholar]
- Lavallé R. Regulation at the level of translation in the arginine pathway of Escherichia coli K12. J Mol Biol. 1970 Jul 28;51(2):449–451. doi: 10.1016/0022-2836(70)90154-3. [DOI] [PubMed] [Google Scholar]
- McLellan W. L., Vogel H. J. Translational repression in the arginine system of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1703–1709. doi: 10.1073/pnas.67.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTE J. C., LE BRAS G., LOVINY T., COHEN G. N. [Retro-inhibition and repression of the homoserine dehydrogenase of Escherichia coli]. Biochim Biophys Acta. 1963 Jan 8;67:16–30. doi: 10.1016/0006-3002(63)91793-1. [DOI] [PubMed] [Google Scholar]
- Patte J. C., Le Bras G., Cohen G. N. Regulation by methionine of the synthesis of a third aspartokinase and of a second homoserine dehydrogenase in Escherichia coli K 12. Biochim Biophys Acta. 1967 Mar 22;136(2):245–247. doi: 10.1016/0304-4165(67)90069-4. [DOI] [PubMed] [Google Scholar]
- Patte J. C., Loviny T., Cohen G. N. Effets inhibiteurs cooperatifs de la L-lysine avec d'autres amino acides sur une aspartokinase de Escherichia coli. Biochim Biophys Acta. 1965 Jun 22;99(3):523–530. [PubMed] [Google Scholar]
- Richaud F., Cohen G. N. Selection of Escherichia coli mutants devoid of one or of both the activities carried by a multifunctional protein. Biochem Biophys Res Commun. 1968 Jan 11;30(1):45–49. doi: 10.1016/0006-291x(68)90710-9. [DOI] [PubMed] [Google Scholar]
- Rothman-Denes L., Martin R. G. Two mutations in the first gene of the histidine operon of Salmonella typhimurium affecting control. J Bacteriol. 1971 Apr;106(1):227–237. doi: 10.1128/jb.106.1.227-237.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLENK F., DEPALMA R. E. The preparation of S-adenosylmethionine. J Biol Chem. 1957 Dec;229(2):1051–1057. [PubMed] [Google Scholar]
- Umbarger H. E. Regulation of amino acid metabolism. Annu Rev Biochem. 1969;38:323–370. doi: 10.1146/annurev.bi.38.070169.001543. [DOI] [PubMed] [Google Scholar]



