Abstract
Glucocorticoid hormones, acting via nuclear receptors, regulate many metabolic processes, including hepatic gluconeogenesis. It recently has been recognized that intracellular glucocorticoid concentrations are determined not only by plasma hormone levels, but also by intracellular 11β-hydroxysteroid dehydrogenases (11β-HSDs), which interconvert active corticosterone (cortisol in humans) and inert 11-dehydrocorticosterone (cortisone in humans). 11β-HSD type 2, a dehydrogenase, thus excludes glucocorticoids from otherwise nonselective mineralocorticoid receptors in the kidney. Recent data suggest the type 1 isozyme (11β-HSD-1) may function as an 11β-reductase, regenerating active glucocorticoids from circulating inert 11-keto forms in specific tissues, notably the liver. To examine the importance of this enzyme isoform in vivo, mice were produced with targeted disruption of the 11β-HSD-1 gene. These mice were unable to convert inert 11-dehydrocorticosterone to corticosterone in vivo. Despite compensatory adrenal hyperplasia and increased adrenal secretion of corticosterone, on starvation homozygous mutants had attenuated activation of the key hepatic gluconeogenic enzymes glucose-6-phosphatase and phosphoenolpyruvate carboxykinase, presumably, because of relative intrahepatic glucocorticoid deficiency. The 11β-HSD-1 −/− mice were found to resist hyperglycamia provoked by obesity or stress. Attenuation of hepatic 11β-HSD-1 may provide a novel approach to the regulation of gluconeogenesis.
Glucocorticoids (cortisol in humans, corticosterone in mice and rats) are an important class of adrenocorticosteroids that regulate many metabolic and homeostatic processes and form a key component of the response to stress (1). Glucocorticoids act via intracellular glucocorticoid receptors and, in some tissues, mineralocorticoid receptors; both are nuclear transcription factors (2). Recently, it has become apparent that glucocorticoid action on target tissues depends not only on circulating steroid concentrations and the cellular expression of receptors, but also on intracellular enzymes that critically determine if glucocorticoids gain access to receptors in active forms.
11β-hydroxysteroid dehydrogenase (11β-HSD) catalyzes the interconversion of cortisol and corticosterone with their inert 11-keto forms [cortisone and 11-dehydrocorticosterone (11-DHC), respectively]. There are two isozymes. 11β-HSD type 2 (11β-HSD-2) is a high affinity (nanomolar KM), NAD-dependent exclusive 11β-dehydrogenase (3, 4), which excludes glucocorticoids from otherwise nonselective mineralocorticoid receptors in aldosterone target tissues, such as the distal nephron (5, 6), and from the feto-placental unit (4). Mutations of the gene encoding 11β-HSD-2 are responsible for the human syndrome of “apparent mineralocorticoid excess,” characterized by illicit activation of renal mineralocorticoid receptors by cortisol, producing sodium retention, severe hypertension, and hypokalemia (7–9).
In contrast, the function(s) of 11β-HSD type 1 (11β-HSD-1), initially purified and cloned from rat liver (10, 11), remain obscure. 11β-HSD-1 shares less than 30% homology with 11β-HSD-2, has a lower affinity for glucocorticoids (micromolar KM), and uses NADP(H) as cosubstrate. High expression of 11β-HSD-1 is found in the liver, lung, adipose tissue, kidney, and brain, largely localized to cells expressing glucocorticoid receptors, but not mineralocorticoid receptors, suggesting that 11β-HSD-1, by analogy to 11β-HSD-2, modulates glucocorticoid access to glucocorticoid receptors (12). 11β-HSD-1 in tissue homogenates and when purified is bidirectional, exhibiting both 11β-dehydrogenase and 11β-reductase reactions, with greater stability of the dehydrogenase activity (10). However, though transfection of Chinese hamster ovary cells with 11β-HSD-1 cDNA encodes bidirectional activity (11), transfection of the same cDNA into other cell lines encodes a predominant 11β-reductase (13, 14), which regenerates active glucocorticoids from inert 11-keto forms. The reductase reaction predominates in intact cells, whereas broken cells, homogenates, and microsomes all show bidirectional activity. Importantly, primary cultures of rat hepatocytes, lung cells, hippocampal cells, and vascular smooth muscle cells (15–18) also show predominant 11β-reductase activity, which parallels 11β-HSD-1 mRNA expression (15). Such glucocorticoid regeneration, if manifest in vivo, might increase effective intracellular glucocorticoid levels, amplifying glucocorticoid activity. To explore this notion further we generated mice bearing targeted disruption of the 11β-HSD-1 gene.
MATERIALS AND METHODS
Cloning and Mapping Mouse 11β-HSD-1 Gene.
A 129/Ola λGEM-12 genomic library was screened with a rat 11β-HSD-1 cDNA probe. A 14-kb 11β-HSD-1 genomic fragment encompassing exons 2–5 was cloned and mapped by partial restriction digestion, subcloning of gene fragments, and crosshybridization of purified fragments. Sequencing of exons 2, 3, 4, and 5 showed complete concordance with the published mouse cDNA sequence (19).
Construction of Replacement Vector and Gene Targeting.
The replacement vector was based on plasmid pBS-βKnpA16 containing the neomycin resistance gene flanked by the human β-actin promoter and the simian virus 40 polyadenylation signal sequence. A 6.5-kb KpnI–SpeI fragment from intron D of the 11β-HSD-1 gene was modified by blunt-ending the SpeI site and subcloned between the KpnI and EcoRV sites of pBS-βKnpA16 to produce pBS-LA1, the 3′ homology arm subclone. The 5′ homology arm was synthesized by generating a 1.2-kb PCR fragment by using oligonucleotides with nested SacI and SpeI restriction sites (oligo 1: TTTGAGCTCTACAAATGAAGAGTTCAGAC; oligo 2: CCCACTAGTGAGAGTTTAAGTCTATTGTT) and subcloned between SacI and SpeI sites of pBS-LA1 to produce pBS-ko16x16. This replacement vector was linearized with KpnI and SacI and electroporated into embryonic stem (ES) cells (line CGR 8) (20). G-418 resistant clones were selected as described (20), and DNA was extracted and analyzed by PCR. Two positive controls were run for each DNA sample to amplify products of ≈1.3 kb: oligo 4 (CACTGCATTCTAGTTGTGGTTTGTCC) and oligo 1 amplified the sequence from the integrated replacement vector, and oligo 3 (TTTGGATCCCACCCAAAGCCAATCATTGC) and oligo 2 amplified from the wild-type allele. PCR with oligo 3 and oligo 4 amplified any specific integration event. Specific recombination was confirmed by Southern blot hybridization of BamHI-digested DNA with a 180-bp PCR-derived external probe located between −87 and +87 relative to the transcription start. Cells from the targeted ES cell clone were injected into C57BL/6 blastocysts and transferred into C57BL/CBA foster mothers. Chimeras were bred to MF1 females, and the progeny was genotyped by Southern analysis of BamHI-digested DNA.
Gene Expression and Enzyme Function Analysis.
Tissue 11β-HSD-1 mRNA expression was determined by Northern analysis, as described (19). 11β-HSD activity was quantitated in homogenates, as described (21). Briefly, tissues were homogenized in Krebs–Ringer bicarbonate buffer and incubated with 200 μM NADP (NAD for 11β-HSD-2), 12 nM [3H]-corticosterone and 0.2% BSA for 60 min, before extraction and analysis of steroids by HPLC. 11β-ketosteroid reductase activity was assayed by using [3H]-11-DHC (8–80nM) at multiple time points by using 0.25 mg protein/ml in 10% glycerol, 1 mM EDTA, 50 mM sodium acetate pH 6.0 buffer, and 200 μM NADPH (22). [3H]-11-DHC was prepared as described (22). The apparent KM was calculated by using Lineweaver–Burke plots.
Analysis of Corticosterone and 11-DHC Metabolites.
Hepatic exposure to corticosterone and 11-DHC was analyzed by quantitation of urinary tetrahydro metabolites (tetrahydrocorticosterone, allo-tetrahydrocorticosterone, and tetrahydro-11-DHC) by using GC-MS. Briefly, urinary steroids together with conjugates were purified from C18 Sep-pak columns, and conjugates were hydrolyzed with β-glucuronidase and rechromatographed. Analysis of trimethylsilyl-methoxime derivatives was carried out by using a Hewlett Packard 5890 gas chromatograph, fitted with a HP-1 crosslinked methylsiloxane column (25 m, id.0.25 mm, ft.0.17 μm) coupled to a Trio-1000 mass spectrometer. Quantitation used deuterated standards (23, 24). Plasma corticosterone levels in morning (basal) and evening (peak) samples were measured by radioimmunoassay (25), modified for microtiter plate scintillation proximity assay (Amersham).
Adrenal Sensitivity to ACTH in Vitro.
Mice were housed singly for 2 days before experimentation. Adrenals were removed from 11β-HSD-1 +/+ and −/− mice, cleaned, halved, and incubated in vitro in 1 ml of MEM (containing 25 mM Hepes and 0.1% wt/vol BSA). After preincubation for 120 min, the medium was changed and adrenals incubated for 60 min to determine basal corticosterone production. ACTH (0.1–10 nmol/liter) was applied for an additional 60 min, and medium was removed and frozen until analysis of corticosterone by radioimmunoassay (as above).
In Vivo Studies Measurement of 11-Hydroxysteroid Reductase Activity in Vivo.
Twenty-four male, 3-month-old 11β-HSD-1 +/+ and −/− mice were bilaterally adrenalectomized under halothane anesthesia and a pellet, 50% 11-DHC (15 mg 11-DHC + 15 mg cholesterol) or placebo (30 mg cholesterol), inserted s.c. After 3 days, mice were decapitated, trunk blood was taken, the thymuses were weighed, and the abdomens were examined to ensure no adrenal tissue remained. Plasma corticosterone was measured by radioimmunoassay. Any circulating corticosterone would be because of the conversion of 11-DHC to corticosterone by tissues in vivo.
Unstressed blood glucose measurements.
Mice were housed singly for 24 hr and fasted overnight. All blood glucose measurements were made between 08.30–09.30 hr. Mice were removed from their cages and loosely restrained in a box. A rapid tail-nick procedure was carried out within 30–60 sec of disturbing the animal, before stress-induced glucose mobilization could occur. Whole blood glucose was measured by the glucose oxidase method by using a One Touch II Glucometer (Lifescan, UK).
Stressed fasting glucose levels.
Mice were fasted overnight and then exposed to a new cage (a potent psychological stressor in rodents) for 60 min. After cervical dislocation, trunk blood samples were obtained and plasma glucose was measured, as described (26).
High-fat diet.
Mice were housed 3–5 per cage and fed either a normal diet (chow) ad libitum or a high-fat diet (60% loose chow, 10% granulated sugar, and 30% margarine fat).
Hepatic enzyme activities and glycogen content.
Mouse liver microsomes and cytosol were prepared, microsomes were disrupted (27), and glucose-6-phosphatase (G6Pase), phosphoenolpyruvate carboxykinase (PEPCK), and glucokinase activities assayed, as described (28–30). Kinetic constants were calculated by nonlinear regression analysis. Glycogen (31) and protein concentrations were measured, as described (32).
RESULTS
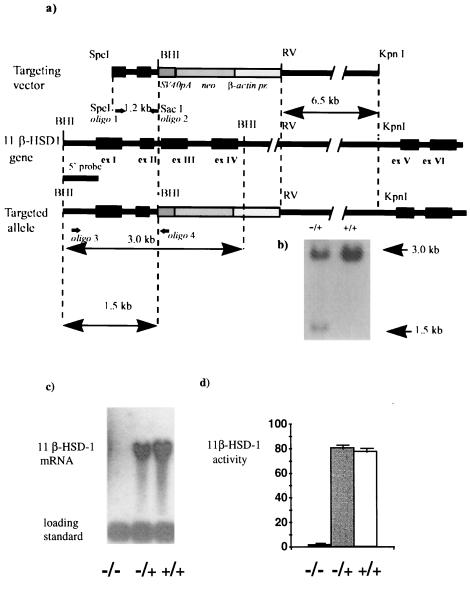
A null mutation of the 11β-HSD-1 locus was generated by replacing the genomic fragment encompassing exons 3 and 4 with a neomycin-resistance cassette, through specific recombination in mouse ES cells (Fig. 1 a and b). Specific recombination was detected in one of 368 G418-resistant colonies screened by PCR. Targeting was confirmed by Southern analysis with 5′ external and 3′ internal probes. Southern analysis with the internal probe confirmed a single site of integration (not shown). Cells from a single targeted ES clone (1C5), confirmed to have a normal karyotype, were injected into C57BL/6 blastocysts. Of six male chimeras born, germ-line transmission was achieved in one, which was mated to MF1 females. Approximately half of the progeny were heterozygous for the disrupted allele. Homozygous (11β-HSD-1 −/−) mice were bred by intercross and backcross of MF1/129 heterozygotes. In 150 animals genotyped, no deviation from Mendelian distribution of alleles was observed, with the distribution of classes in the intercross 44 (+/+):78 (+/−):34 (−/−). No 11β-HSD-1 mRNA was detected by Northern analysis of liver RNA from 11β-HSD-1 −/− homozygous mutants, whereas heterozygotes (11β-HSD-1 +/−) showed ≈50% reduction of 11β-HSD-1 mRNA expression (Fig. 1c). Hepatic 11β-HSD activity was <5% of wild-type levels in homozygous mutants (Fig. 1d). There was no detectable 11β-ketosteroid reductase activity in liver homogenates from 11β-HSD −/− mutant mice; the Km for 11-DHC (11β-reductase) was 120 ± 10 nM in wild-type liver. No 11β-HSD-1 mRNA or enzyme activity was detected in −/− brains. Activity of the 11β-HSD-2 isozyme in the kidney appeared unaffected in 11β-HSD-1 −/− mice (renal conversion of corticosterone to 11-DHC was 81.9 ± 2.1% in wild type and 76.2 ± 1.9% in null mice, n = 4 and 5, respectively). 11β-HSD-1 −/− mice showed no detectable changes in blood pressure (mean blood pressure was measured by cannulation of the aorta in anesthetized animals, wild type 105 ± 11 mm Hg, 11β-HSD-1 −/− 108 ± 12 mm Hg), urinary electrolytes (urinary sodium, wild type 102 ± 4 mmol/liter, 11β-HSD-1 −/− 101 ± 14 mmol/liter; urinary potassium wild type 101 ± 6 mmol/liter, −/− 95 ± 13 mmol/liter; urinary creatinine, wild type 1.3 ± 0.4 mmol/liter, −/− 1.0 ± 0.4 mmol/liter), or plasma electrolytes (e.g., plasma sodium, wild type 149 ± 0.6 mmol/liter, −/− 151 ± 1.6 mmol/liter) to suggest mineralocorticoid excess. No major visible abnormalities were found in 11β-HSD-1 −/− mice. Birth weight (wild type 1.45 ± 0.07 g, −/− 1.46 ± 0.08 g), postnatal growth, and development were indistinguishable from wild-type litter mates. Homozygous males and females were fertile, delivering litters of 8–12 pups at 21 days gestation.
Figure 1.
Targeted inactivation of 11β-HSD-1 gene. (a) Structure of the targeting vector, the 11β-HSD-1 locus, and the targeted allele. RV, EcoRV; BHI, BamHI. Synthesis of 5′ homology arm, 5′ external probe, and initial screening of colonies was done by PCR with oligos 1, 2, 3, and 4 as shown. (b) Southern blot hybridization analysis of BamHI-digested genomic DNA from neomycin-resistant targeted (+/−) and nontargeted (+/+) ES clones. (c) Northern blot analysis of 11β-HSD-1 expression. Thirty micrograms of total RNA from liver of homozygous (−/−) and heterozygous (+/−) mutants and wild type (+/+) litter mates was hybridized with 11β-HSD-1 and control (U-1) cDNA probes. (d) 11β-HSD activity in the liver homogenates of the same animals expressed as % conversion corticosterone to 11-DHC (n = 4 for −/−, n = 5 for −/+, and n = 5 for +/+).
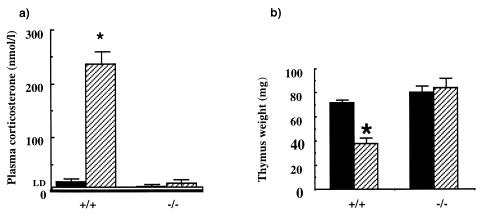
To investigate the effect of 11β-HSD-1 gene knockout on the net production of corticosterone from exogenous 11-DHC, adrenalectomized wild-type and −/− mice were implanted with 11-DHC pellets (15 mg) and plasma corticosterone was measured 3 days later. Whereas wild-type adrenalectomized mice could readily convert 11-DHC to active corticosterone, achieving plasma levels ≈250 nmol/liter, in −/− animals plasma corticosterone remained undetectable (<10 nmol/liter; Fig. 2a). Moreover, 11-DHC pellets led to thymic involution (a glucocorticoid-dependent process) in wild type, but not −/− mice (Fig. 2b). These data demonstrate that 11β-HSD-1 is the sole 11β-reductase activity converting inert 11-ketosteroids into active glucocorticoids under physiological conditions and can generate sufficient corticosterone to confer physiological effects.
Figure 2.
The effect of dehydrocorticosterone (11-DHC) or vehicle pellets in vivo on plasma corticosterone and thymus weight (Inset) in adrenalectomized 11β-HSD-1 +/+ and −/− mice. The black columns represent data obtained from mice with vehicle pellets, and the hatched columns mice with A pellets. LD, limit of detection. Values are means ± SEM, n = 5–6. ∗, P < 0.05 compared with vehicle control (t test).
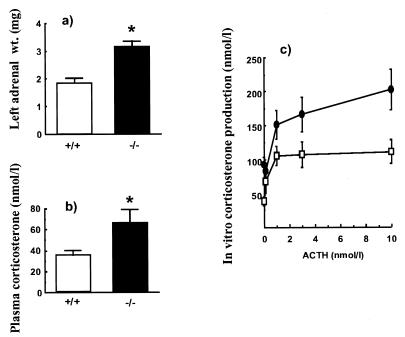
The adrenal glands of male 11β-HSD-1 −/− mice were significantly enlarged (3.15 ± 0.2 mg; n = 18) in comparison to weight- and age-matched wild-type controls (1.85 ± 0.17 mg; n = 16; P < 0.05 Student’s t test); morphometric analysis suggested adrenocortical hyperplasia. Moreover, the adrenals of male 11β-HSD-1 −/− mice incubated in vitro showed increased basal and ACTH-stimulated corticosterone secretion (Fig. 3).
Figure 3.
(a) Adrenal weights, (b) basal, unstressed, plasma corticosterone levels in vivo, and (c) ACTH-stimulated corticosterone production from adrenal glands in vitro of 11β-HSD-1 +/+ and −/− mice. Open bars and symbols are wild type. Solid bars and symbols are 11β-HSD-1 −/− mice. ACTH (0–10 nmol/liter) was applied to adrenal glands in vitro for 60 min. Results are means ± SEM, n = 8–10. ∗, P < 0.05 compared with +/+ control (t test). Two-way ANOVA showed a significant effect of dose of ACTH (P = 0.02) and mouse genotype (P = 0.002), with no interaction between the two variables (P = 0.47).
In vivo and basal (morning, diurnal nadir) plasma corticosterone levels were higher in 11β-HSD-1 −/− mice (62 ± 14 nmol/liter, n = 10) than in wild types (33 ± 4 nmol/liter, P < 0.05, n = 10). Peak (evening) levels of plasma corticosterone did not differ significantly (149 ± 20 nmol/liter in wild type, 106 ± 20 nmol/liter in −/− mice). The ratio of tetrahydro 11-DHC to tetrahydro-corticosterone derivatives, largely generated in the liver, in pooled 24-hr urine samples was 0.62. This ratio predicts the concentration of 11-DHC reaching the liver in wild-type mice to be in the limits between 20 and 90 nM.
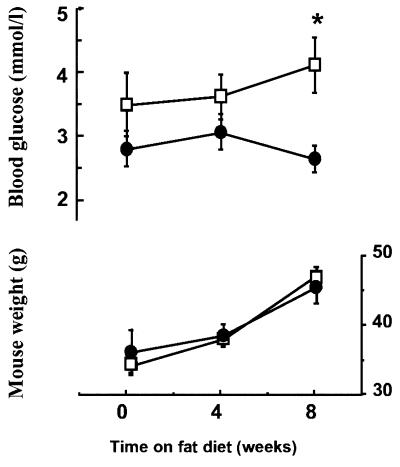
To determine nonstressed glucose levels, mice were habituated to rapid sampling by tail nick without handling. This experiment revealed no fasting hypoglycemia in −/− mice. However, feeding the mice a high-fat diet, previously reported to produce obesity and defective glycemic control (33), showed that obese 11β-HSD-1 −/− mice had significantly lower fasting plasma glucose levels than weight-matched wild-type controls (Fig. 4). Similarly, after 24-hr starvation combined with novel environment stress, plasma glucose levels were significantly lower in −/− mice (5.6 ± 0.4 mmol/liter; n = 14) than in wild types (6.7 ± 0.3 mmol/liter; n = 13; P < 0.05). 11β-HSD-1 −/− mice had normal basal levels of hepatic G6Pase and PEPCK mRNA and activity. However, on starvation, 11β-HSD-1 −/− mice failed to show the normal induction of G6Pase and PEPCK (Table 1). The effects on G6Pase were particularly striking and predominantly affected the enzyme itself rather than the G6P transporter, because similar effects occurred in intact and disrupted microsomes. Hepatic glucokinase and its fall on starvation were unaltered in the −/− animals. However, liver glycogen levels in −/− mice were significantly increased in the fed state compared with wild types (Table 1).
Figure 4.
Effect of a high-fat diet on body weight and fasting blood glucose levels in 11β-HSD-1 +/+ and −/− mice. Each value indicates mean ± SEM, n = 8–10/group. ∗, P < 0.05 significant to corresponding −/− value.
Table 1.
Gluconeogenic enzyme activities and glycogen stores in starved and fed 11 β-HDS-1 −/− mutants and 11 β-HSD-1 +/+ wild-type controls
| 11β-HSD-1 −/−
|
11β-HSD-1 +/+
|
|||
|---|---|---|---|---|
| Fed (n = 8) | Starved (n = 8) | Fed (n = 8) | Starved (n = 8) | |
| G6Pase, intact microsomes | ||||
| Vmax (μmol/min·mg) | 0.42 ± 0.04 | 0.42 ± 0.05* | 0.43 ± 0.07 | 0.63 ± 0.04† |
| KM (mM) | 5.26 ± 0.42 | 5.93 ± 0.57* | 6.06 ± 0.58 | 7.86 ± 0.77† |
| G6Pase, disrupted microsomes | ||||
| Vmax (μmol/min·mg) | 0.54 ± 0.03 | 0.64 ± 0.06 | 0.53 ± 0.02 | 0.81 ± 0.07† |
| KM (mM) | 0.40 ± 0.06 | 0.44 ± 0.09 | 0.56 ± 0.13 | 0.50 ± 0.10 |
| PEPCK | ||||
| Vmax (nmol/min·mg) | 3.5 ± 0.5 | 11.3 ± 1.4*† | 5.3 ± 0.5 | 15.7 ± 1.7† |
| Glucokinase | ||||
| Vmax (nmol/min·mg) | 28.4 ± 3.0 | 19.6 ± 1.8† | 29.4 ± 4.3 | 22.8 ± 2.6 |
| Glycogen μg/mg protein | 528 ± 99* | 77.5 ± 8.1† | 302 ± 37.9 | 73.8 ± 8.2† |
| Animal weight grams | 30.2 ± 1.2 | 25.9 ± 1.1† | 27.1 ± 2.0 | 23.3 ± 0.9 |
G6Pase and glycogen are per mg microsomal protein. PEPCK and glucokinase are per mg cytosolic protein.
*P < 0.05 compared with corresponding 11β-HSD-1 +/+ value.
P < 0.05 compared with corresponding fed value.
DISCUSSION
Our data suggest that 11β-HSD-1 is a unique enzyme in the mouse, converting inert 11-keto corticosteroids into active glucocorticoids, and indicate this conversion has important consequences for intracellular glucocorticoid action, notably in the liver. The mice appear normal at birth, findings in concert with the low expression of this isozyme in most fetal tissues (34). Although 11β-HSD-1 is expressed in rodent placenta and 11β-HSD inhibitors reduce birth weight in rats (26, 35), no alterations in birth weight in 11β-HSD-1 −/− mice were found, suggesting these studies reflect effects on 11β-HSD-2, itself highly expressed in fetus and placenta (36). Both male and female 11β-HSD-1 −/− mice are fertile and produce normal litters. Although 11β-HSD-1 is expressed in the rat testis (37) and ovary (38) and has been suggested to regulate fertility in males (39), this does not appear to be the case, at least in mice.
In the absence of 11β-HSD-1 reductase, inert 11-keto corticosteroids cannot be reduced to active 11-hydroxy forms. Our data suggest that significant amounts of 11-DHC reach the liver, as judged by the ≈2:3 ratio of urinary tetrahydro-11-DHC metabolites to tetrahydrocorticosterone metabolites (largely generated in liver), allowing an estimate of plasma 11-DHC levels of around 50 nM, a value according with plasma levels of cortisone, the human equivalent (40). The 120 nM KM for reduction of 11-DHC in wild-type liver is also clearly appropriate to metabolize nanomolar levels of circulating 11-DHC; enzyme activity was not detected in the liver homogenates of 11β-HSD-1 −/− animals.
If the lack of 11-DHC reactivation to corticosterone is of importance in overall glucocorticoid production and tissue activation, it might be anticipated that there would be increased drive from the hypothalamic-pituitary-adrenal axis to restore homeostasis. Intriguingly, adrenal hyperplasia has been described in a unique female patient with presumed 11β-reductase deficiency (41). This contention is further supported in 11β-HSD-1 null mice, in which adrenal weight is increased, caused, in large part, by increased adrenal cortical size. This finding appears to be of functional significance because the null mice showed increased secretion of corticosterone to ACTH stimulation in vitro. Indeed, plasma corticosterone levels actually were elevated at the diurnal nadir (morning). This result suggests not only increased adrenal corticosterone secretion in vivo, but also relative insensitivity of the hypothalamic-pituitary-adrenal axis to central glucocorticoid inhibition; 11β-HSD-1 is expressed in the hippocampus, hypothalamus, and pituitary in wild-type, but not −/− mice, and in normal rats (42–44). Loss of function of central 11β-HSD-1 reductase thus may attenuate effective glucocorticoid feedback, as appears to occur at least for some hypothalamic corticotroph secretagogues (44).
Glucocorticoids stimulate transcription of hepatic gluconeogenic enzymes and thus play a major role in the enhancement of liver glucose output during starvation or stress (45). PEPCK and G6Pase catalyze the rate-limiting steps of gluconeogenesis. Transcription of genes encoding both enzymes is regulated by classical glucocorticoid-inducible promoters (46, 47) and is markedly attenuated in glucocorticoid receptor-deficient mice (48). Our results indicate that glucocorticoid reactivation by 11β-HSD-1 also plays a critical role in the up-regulation of these genes during starvation. Thus in 11β-HSD-1 −/− mice, the expected induction of hepatic G6Pase mRNA and enzyme activity on starvation was lost and PEPCK induction was attenuated, the lesser effect presumably reflecting complex regulation of the PEPCK promoter by both glucocorticoid and cAMP response elements (49) and mirroring data from glucocorticoid receptor knockout mice (48). Similarly, elevated liver glycogen in fed null mice presumably reflects attenuated glycogenolysis, a pathway normally stimulated by glucocorticoids in the fed state (Table 1) (45). These results are unlikely merely to reflect generalized or nonspecific effects of the mutation on liver function, because glucokinase activity and its fall with starvation (which show complex regulation) are unaffected in −/− mice. Presumably in consequence of these changes, the null mice resist the hyperglycemia seen in the wild-type litter mates with stress or obesity. Thus, the 11β-HSD-1 −/− liver appears to reproduce a phenotype of partial glucocorticoid deficiency, despite somewhat increased basal plasma corticosterone levels.
These experiments suggest that intracellular regeneration of corticosterone in the liver from inert circulating 11-DHC contributes more to effective intracellular corticosterone concentrations than plasma corticosterone itself. Indeed, 11-DHC circulates mainly unbound, whereas corticosterone is predominantly bound to corticosteroid binding globulin (CBG). CBG has a substantial excess capacity for corticosterone (50) such that for most of the day “free” corticosterone concentrations are low (<5 nmol/liter), only reaching levels comparable to 11-DHC during the diurnal peak or stress response. We believe that by eliminating 11β-HSD-1 gene function we have created an animal model of relative intracellular glucocorticoid deficiency. This model will be particularly useful for investigating the action of glucocorticoids on tissues (liver, brain, lung, and vasculature) where reactivation of 11-keto steroids by 11β-HSD-1 may occur. The study also raises the notion of 11-DHC and cortisone as prohormones, with plasma levels not subjected to wide diurnal fluctuations (40) or sequestration by CBG (51), and which may possess higher tissue specificity because they can act only on tissues expressing 11β-HSD-1. Finally, the resistance of 11β-HSD-1 −/− mice to stress and obesity-related hyperglycemia suggests that inhibition of this enzyme may attenuate gluconeogenesis (52). The relevance of this finding to disease processes, such as noninsulin-dependent diabetes mellitus, remains to be explored.
Acknowledgments
We thank Austin Smith and Matthew Sharp for advice on ES cell technology, Jan Ure and Jenny Nichols for microinjections, Meng Li and William Skarnes for pBS-bKnpA, Craig Watt for animal husbandry, and Mark Lindsay for plasma glucose and electrolyte estimations. The work was funded by a Programme Grant from the Wellcome Trust (C.R.W.E., J.R.S., and J.J.M.), a Wellcome Senior Clinical Fellowship (J.R.S.), and a Wellcome Career Development Fellowship (M.C.H.), and it was supported by the Commission of the European Communities concerted action Transgeneur.
ABBREVIATIONS
- 11β-HSD-1
11β-hydroxysteroid dehydrogenase type 1
- 11-DHC
11-dehydrocorticosterone
- ES
embryonic stem
- PEPCK
phosphoenolpyruvate carboxykinase
- G6Pase
glucose 6-phosphatase
References
- 1.Miller W L, Tyrrell J B. In: Endocrinology and Metabolism. 3rd Ed. Felig P, Baxter J D, Frohman L, editors. New York: McGraw Hill; 1995. pp. 555–711. [Google Scholar]
- 2.Evans R M. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albiston A L, Obeyesekere V R, Smith R E, Krozowski Z S. Mol Cell Endocrinol. 1994;105:R11–R17. doi: 10.1016/0303-7207(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 4.Brown R W, Chapman K E, Kotelevtsev Y, Leckie C, Lindsay R S, Lyons V, Murad P, Mullins J J, Edwards C R W, Seckl J R. Biochem J. 1996;313:1007–1017. doi: 10.1042/bj3131007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards C R W, Stewart P M, Burt D, Brett L, McIntyre M A, Sutanto W S, de Kloet E R, Monder C. Lancet. 1988;2:986–989. doi: 10.1016/s0140-6736(88)90742-8. [DOI] [PubMed] [Google Scholar]
- 6.Funder J W, Pearce P T, Smith R, Smith A I. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- 7.Wilson R C, Krozowski Z S, Lee K, Obeyesekere V R, Razzaghy-Azar M, Harbison M D, Wei J Q, Shackleton C, Funder J W, New M I. J Clin Endocrinol Metab. 1995;80:2263–2266. doi: 10.1210/jcem.80.7.7608290. [DOI] [PubMed] [Google Scholar]
- 8.Mune T, Rogerson F, Nikkila H, Agarwal A K, White P C. Nat Genet. 1995;10:394–399. doi: 10.1038/ng0895-394. [DOI] [PubMed] [Google Scholar]
- 9.Stewart P M, Krozowski Z S, Gupta A, Milford D V, Howie A J, Sheppard M C, Whorwood C B. Lancet. 1996;347:88–91. doi: 10.1016/s0140-6736(96)90211-1. [DOI] [PubMed] [Google Scholar]
- 10.Lakshmi V, Monder C. Endocrinology. 1988;123:2390–2398. doi: 10.1210/endo-123-5-2390. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal A K, Monder C, Eckstein B, White P C. J Biol Chem. 1989;264:18939–18943. [PubMed] [Google Scholar]
- 12.Whorwood C B, Franklyn J A, Sheppard M C, Stewart P M. J Steroid Biochem Mol Biol. 1991;41:21–28. doi: 10.1016/0960-0760(92)90220-d. [DOI] [PubMed] [Google Scholar]
- 13.Low S C, Chapman K E, Edwards C R W, Seckl J R. J Mol Endocrinol. 1994;13:167–174. doi: 10.1677/jme.0.0130167. [DOI] [PubMed] [Google Scholar]
- 14.Duperrex H, Kenouch S, Gaegeler H-P, Seckl J R, Edwards C R W, Farman N, Rossier B C. Endocrinology. 1993;132:612–619. doi: 10.1210/endo.132.2.8425481. [DOI] [PubMed] [Google Scholar]
- 15.Jamieson P M, Chapman K E, Edwards C R W, Seckl J R. Endocrinology. 1995;136:4754–4761. doi: 10.1210/endo.136.11.7588203. [DOI] [PubMed] [Google Scholar]
- 16.Rajan V, Edwards C R W, Seckl J R. J Neurosci. 1996;16:65–70. doi: 10.1523/JNEUROSCI.16-01-00065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hundertmark S, Buhler H, Ragosch V, Dinkelborg L, Arabin B, Weitzel H K. Endocrinology. 1995;136:2573–2578. doi: 10.1210/endo.136.6.7750479. [DOI] [PubMed] [Google Scholar]
- 18.Brem A S, Bina R B, King T, Morris D J. Steroids. 1995;60:406–410. doi: 10.1016/0039-128x(94)00074-m. [DOI] [PubMed] [Google Scholar]
- 19.Rajan V, Chapman K E, Lyons V, Jamieson P, Mullins J J, Edwards C R W, Seckl J R. J Steroid Biochem Mol Biol. 1995;52:141–147. doi: 10.1016/0960-0760(94)00159-j. [DOI] [PubMed] [Google Scholar]
- 20.Clark A F, Sharp M G F, Morley S D, Fleming S, Peters J, Mullins J J. J Biol Chem. 1997;272:18185–18190. doi: 10.1074/jbc.272.29.18185. [DOI] [PubMed] [Google Scholar]
- 21.Low S C, Moisan M-P, Edwards C R W, Seckl J R. J Neuroendocrinol. 1994;6:285–290. doi: 10.1111/j.1365-2826.1994.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 22.Brown R W, Chapman K E, Edwards C R W, Seckl J R. Endocrinology. 1993;132:2614–2621. doi: 10.1210/endo.132.6.8504762. [DOI] [PubMed] [Google Scholar]
- 23.Shackleton C H L, Honour J. Clin Chim Acta. 1976;69:267–283. doi: 10.1016/0009-8981(76)90505-2. [DOI] [PubMed] [Google Scholar]
- 24.Best R, Walker B R. Clin Endocrinol. 1997;47:231–236. doi: 10.1046/j.1365-2265.1997.2471061.x. [DOI] [PubMed] [Google Scholar]
- 25.MacPhee I A M, Antoni F A, Mason D W. J Exp Med. 1989;169:431–445. doi: 10.1084/jem.169.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindsay R S, Lindsay R M, Waddell B, Seckl J R. Diabetologia. 1996;39:1299–1305. doi: 10.1007/s001250050573. [DOI] [PubMed] [Google Scholar]
- 27.Blair J N, Burchell A. Biochem Biophys Acta. 1988;964:161–167. doi: 10.1016/0304-4165(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 28.Burchell A, Hume R, Burchell B. Clin Chim Acta. 1988;173:183–192. doi: 10.1016/0009-8981(88)90256-2. [DOI] [PubMed] [Google Scholar]
- 29.Petrescu I, Bojan O, Saied M, Barzu O, Schmidt F, Kuhnle H F. Anal Biochem. 1979;96:279–281. doi: 10.1016/0003-2697(79)90582-7. [DOI] [PubMed] [Google Scholar]
- 30.Davidson, T. A. & Arion, W. J. (1987) Arch. Biochem. Biophys. 156–157. [DOI] [PubMed]
- 31.Van Handel E. Anal Biochem. 1965;11:256–265. doi: 10.1016/0003-2697(65)90013-8. [DOI] [PubMed] [Google Scholar]
- 32.Peterson G L. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 33.Ikemoto S, Thompson K S, Takahashi M, Itakura H, Lane M D, Ezaki O. Proc Natl Acad Sci USA. 1995;92:3096–3099. doi: 10.1073/pnas.92.8.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz, R., Brown, R. W. & Seckl, J. R. (1997) J. Neurosci., in press. [DOI] [PMC free article] [PubMed]
- 35.Lindsay R S, Lindsay R M, Edwards C R W, Seckl J R. Hypertension. 1996;27:1200–1204. doi: 10.1161/01.hyp.27.6.1200. [DOI] [PubMed] [Google Scholar]
- 36.Brown R W, Diaz R, Robson A C, Kotelevtsev Y, Mullins J J, Kaufman M H, Seckl J R. Endocrinology. 1996;137:794–797. doi: 10.1210/endo.137.2.8593833. [DOI] [PubMed] [Google Scholar]
- 37.Phillips M D, Lakshmi V, Monder C. Endocrinology. 1989;125:209–216. doi: 10.1210/endo-125-1-209. [DOI] [PubMed] [Google Scholar]
- 38.Benediktsson R, Yau J L W, Low S C, Brett L, Cooke B A, Edwards C R W, Seckl J R. J Endocrinol. 1992;135:53–58. doi: 10.1677/joe.0.1350053. [DOI] [PubMed] [Google Scholar]
- 39.Monder C, Miroff Y, Marandici A, Hardy M P. Endocrinology. 1994;134:1199–1204. doi: 10.1210/endo.134.3.8119160. [DOI] [PubMed] [Google Scholar]
- 40.Walker B R, Campbell J C, Fraser R, Stewart P M, Edwards C R W. Clin Endocrinology. 1992;37:483–492. doi: 10.1111/j.1365-2265.1992.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 41.Phillipou G, Higgins B A. J Steroid Biochem. 1985;22:435–436. doi: 10.1016/0022-4731(85)90451-0. [DOI] [PubMed] [Google Scholar]
- 42.Moisan M-P, Seckl J R, Edwards C R W. Endocrinology. 1990;127:1450–1455. doi: 10.1210/endo-127-3-1450. [DOI] [PubMed] [Google Scholar]
- 43.Sakai R R, Lakshmi V, Monder C, McEwen B S. J Neuroendocrinol. 1992;4:101–106. doi: 10.1111/j.1365-2826.1992.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 44.Seckl J R, Dow R C, Low S C, Edwards C R W, Fink G. J Endocrinol. 1993;136:471–477. doi: 10.1677/joe.0.1360471. [DOI] [PubMed] [Google Scholar]
- 45.Pilkis S J, Granner D K. Annu Rev Physiol. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- 46.Imai E, Stromstedt P E, Quinn P G, Carlstedt-Duke J, Gustafsson J-A, Granner D K. Mol Cell Biol. 1990;10:4712–4719. doi: 10.1128/mcb.10.9.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lange A J, Argaud D, Elmaghrabi M R, Pan W S, Maitra S R, Pilkis S J. Biochem Biophys Res Commun. 1994;201:302–309. doi: 10.1006/bbrc.1994.1702. [DOI] [PubMed] [Google Scholar]
- 48.Cole T J, Blendy J A, Monaghan A P, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 49.Imai E, Miner J A, Mitchell K R, Yamamoto K R, Granner D K. J Biol Chem. 1993;268:5353–5356. [PubMed] [Google Scholar]
- 50.Gayrard V, Alvinerie M, Toutain P L. Domest Anim Endocrinol. 1996;13:35–45. doi: 10.1016/0739-7240(95)00042-9. [DOI] [PubMed] [Google Scholar]
- 51.Meikle A W. In: Endocrinology. DeGroot L J, editor. Philadelphia: Saunders; 1989. pp. 1610–1632. [Google Scholar]
- 52.Walker B R, Connacher A A, Lindsay R M, Webb D J, Edwards C R W. J Clin Endocrinol Metab. 1995;80:3155–3159. doi: 10.1210/jcem.80.11.7593419. [DOI] [PubMed] [Google Scholar]