Abstract
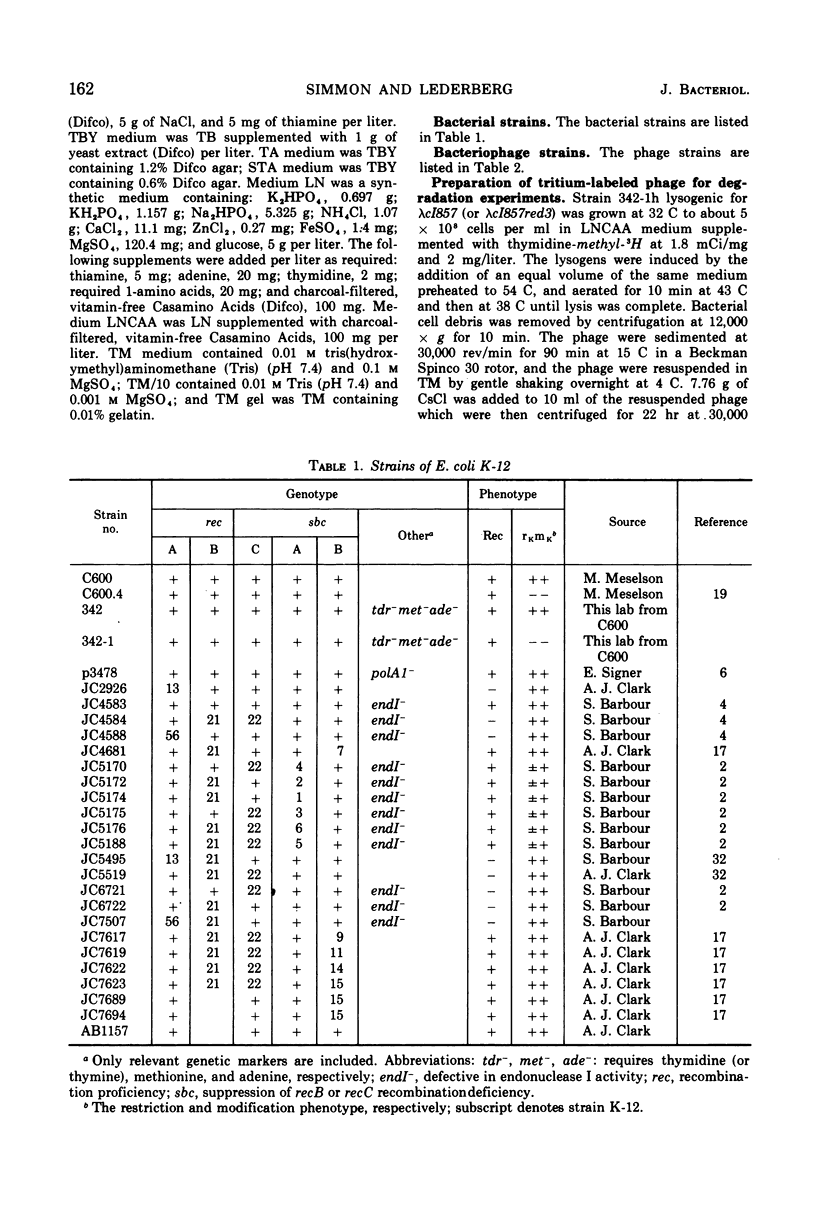
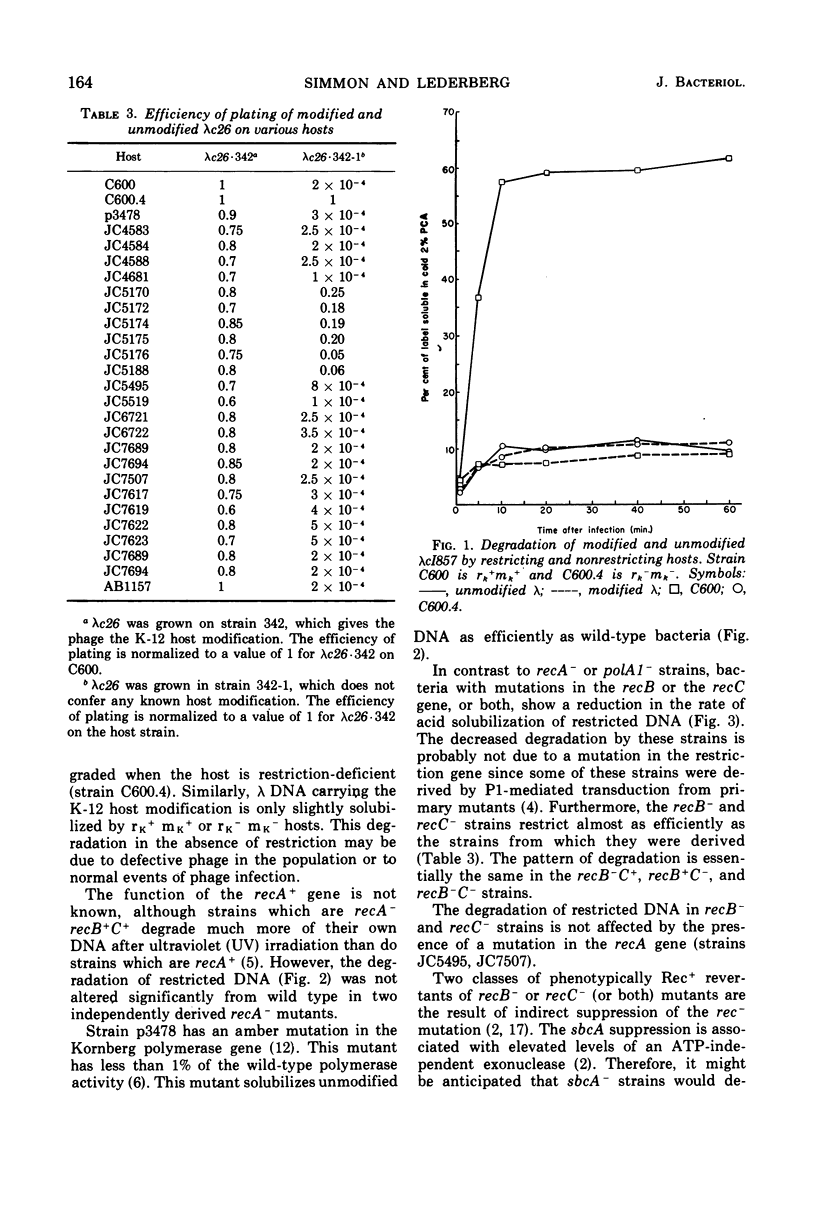
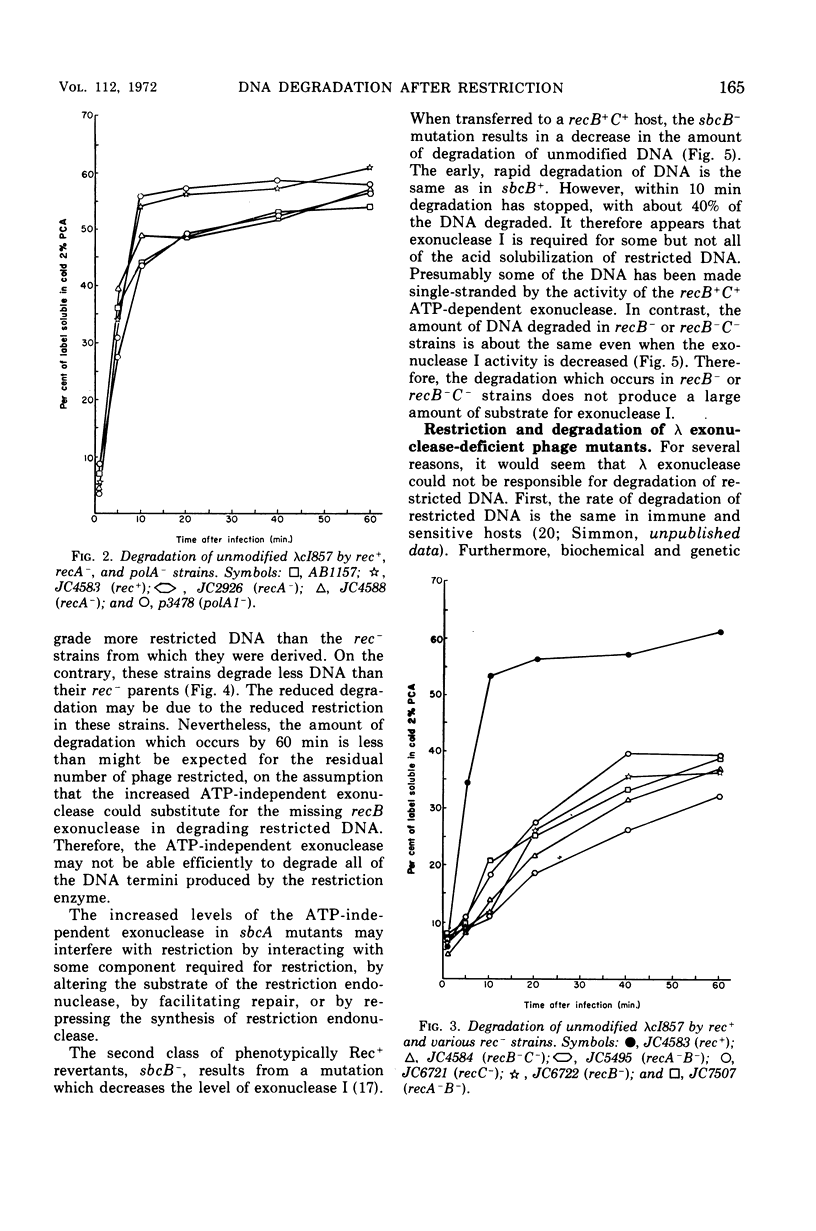
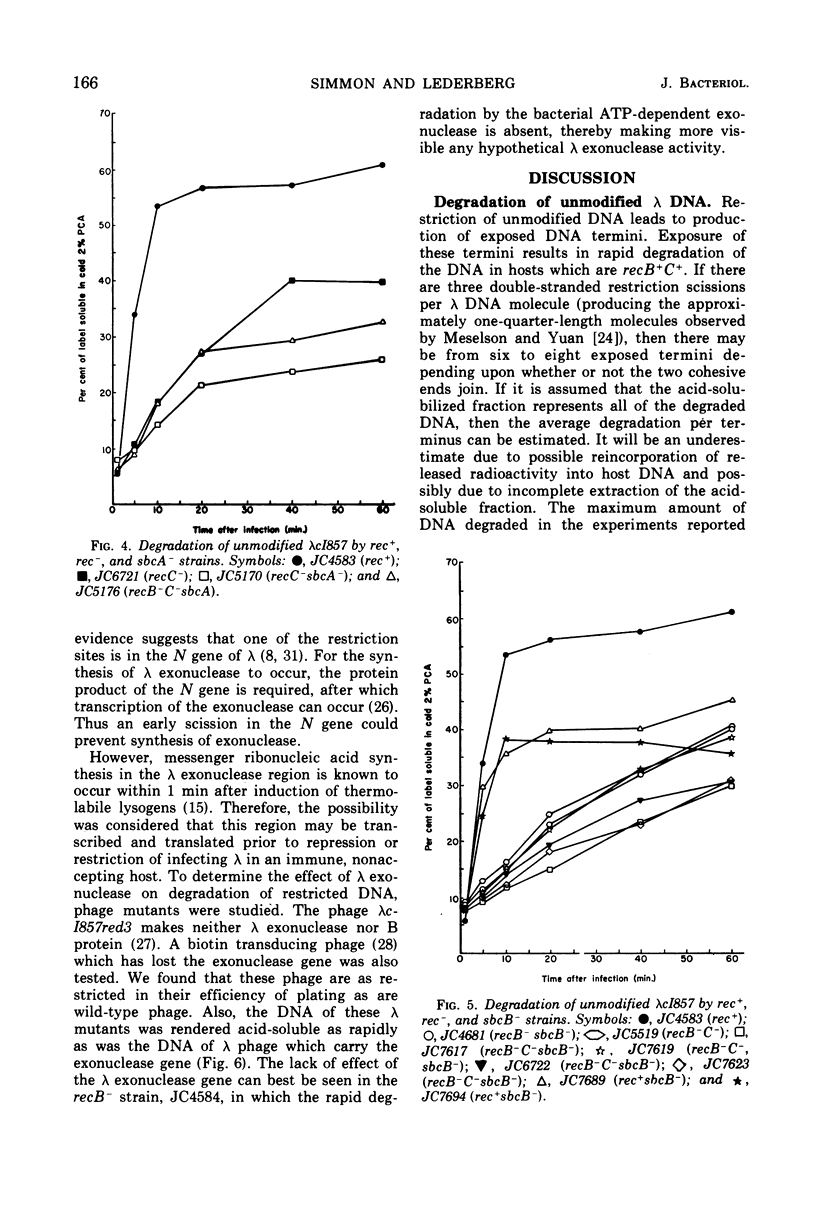
Wild-type bacteria which restrict the deoxyribonucleic acid (DNA) of infecting phage when the phage do not carry the proper host modification rapidly degrade that restricted DNA to acid-soluble products. The purified restriction enzyme acts as an endonuclease in vitro to cleave restrictable DNA and does not further degrade the DNA fragments produced. We have examined mutants of Escherichia coli K-12 which lack various nucleases in order to determine which nucleases are involved in the rapid acid solubilization in vivo of unmodified λ DNA following restriction. Bacteria which are wild type, recA−, or polA1− degrade about 50% of the unmodified phage DNA within 10 min of infection, with little subsequent degradation. Mutants which are recB− or recC− degrade unmodified DNA very slowly, solubilizing about 15% of the DNA by 10 min after infection. Two classes of phenotypic revertants of recB−/C− mutants were also tested. Bacteria which are sbcA− restrict poorly and do not degrade much of the restricted DNA. Bacteria which are sbcB− restrict normally. This mutation does not appear to affect degradation of restricted phage DNA in recB−/C− mutants, but such degradation is decreased in recB+/C+ bacteria. The presence of a functional λ exonuclease gene is not required for degradation after restriction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Nagaishi H., Templin A., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. II. Rec+ revertants caused by indirect suppression of rec- mutations. Proc Natl Acad Sci U S A. 1970 Sep;67(1):128–135. doi: 10.1073/pnas.67.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttin G., Wright M. Enzymatic DNA degradation in E. coli: its relationship to synthetic processes at the chromosome level. Cold Spring Harb Symp Quant Biol. 1968;33:259–269. doi: 10.1101/sqb.1968.033.01.030. [DOI] [PubMed] [Google Scholar]
- Capaldo-Kimball F., Barbour S. D. Involvement of recombination genes in growth and viability of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):204–212. doi: 10.1128/jb.106.1.204-212.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Chamberlin M., Boyce R. P., Howard-Flanders P. Abnormal metabolic response to ultraviolet light of a recombination deficient mutant of Escherichia coli K12. J Mol Biol. 1966 Aug;19(2):442–454. doi: 10.1016/s0022-2836(66)80015-3. [DOI] [PubMed] [Google Scholar]
- DUSSOIX D., ARBER W. Host specificity of DNA produced by Escherichia coli. II. Control over acceptance of DNA from infecting phage lambda. J Mol Biol. 1962 Jul;5:37–49. doi: 10.1016/s0022-2836(62)80059-x. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Franklin N. C., Dove W. F. Genetic evidence for restriction targets in the DNA of phages lambda and phi 80. Genet Res. 1969 Oct;14(2):151–157. doi: 10.1017/s0016672300001981. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R., Howe M. New mutations in the S cistron of bacteriophage lambda affecting host cell lysis. Virology. 1969 May;38(1):200–202. doi: 10.1016/0042-6822(69)90148-2. [DOI] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. An endonuclease activity from Escherichia coli absent from certain rec- strains. Proc Natl Acad Sci U S A. 1970 Sep;67(1):434–441. doi: 10.1073/pnas.67.1.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso R. J., Paigen K. The effect of amino acids on host-controlled restriction of lambda phage. Virology. 1968 Sep;36(1):1–8. doi: 10.1016/0042-6822(68)90110-4. [DOI] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Haefner K. Spontaneous lethal sectoring, a further feature of Escherichia coli strains deficient in the function of rec and uvr genes. J Bacteriol. 1968 Sep;96(3):652–659. doi: 10.1128/jb.96.3.652-659.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IHLER G., MESELSON M. GENETIC RECOMBINATION IN BACTERIOPHAGE LAMBDA BY BREAKAGE AND JOINING OF DNA MOLECULES. Virology. 1963 Sep;21:7–10. doi: 10.1016/0042-6822(63)90297-6. [DOI] [PubMed] [Google Scholar]
- KORN D., WEISSBACH A. THE EFFECT OF LYSOGENIC INDUCTION ON THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI K12-LAMBDA. I. APPEARANCE OF A NEW EXONUCLEASE ACTIVITY. J Biol Chem. 1963 Oct;238:3390–3394. [PubMed] [Google Scholar]
- Konrad M. Gene expression in bacteriophage lambda. II. Regulation of RNA synthesis in vivo by the N protein. J Mol Biol. 1970 Nov 14;53(3):389–400. doi: 10.1016/0022-2836(70)90073-2. [DOI] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG S., MESELSON M. DEGRADATION OF NON-REPLICATING BACTERIOPHAGE DNA IN NON-ACCEPTING CELLS. J Mol Biol. 1964 May;8:623–628. doi: 10.1016/s0022-2836(64)80112-1. [DOI] [PubMed] [Google Scholar]
- LEDERBERG S. Suppression of the multiplication of heterologous bacteriophages in lysogenic bacteria. Virology. 1957 Jun;3(3):496–513. doi: 10.1016/0042-6822(57)90006-5. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R. The deoxyribonucleases of Escherichia coli. I. Purification and properties of a phosphodiesterase. J Biol Chem. 1960 May;235:1479–1487. [PubMed] [Google Scholar]
- LURIA S. E. Host-induced modifications of viruses. Cold Spring Harb Symp Quant Biol. 1953;18:237–244. doi: 10.1101/sqb.1953.018.01.034. [DOI] [PubMed] [Google Scholar]
- Lederberg S. Genetics of host-controlled restriction and modification of deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1966 Mar;91(3):1029–1036. doi: 10.1128/jb.91.3.1029-1036.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protass J. J., Korn D. Function of the N cistron of bacteriophage lambda. Proc Natl Acad Sci U S A. 1966 May;55(5):1089–1095. doi: 10.1073/pnas.55.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUSSMAN R., JACOB F. [On a thermosensitive repression system in the Escherichia coli lambda bacteriophage]. C R Hebd Seances Acad Sci. 1962 Feb 19;254:1517–1519. [PubMed] [Google Scholar]
- Shulman M. J., Hallick L. M., Echols H., Signer E. R. Properties of recombination-deficient mutants of bacteriophage lambda. J Mol Biol. 1970 Sep 28;52(3):501–520. doi: 10.1016/0022-2836(70)90416-x. [DOI] [PubMed] [Google Scholar]
- Signer E. R., Manly K. F., Brunstetter M. A. Deletion mapping of the c-3-N region of bacteriophage. Virology. 1969 Sep;39(1):137–141. doi: 10.1016/0042-6822(69)90356-0. [DOI] [PubMed] [Google Scholar]
- Signer E. R., Weil J. Recombination in bacteriophage lambda. I. Mutants deficient in general recombination. J Mol Biol. 1968 Jul 14;34(2):261–271. doi: 10.1016/0022-2836(68)90251-9. [DOI] [PubMed] [Google Scholar]
- Terzi M. Physiology of bacteriophage lambda in the restrictive host. J Mol Biol. 1968 May 28;34(1):165–173. doi: 10.1016/0022-2836(68)90242-8. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


