Abstract
The speed of absorption of dietary amino acids by the gut varies according to the type of ingested dietary protein. This could affect postprandial protein synthesis, breakdown, and deposition. To test this hypothesis, two intrinsically 13C-leucine-labeled milk proteins, casein (CAS) and whey protein (WP), of different physicochemical properties were ingested as one single meal by healthy adults. Postprandial whole body leucine kinetics were assessed by using a dual tracer methodology. WP induced a dramatic but short increase of plasma amino acids. CAS induced a prolonged plateau of moderate hyperaminoacidemia, probably because of a slow gastric emptying. Whole body protein breakdown was inhibited by 34% after CAS ingestion but not after WP ingestion. Postprandial protein synthesis was stimulated by 68% with the WP meal and to a lesser extent (+31%) with the CAS meal. Postprandial whole body leucine oxidation over 7 h was lower with CAS (272 ± 91 μmol⋅kg−1) than with WP (373 ± 56 μmol⋅kg−1). Leucine intake was identical in both meals (380 μmol⋅kg−1). Therefore, net leucine balance over the 7 h after the meal was more positive with CAS than with WP (P < 0.05, WP vs. CAS). In conclusion, the speed of protein digestion and amino acid absorption from the gut has a major effect on whole body protein anabolism after one single meal. By analogy with carbohydrate metabolism, slow and fast proteins modulate the postprandial metabolic response, a concept to be applied to wasting situations.
Keywords: amino acid turnover, postprandial protein anabolism, milk protein, stable isotopes
Dietary carbohydrates are commonly classified as slow and fast because it now is well recognized that their structure affects their speed of absorption, which in turn has a major impact on the metabolic and hormonal response to a meal (1). On the other hand, little is known about whether postprandial protein kinetics are affected by the speed of absorption of dietary amino acids; the latter is very variable, depending on gastric and intestinal motility, luminal digestion, and finally mucosal absorption. This lack of data is due to the fact that postprandial amino acid kinetics have been studied almost exclusively during continuous feeding, obtained either by a nasogastric infusion or by small repeated meals (2–7). Measurements are done 2–4 h after initiation of feeding, once isotopic and substrate steady-state is achieved. Under these conditions, any difference related to the speed of dietary amino acid absorption is blunted.
There is, however, indirect evidence that this parameter might be of importance. Indeed, the postprandial amino acid levels differ a lot depending on the mode of administration of a dietary protein; a single protein meal results in an acute but transient peak of amino acids (9–11) whereas the same amount of the same protein given in a continuous manner, which mimics a slow absorption, induces a smaller but prolonged increase (12). Amino acids are potent modulators of protein synthesis, breakdown, and oxidation, so such different patterns of postprandial amino acidemia might well result in different postprandial protein kinetics and gain. Of interest, whole body leucine balance, an index of protein deposition, was shown recently to differ under these two circumstances (13).
Therefore, our hypothesis was that the speed of absorption by the gut of amino acids derived from dietary proteins might affect whole body protein synthesis, breakdown, and oxidation, which in turn control protein deposition. To test this hypothesis, we compared those parameters, assessed by leucine kinetics, after ingestion of a single meal containing either whey protein (WP) or casein (CAS), taken as paradigms for “fast” and “slow” proteins, respectively. Indeed, WP is a soluble protein whereas CAS clots into the stomach, which delays its gastric emptying and thus probably results in a slower release of amino acids (14). Speed of amino acid absorption was directly assessed by using a newly developed tracer, i.e., milk protein fractions intrinsically labeled with l-[1-13C]leucine (15). Leucine kinetics were modelized by using non-steady-state equations as recently described (16). Our results demonstrate that amino acids derived from CAS are indeed slowly released from the gut and that slow and fast proteins differently modulate postprandial changes of whole body protein synthesis, breakdown, oxidation, and deposition.
MATERIALS AND METHODS
Materials.
l-[1-13C]leucine (99 mol percent excess) and l-[5,5,5-2H3]leucine (97 mol percent excess) were supplied by Mass Trace (Woburn, MA). Isotopic and chemical purity of leucine was checked by gas chromatography–mass spectrometry. Solutions of tracers were tested for sterility and pyrogenicity, prepared in sterile pyrogen-free water, and membrane-filtered through 0.22-μm filters.
Two milk protein fractions, CAS and WP, intrinsically labeled with l-[1-13C]leucine were obtained by infusing lactating cows as described (15). Labeled milk proteins were mixed with unlabeled proteins to obtain sufficient amounts of protein of appropriate enrichments. Those mixtures then were referred to as 13C-WP for 13C leucine-intrinsically labeled WP and 13C-CAS for 13C leucine-intrinsically labeled CAS. Their final leucine enrichments and contents were checked by gas chromatography–mass spectrometry after hydrolysis.
Subjects.
Sixteen young healthy subjects participated in the different studies. All subjects had a normal physical examination (mean ± SD: age 24 ± 4 years; body mass index 21.9 ± 1.8 kg/m2). They maintained their usual physical activity, with an usual protein–energy intake of ≈38 kcal⋅kg−1⋅d−1 (16% protein) during the 3 days before the study. A written informed consent was obtained from each participant. The experimental protocol was approved by the Ethical Committee of Clermont–Ferrand.
Experimental Protocol.
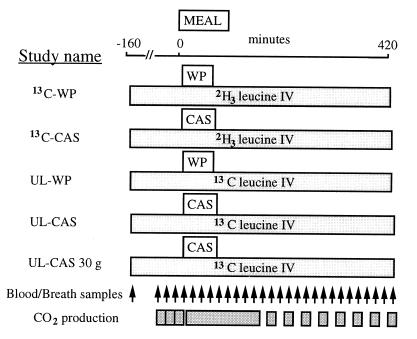
Fig. 1 and Table 1: Five separate protocols were performed in the volunteers who were assigned to receive: (i) 30 g (i.e., 336 mmol N) of labeled WP (13C-WP study) with i.v. infused [5,5,5-2H3]leucine (n = 6 subjects, body mass index 22 ± 1 kg/m2, age 24 ± 4 years); (ii) 43 g (i.e., 479 mmol N) of labeled CAS (13C-CAS study) (same amount of dietary leucine as in the 30-g WP meal) with i.v. infused [5,5,5-2H3]leucine (n = 6, 22 ± 2 kg/m2, 23 ± 3 years); (iii) 30 g (i.e., 336 mmol N) of unlabeled WP (UL-WP study) with i.v. infused l-[1-13C]leucine (n = 5, 22 ± 1 kg/m2, 25 ± 5 years); (iv) 40 g (i.e., 446 mmol N) of unlabeled CAS (UL-CAS) with i.v. infused l-[1-13C]leucine (n = 6, 22 ± 2 kg/m2, 23 ± 3 years); or (v) finally, a last study (UL-CAS 30 g) was performed in three subjects to assess the response to an intake of 30 g (i.e., 336 mmol N) of unlabeled CAS (isonitrogenous to WP but with a lower leucine content), with an i.v. l-[1-13C]leucine infusion.
Figure 1.
Protocol design of the different studies with the labeled (13C-WP and 13C-CAS studies) and unlabeled (UL-WP and UL-CAS studies) milk protein fractions.
Table 1.
Tracer infusion rates and protein and leucine intakes in the 13C-labeled (13C-WP and 13C-CAS) and unlabeled (UL-WP and UL-CAS) WP and CAS studies
| 13C-WP | UL-WP | 13C-CAS | UL-CAS | UL-CAS 30 g | |
|---|---|---|---|---|---|
| i.v. [13C]leucine, μmol⋅kg−1⋅min−1 | — | 0.059 ± 0.006 | — | 0.063 ± 0.003 | 0.060 ± 0.002 |
| i.v. [2H3]leucine, μmol⋅kg−1⋅min−1 | 0.046 ± 0.004 | — | 0.040 ± 0.015 | — | — |
| Dietary protein intake, g⋅kg−1 | 0.45 ± 0.04 | 0.45 ± 0.05 | 0.61 ± 0.03 | 0.57 ± 0.01 | 0.45 ± 0.02 |
| Dietary leucine intake, μmol⋅kg−1 | 382 ± 32 | 359 ± 41 | 380 ± 20 | 379 ± 3 | 291 ± 13 |
| Dietary [13C]leucine enrichment, MPE | 3.42 ± 0.27 | — | 3.40 ± 0.10 | — | — |
Values are means ± SD. MPE, mol percent excess.
Studies using [1-13C]leucine as an i.v. tracer and unlabeled dietary proteins (UL-WP and UL-CAS studies) were intended for the measurement of total leucine oxidation before and during the meal in nonsteady-state as already described (16). Studies with ingested labeled proteins and i.v. deuterated leucine (13C-WP and 13C-CAS studies) allowed us to determine total leucine flux, rate of appearance of exogenous (i.e., dietary) leucine, endogenous leucine production, and exogenous leucine oxidation. Tracers infusion rates, amounts of dietary leucine, and leucine enrichments in the diet are indicated in Table 1.
After an overnight fast of 10 h, a catheter was inserted in a retrograde fashion into a dorsal vein of the hand for blood sampling after introduction of the hand in a 60°C heated ventilated box. A second catheter was inserted into a vein of the contralateral arm for tracer infusion. At 7:30 a.m. (T-160), a primed (60 × infusion rate/min) continuous infusion of l-[5,5,5-2H3]leucine (13C-WP or 13C-CAS studies) or [1-13C]leucine (UL-WP or UL-CAS studies) was begun and continued for 580 min (Table 1). After 160 min of infusion (T0), the protein meal, prepared just before the study, was ingested as a liquid diet in <5 min.
As shown on Fig. 1, blood and breath samples were taken before any infusion, at the isotopic plateau of the i.v. tracer before the meal, and every 20 min after meal ingestion. The plasma supernatant was separated, added with an internal standard, and then stored at −20°C until further analysis. Breath samples were kept in Vacutainers (Becton Dickinson). Total carbon dioxide production rates were measured three times for 20 min in the postabsorptive state, then continuously between T0 and T120, and for 20 min every 40 min after T120, by open circuit indirect calorimetry (Deltatrac, Datex, Geneva, Switzerland).
Analytical Methods.
Plasma 13C and 2H3 leucine and ketoisocaproate (KIC) enrichments and concentrations were measured by gas chromatography–mass spectrometry (Hewlett–Packard 5971A) as described (16). Appropriate corrections for the contribution of the M+1 to the M+3 labeling were made (17). 13C enrichments of CO2 were measured on a gas chromatography isotope ratio mass spectrometer (μGas system, Fisons, Loughborough, U.K.) as described (16).
The plasma level of insulin was measured by radioimmunoassay (CIS, Gif-sur-Yvette, France), and plasma amino acid concentrations were determined after deproteinization with sulfosalicylic acid by ion exchange liquid chromatography (Beckman LS 6300) at 0, 100, and 300 min.
Calculations.
Leucine fluxes were calculated from the time-dependent evolution of plasma leucine and KIC enrichments and concentrations in the nonsteady-state according to the dual tracer methodology and by using a precursor pool model as described (16). The rate of total leucine appearance into the peripheral circulation (Total Leu Ra) is the sum of the entry rate of exogenous (dietary) leucine (Exo Leu Ra) plus the entry rate of endogenous leucine derived from protein breakdown (Endo Leu Ra). Total Leu Ra includes the i.v. infused labeled leucine. Similarly, total leucine rate of disappearance (Total Leu Rd) from plasma is the sum of the fluxes of leucine, either oxidized (Total Leu Ox) or used for protein synthesis (Total nonoxidative leucine disposal or Total NOLD).
The equations used have been reported in detail (16). In brief, Total Leu Ra was calculated from the i.v. tracer infusion rate corrected for its time-dependent appearance into the plasma and from the plasma leucine enrichment of the i.v. tracer (i.e., 13C for UL-CAS and UL-WP studies or 2H3 for 13C-CAS and 13C-WP studies). In the 13C-CAS and 13C-WP studies, Exo Leu Ra, which is the plasma entry rate of the dietary leucine, was calculated from the simultaneous enrichments of the infused and ingested tracer, according to the Proietto’s transposition of Steele’s equations (18). Endo Leu Ra, an index of whole body protein breakdown, was then obtained by subtracting Exo Leu Ra and the tracer infusion rate from Total Leu Ra.
Postabsorptive and postprandial Total (i.e., endogenous and dietary) Leu Ox and NOLD were obtained from the UL-CAS and UL-WP studies. Total Leu Ox was obtained by dividing the 13CO2 production rate by the plasma 13C KIC enrichment. Total Leu Rd was calculated as Total Leu Ra minus the time-dependent changes in leucine pool size. Total NOLD was therefore Total Leu Rd minus Total Leu Ox.
Postprandial oxidation of the exogenous (i.e., dietary) leucine was determined by the ratio between the 13CO2 excretion and the dietary 13C leucine enrichments (13C-CAS and 13C-WP studies). Total recovery of the oxidized dietary leucine then was calculated over 420 min. This recovery also corresponds to the ratio between the tracer recovered as 13CO2 and the amount of tracer ingested.
Postprandial leucine balance was finally calculated over a 420-min period by subtracting the integrated area under the curve of Total Leu Ox from the leucine intake, assuming that all of the leucine that was administered was absorbed given the high digestibility of milk proteins (19).
In all of the calculations, the constants for leucine distribution volume (0.5 liter⋅kg−1), the correction factor of the pool size for instant mixing (0.25), and the CO2 recovery factor (0.8) were the same as those previously used (16).
Statistical Analysis.
Results are expressed as means ± SD. In Figs. 2, 3, 4, moving averages were calculated on three consecutive time points. To examine the effects of the two diets (CAS or WP) on leucine kinetics at individual time points, a two-way ANOVA, followed by the F Scheffe test for multiple comparisons, was used (StatView, Abacus Concepts, Berkeley, CA). The same test was used to analyze the differences of amino acid concentrations at 100 and 300 min. Within each study (CAS or WP), differences between postprandial values and baseline were assessed by using Bonferroni multiple test comparisons (SuperANOVA, Abacus Concepts). Differences between the areas under the curve of leucine oxidation and leucine balances were assessed by unpaired t tests.
Figure 2.
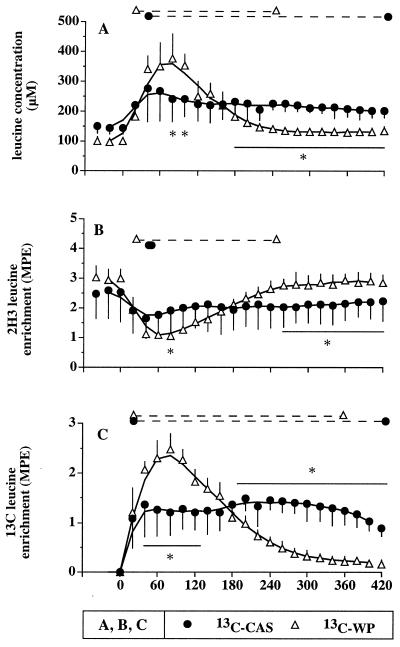
(A) Plasma leucine concentrations, (B) enrichments of the i.v. infused 2H3 tracer, and (C) enrichments of the orally administered 13C tracer, after a labeled WP meal (13C-WP study) and a CAS meal (13C-CAS study). ∗, Statistical differences between the two protein meals (P < 0.05). The dashed lines at the top of each panel indicate a significant difference from baseline (P < 0.05) within each study.
Figure 3.
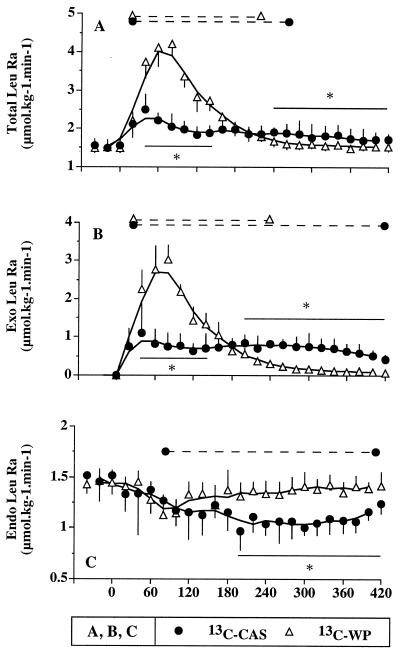
(A) Total leucine rate of appearance, (B) exogenous leucine rate of appearance, and (C) endogenous leucine rate of appearance after labeled WP ingestion (13C-WP study; open triangles) and labeled CAS ingestion (13C-CAS study; closed circles). ∗, Statistical differences between the two protein meals (P < 0.05). The dashed lines at the top of each panel indicate a significant difference from baseline (P < 0.05) within each study.
Figure 4.
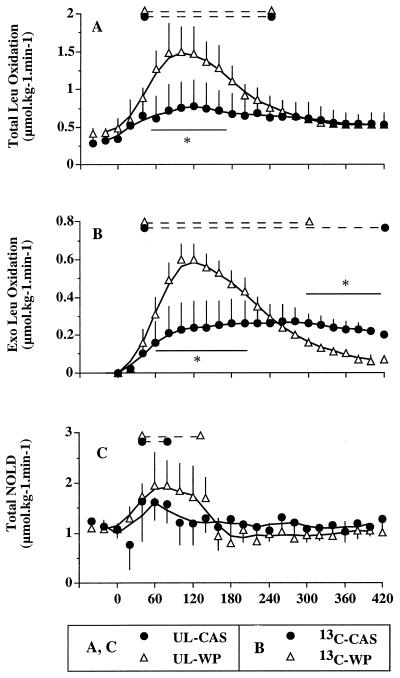
(A) Total leucine oxidation after unlabeled milk protein ingestion (UL-WP and UL-CAS studies), (B) exogenous leucine oxidation after labeled protein ingestion (13C-WP, open triangles; 13C-CAS, closed circles), (C) and Total NOLD (i.e., protein synthesis) from UL-WP and UL-CAS studies. ∗, Statistical differences between the two protein meals (P < 0.05). The dashed lines at the top of each panel indicate a significant difference from baseline (P < 0.05) within each study.
RESULTS
Plasma Amino Acids and Insulin Concentrations After Meal Ingestion.
Postprandial amino acid increases over baseline were identical whether the proteins were labeled or not. Those increases were different between CAS and WP (Table 2). The two protein meals were matched for leucine content but were not isonitrogenous, and amino acid intake was higher with CAS. Despite this higher amino acid intake, amino acid concentrations increased less with CAS than with WP at 100 min. By contrast, at 300 min, most amino acids remained at higher concentrations with CAS whereas they returned to basal levels with WP. These two particular plasma amino acid profiles also are illustrated by leucine concentration determined at each time point in Fig. 2A.
Table 2.
Amino acid intake, baseline plasma amino acid concentrations, and relative increase (%) above baseline of amino acid concentrations after 100 and 300 min
| Amino acid | Amino acid intake, μmol/kg
|
Baseline amino acid concentration, μM | 100 min, increase above baseline, %
|
300 min, increase above baseline, %
|
|||
|---|---|---|---|---|---|---|---|
| CAS | WP | CAS | WP | CAS | WP | ||
| Asp | 509 | 202 | 7 ± 2 | 44 ± 93 | 113 ± 58* | 6 ± 41 | −6 ± 25 |
| Thr | 242 | 126 | 114 ± 21 | 44 ± 15* | 110 ± 25*† | 27 ± 18* | 6 ± 12 |
| Ser | 247 | 156 | 104 ± 17 | 18 ± 12* | 48 ± 17* | 11 ± 17 | −4 ± 6† |
| Asn | — | — | 65 ± 19 | 32 ± 22* | 71 ± 25*† | 18 ± 22 | −26 ± 25 |
| Glu | 773 | 670 | 110 ± 43 | 14 ± 11* | 46 ± 16* | 12 ± 12* | −10 ± 7*† |
| Gln | — | — | 513 ± 87 | 5 ± 10 | 21 ± 7* | 7 ± 17 | −1 ± 9 |
| Pro | 304 | 398 | 175 ± 31 | 97 ± 34* | 68 ± 31* | 67 ± 23* | −5 ± 15† |
| Gly | 128 | 87 | 190 ± 29 | −1 ± 7 | −2 ± 11* | −4 ± 10 | −21 ± 11* |
| Ala | 328 | 128 | 291 ± 83 | 10 ± 12 | 45 ± 28* | −7 ± 13 | −24 ± 14* |
| Val | 251 | 203 | 226 ± 39 | 54 ± 9* | 97 ± 15* | 48 ± 17* | 13 ± 6† |
| Met | 82 | 73 | 20 ± 5 | 81 ± 38* | 172 ± 75* | 34 ± 21* | 2 ± 41† |
| Ile | 222 | 135 | 60 ± 13 | 90 ± 26* | 274 ± 64*† | 62 ± 43* | 11 ± 14† |
| Leu | 382 | 380 | 132 ± 25 | 77 ± 24* | 236 ± 56*† | 61 ± 30* | 29 ± 11*† |
| Tyr | 119 | 114 | 58 ± 18 | 75 ± 41* | 86 ± 28* | 40 ± 22* | −8 ± 14† |
| Phe | 126 | 119 | 49 ± 10 | 34 ± 17* | 46 ± 21* | 19 ± 12* | −12 ± 11† |
| Lys | 379 | 183 | 174 ± 53 | 104 ± 124* | 140 ± 54*† | 78 ± 131* | 8 ± 11 |
| His | 68 | 68 | 90 ± 12 | 16 ± 18 | 32 ± 19*† | 14 ± 10* | 6 ± 10 |
| Arg | 89 | 67 | 69 ± 19 | 48 ± 22* | 78 ± 30* | 17 ± 22 | −7 ± 16 |
P < 0.05 vs. baseline;
P < 0.05 CAS vs. WP.
Plasma insulin levels similarly increased after both meals. The values were, at 0, 40, and 300 min, 6.2 ± 2.4, 16.8 ± 12.8, and 6.3 ± 3.4 μU/ml, and 7.5 ± 1.3, 19.8 ± 5.3, and 6.1 ± 1.4 μU/ml for CAS and WP, respectively.
Tracer Enrichments.
The pattern of the i.v. tracer enrichment after protein ingestion was similar whatever the infused tracer was ([2H3]- or [13C]leucine). Therefore, for the sake of simplicity, only the 2H3 tracer values are presented on Fig. 2B. After WP ingestion, the i.v. tracer enrichment reached a nadir (−62% vs. baseline) at 60 min and was back to baseline after 4 h. After CAS ingestion, the nadir was less pronounced (−30% at 40 min), but the dilution of the i.v. tracer remained fairly constant throughout the study. The difference between the two patterns was significant at time 80 and from 260 to 420 min.
A mirror image was obtained for the oral tracer (Fig. 2C). The 13C tracer appeared within 20 min into the plasma for both proteins. There was a high and transient (4–5 h) peak with WP. By contrast, 13C leucine derived from CAS plateaued during 6–7 h. Again, the difference between the two patterns was significant from 40 to 120 and from 180 to 420 min.
Leucine Kinetics.
Leucine rates of appearance. Fig. 3 A–C: Total Leu Ra dramatically increased after ingestion of WP (from 1.49 ± 0.13 to 4.21 ± 0.33 μmol⋅kg−1⋅min−1 at 80 min). It increased less after the CAS meal (from 1.55 ± 0.18 to 2.05 ± 0.32 μmol⋅kg−1⋅min−1, at 80 min, P < 0.01 CAS vs. WP and vs. baseline) (Fig. 3A). Total Leu Ra then returned to basal values after 360 min for 13C-WP (1.49 ± 0.08 μmol⋅kg−1⋅min−1 at 360 min) but remained elevated for 13C-CAS (1.74 ± 0.26 μmol⋅kg−1⋅min−1 at 360 min, P < 0.05 for CAS vs. WP).
Exo Leu Ra followed the same pattern with a smaller but prolonged influx of dietary leucine from the CAS meal compared with the WP meal (Fig. 3B). The areas under the curve were 300 ± 35 and 356 ± 35 μmol⋅kg−1 for CAS and WP, respectively (not significant).
Endo Leu Ra, an index of protein breakdown, did not significantly change after the WP meal. It was progressively and durably inhibited from 120 to 420 min with CAS. Mean Endo Leu Ra between 180 and 420 min were 1.08 ± 0.07 and 1.38 ± 0.04 μmol⋅kg−1⋅min−1 for CAS and WP, respectively.
Leucine oxidation.
Fig. 4 A and B: Total Leu Ox, determined from the UL-WP and UL-CAS studies (i.v. infused 13C leucine), increased with both proteins (Fig. 4A). With WP, the magnitude of the increase (from 0.48 ± 0.13 at baseline to 1.50 ± 0.33 μmol⋅kg−1⋅min−1 at 100 min) was much larger than with CAS (0.35 ± 0.11 at baseline to 0.78 ± 0.34 μmol⋅kg−1⋅min−1 at 120 min). Total Leu Ox returned to baseline 420 min after WP ingestion; after CAS ingestion, it remained slightly elevated (0.53 ± 0.15 μmol⋅kg−1⋅min−1) but not statistically different from baseline. Postprandial Total Leu Ox over 420 min, expressed as areas under the curve, was 373 ± 56 μmol⋅kg−1 and 272 ± 91 μmol⋅kg−1, respectively, for WP and CAS (P < 0.05).
Exo Leu Ox, displayed in Fig. 4B, increased rapidly after WP ingestion and was completed after 420 min; the areas under the curve represented 31% of the ingested leucine. The time course was different after CAS ingestion, with a pseudo-plateau between 120 and 360 min. It did not return to baseline at the end of the study, and it represented 24% of the ingested leucine, a value not significantly different from the one of WP.
NOLD.
Fig. 4C: Total NOLD (i.e., total protein synthesis) was stimulated by 68% and 31% (average from 40 to 140 min) with WP and CAS, respectively, the difference between the two diets being not significant although there was a trend for a higher protein synthesis with WP.
Postprandial leucine balance.
Postprandial leucine balance over 420 min was positive for the CAS meal (141 ± 96 μmol⋅kg−1) and not different from zero for the WP meal (11 ± 36 μmol⋅kg−1; P < 0.05, CAS vs. WP). In the last study (UL-CAS 30 g), three subjects received an amount of CAS isonitrogenous to the WP meal but with a lower leucine content. Total Leu Ox was 268 ± 36 μmol⋅kg−1 (areas under the curve), and postprandial leucine balance over 420 min was positive (48 ± 33 μmol⋅kg−1).
DISCUSSION
The impact on postprandial protein metabolism of the speed of amino acid absorption by the gut has not been estimated previously in humans. Under the conditions of this study, i.e., a single protein meal with no energy added, two dietary proteins have different metabolic fates and uses. After WP ingestion, the plasma appearance of dietary amino acids is fast, high, and transient. This amino acid pattern is associated with an increased protein synthesis and oxidation and no change in protein breakdown. By contrast, the plasma appearance of dietary amino acids after a CAS meal is slower, lower, and prolonged with a different whole body metabolic response: Protein synthesis slightly increases, oxidation is moderately stimulated, but protein breakdown is markedly inhibited. The latter metabolic profile results in a better leucine balance.
The two proteins differed by their amino acid composition. In particular, leucine content was higher in WP (11% wt/wt) than in CAS (8% wt/wt). Therefore, it was not possible to have two meals of identical leucine and nitrogen content. Because nitrogen content could affect postprandial balance, we performed an additional study during which nitrogen content was matched for WP and CAS. Under these conditions, and even though the leucine intake from the CAS was lower, leucine balance remained better with CAS than with WP; this makes it highly unlikely that the better leucine balance obtained with 43 g of CAS was caused by the larger nitrogen intake. More generally, amino acid composition of the meal could affect postprandial protein metabolism, if an essential amino acid is rate limiting for protein synthesis. This possibility is unlikely because CAS and WP were given in large amounts and have a high digestibility (19) and a well balanced amino acid score.
Studying amino acid kinetics after a single meal raises specific methodological questions. Among those, the use of nonsteady-state equations and, in particular, the problems of distribution volume, have been discussed in detail in our previous work (16). Also, we had demonstrated that only a tracer incorporated into the protein adequately represents the fate of the ingested amino acids by comparing labeled WP and WP added with free [13C]leucine. This is even more true for CAS: In preliminary studies (data not shown), we assessed leucine kinetics after ingestion of CAS with free labeled leucine added. The pattern of tracer appearance was completely different with a very fast and transient appearance of the oral tracer; as expected, this lead to aberrant results for leucine kinetics. Thus, intrinsically labeled proteins constitute an unique tool for assessing the speed of absorption of a protein.
A classic methodological issue is the CO2 recovery factor to be used for leucine oxidation calculations. The CO2 recovery factor that we used in this study was a constant factor of 0.8 throughout the study protocol because it is a usual factor value in the fed studies and because the CO2 recovery in nonsteady-state has not been explored in our conditions. Because the CO2 recovery is proportionally related to CO2 production (20), it could be expected that the recovery factor would have been slightly higher immediately after the meal. When the recovery factor was calculated from CO2 production in our study, values between 0.72 and 0.76 for CAS and between 0.72 and 0.78 for WP were found, and the consequences on leucine oxidation results were negligible. Also, Total Leu Ox was not back to baseline at the end of the study, particularly for the CAS, which might interfere with the calculations of the area under the curve of oxidation and hence leucine balance. However, when extrapolating the decay curve to baseline (which was reached at a theoretical time of 660 min), our estimate of Total Leu Ox after the CAS meal increased by only 8% (+23 μmol⋅kg−1), and this does not affect our conclusions.
We measured Total Leu Ox during an i.v. tracer infusion, as do most authors (e.g., as in ref. 13). This raises some uncertainties concerning the fate of dietary amino acids that are oxidized in the splanchnic area. Indeed, the extent to which the meal-derived leucine is oxidized in this area is questionable because the i.v. infused labeled leucine must be fully equilibrated with whole body leucine and KIC pools. In the fasted state, liver contribution to leucine oxidation is very small because only 10% of the first-pass leucine uptake is oxidized (21). This figure could be different after the ingestion of one single meal but, in any case, would probably be higher with WP than with CAS because of the higher initial splanchnic load of amino acids, thus leading to a larger underestimate of Total Leu Ox with WP than with CAS.
This study demonstrates that dietary amino acid absorption is faster with WP than with CAS. Our methodology does not allow identification of the rate limiting step(s) that might be gastric emptying and/or luminal hydrolysis and/or amino acid mucosal absorption. It is very likely, however, that a slower gastric emptying was mostly responsible for the slower appearance of amino acids into the plasma. Indeed, CAS clots into the stomach whereas WP is rapidly emptied from the stomach into the duodenum (14). Thus, after WP ingestion, large amounts of dietary amino acids (≈25 mmol of leucine) flood the small body pool (≈5 mmol) in a short time, resulting in a dramatic increase in amino acid concentrations. This is probably responsible for the stimulation of leucine oxidation and protein synthesis, as recently suggested by Giordano et al. (8), who artificially elevated plasma amino acid concentrations by i.v. infusions of amino acids. This dramatic stimulation of protein synthesis and absence of protein breakdown inhibition is quite different from the pattern observed with classic feeding studies (22). By contrast, with CAS, plasma amino acid concentrations are lower, resulting in a lower oxidation and in a lesser increase of protein synthesis but also in an inhibition of protein breakdown. This metabolic response is similar to what is usually demonstrated during steady-state studies (2–7, 17, 23–26), and it is noticeable that postprandial plasma amino acid concentrations were actually at a near steady state. Explanation for the difference of inhibition of protein breakdown between WP and CAS is unclear; protein breakdown is classically inhibited by insulin (27–29), but plasma insulin concentrations were not different between the two meals. Although their effects are less powerful than insulin, amino acids also inhibit protein breakdown (27). The absence of any change of protein breakdown with WP, despite very high amino acid concentrations, suggests that a prolonged enough time of hyperaminoacidemia would be needed to obtain a significant protein breakdown inhibition. Finally, with respect to the issue of leucine balance, it is noticeable that Young’s (13) group recently reported a 24-h leucine balance, which was better with three discrete meals than with multiple small repeated meals, the latter circumstance somewhat mimicking a slow protein absorption. This study and ours are not, however, directly comparable in numerous respects, such as the meal composition, which included carbohydrate in Young’s study.
In conclusion, we demonstrate that the speed of amino acid absorption after protein ingestion has a major impact on the postprandial metabolic response to a single protein meal. The slowly absorbed CAS promotes postprandial protein deposition by an inhibition of protein breakdown without excessive increase in amino acid concentration; by contrast, a fast dietary protein stimulates protein synthesis but also oxidation. This impact of amino acid absorption speed on protein metabolism is true when proteins are given alone, but as for carbohydrate, this might be blunted in more complex meals that could affect gastric emptying (lipids) and/or insulin response (carbohydrate); thus, further studies are needed to confirm the specific roles of nonprotein substrates on whole body protein metabolism. This concept of slow and fast proteins could be applied to circumstances in which protein deposition has to be improved (i.e., protein–energy malnutrition) and in which excessive protein intakes have to be avoided (elderlies, renal diseases).
Acknowledgments
We thank Michel Genest, Paulette Rousset, and Liliane Morin for their technical help, Sandrine Corny for tracer preparations, and Olivier Ballèvre for helpful discussion. This study was supported by Grant 94G0079 from the Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche, and Grants from Région Auvergne and Institut National de la Recherche Agronomique.
ABBREVIATIONS
- KIC
ketoisocaproate
- CAS
casein
- WP
whey protein
- 13C-CAS
13C-leucine-labeled CAS
- 13C-WP
13C-leucine-labeled WP
- UL-CAS
unlabeled CAS
- UL-WP
unlabeled WP
- Ra
rate of appearance
- Rd
rate of disappearance
- Ox
oxidation
- NOLD
nonoxidative leucine disposal
References
- 1.Jenkins D J A, Wolever T M S, Taylor R H, Barker H, Fielden H, Baldwin J M, Bowling A C, Newman H C, Jenkins A L, Goff D U. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 2.Motil K J, Matthews D E, Bier D M, Burke J F, Munro H N, Young V R. Am J Physiol. 1981;240:E712–E721. doi: 10.1152/ajpendo.1981.240.6.E712. [DOI] [PubMed] [Google Scholar]
- 3.Beaufrère B, Horber F, Schwenk F, Marsh H, Haymond M W. Am J Physiol. 1989;257:E712–E721. doi: 10.1152/ajpendo.1989.257.5.E712. [DOI] [PubMed] [Google Scholar]
- 4.Melville S, Nurlan M A M, Hardy K C M, Broom J, Milne E, Calder A G, Garlick P J. Metabolism. 1989;38:248–255. doi: 10.1016/0026-0495(89)90083-8. [DOI] [PubMed] [Google Scholar]
- 5.Pacy P J, Price G M, Halliday D, Millward D J. Clin Sci. 1994;86:103–118. doi: 10.1042/cs0860103. [DOI] [PubMed] [Google Scholar]
- 6.Collin-Vidal C, Cayol M, Obled C, Ziegler F, Bommelaer G, Beaufrère B. Am J Physiol. 1994;267:E907–E914. doi: 10.1152/ajpendo.1994.267.6.E907. [DOI] [PubMed] [Google Scholar]
- 7.Gibson N R, Fereday A, Cox M, Halliday D, Pacy P J, Millward D J. Am J Physiol. 1996;270:E282–E291. doi: 10.1152/ajpendo.1996.270.2.E282. [DOI] [PubMed] [Google Scholar]
- 8.Giordano M, Castellino P, DeFronzo R A. Diabetes. 1996;45:393–399. doi: 10.2337/diab.45.4.393. [DOI] [PubMed] [Google Scholar]
- 9.Wahren J, Felig P, Hagenfeldt L. J Clin Invest. 1976;57:987–999. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elia M, Livesey G. Clin Sci. 1983;64:517–526. doi: 10.1042/cs0640517. [DOI] [PubMed] [Google Scholar]
- 11.Bergström J, Fürst P, Vinnars E. Clin Sci. 1990;79:331–337. doi: 10.1042/cs0790331. [DOI] [PubMed] [Google Scholar]
- 12.Wolever T M S. Am J Med Sci. 1994;307:97–101. doi: 10.1097/00000441-199402000-00005. [DOI] [PubMed] [Google Scholar]
- 13.El-Khoury A E, Sànchez M, Fukagawa N K, Gleason R E, Tsay R H, Young V R. Am J Clin Nutr. 1995;62:579–590. doi: 10.1093/ajcn/62.3.579. [DOI] [PubMed] [Google Scholar]
- 14.Mahé S, Roos N, Benamouzig R, Davin L, Luengo C, Gagnon L, Gausserès N, Rautureau J, Tomé D. Am J Clin Nutr. 1996;63:546–552. doi: 10.1093/ajcn/63.4.546. [DOI] [PubMed] [Google Scholar]
- 15.Boirie Y, Fauquant J, Rulpin H, Maubois J L, Beaufrère B. J Nutr. 1995;125:92–98. doi: 10.1093/jn/125.1.92. [DOI] [PubMed] [Google Scholar]
- 16.Boirie Y, Gachon P, Corny S, Fauquant J, Maubois J L, Beaufrère B. Am J Physiol. 1996;271:E1083–E1091. doi: 10.1152/ajpendo.1996.271.6.E1083. [DOI] [PubMed] [Google Scholar]
- 17.Biolo G, Tessari P, Inchiostro S, Brutomesso D, Fongher C. Am J Physiol. 1992;262:E455–E463. doi: 10.1152/ajpendo.1992.262.4.E455. [DOI] [PubMed] [Google Scholar]
- 18.Proietto J, Rohner-Jeanrenaud F, Ionescu E, Terrettaz J, Sauter J F, Jeanrenaud B. Am J Physiol. 1987;252:E77–E84. doi: 10.1152/ajpendo.1987.252.1.E77. [DOI] [PubMed] [Google Scholar]
- 19.WHO/FAO/UNU Report. Energy and Protein Requirements. WHO Technical Report Series 724. Geneva: World Health Organization; 1985. pp. 132–134. [PubMed] [Google Scholar]
- 20.Hoerr R A, Yu Y-M, Wagner A, Burke J F, Young V R. Am J Physiol. 1989;257:E426–E438. doi: 10.1152/ajpendo.1989.257.3.E426. [DOI] [PubMed] [Google Scholar]
- 21.Matthews D, Marano M A, Campbell R G. Am J Physiol. 1993;264:E109–E118. doi: 10.1152/ajpendo.1993.264.1.E109. [DOI] [PubMed] [Google Scholar]
- 22.Waterlow J C. Annu Rev Nutr. 1995;15:57–92. doi: 10.1146/annurev.nu.15.070195.000421. [DOI] [PubMed] [Google Scholar]
- 23.Cayol M, Boirie Y, Rambourdin F, Prugnaud J, Gachon P, Beaufrère B, Obled C. Am J Physiol. 1997;272:E584–E591. doi: 10.1152/ajpendo.1997.272.4.E584. [DOI] [PubMed] [Google Scholar]
- 24.Garlick P J, McNurlan M A, Ballmer P E. Diabetes Care. 1991;14:1189–1198. doi: 10.2337/diacare.14.12.1189. [DOI] [PubMed] [Google Scholar]
- 25.Hoerr R A, Matthews D E, Bier D M, Young V R. Am J Physiol. 1993;264:E567–E575. doi: 10.1152/ajpendo.1993.264.4.E567. [DOI] [PubMed] [Google Scholar]
- 26.Tessari P, Pehling G, Nissen S L, Gerich J E, Service F J, Rizza R A, Haymond M W. Diabetes. 1988;37:512–519. doi: 10.2337/diab.37.5.512. [DOI] [PubMed] [Google Scholar]
- 27.Castellino P, Luzi L, Simonson D, Haymond M W, Fronzo R A D. J Clin Invest. 1987;80:1784–1793. doi: 10.1172/JCI113272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tessari P, Inchiostro S, Biolo G, Trevisan R, Fantin G, Marescotti M C, Iori E, Tiengo A, Crepaldi G. J Clin Invest. 1987;79:1062–1069. doi: 10.1172/JCI112919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tessari P, Inchiostro S, Biolo G, Vincenti E, Sabadin L. J Clin Invest. 1991;88:27–33. doi: 10.1172/JCI115287. [DOI] [PMC free article] [PubMed] [Google Scholar]