Abstract
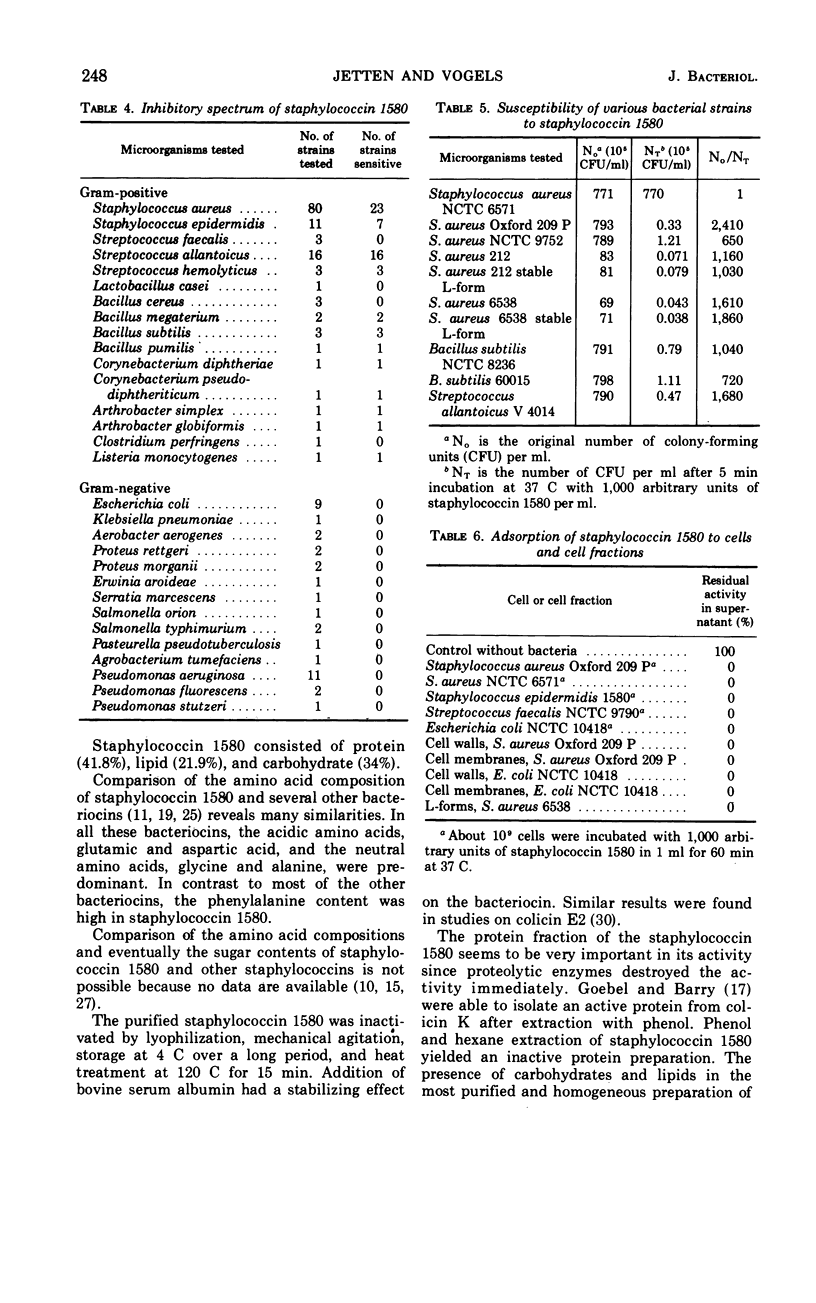
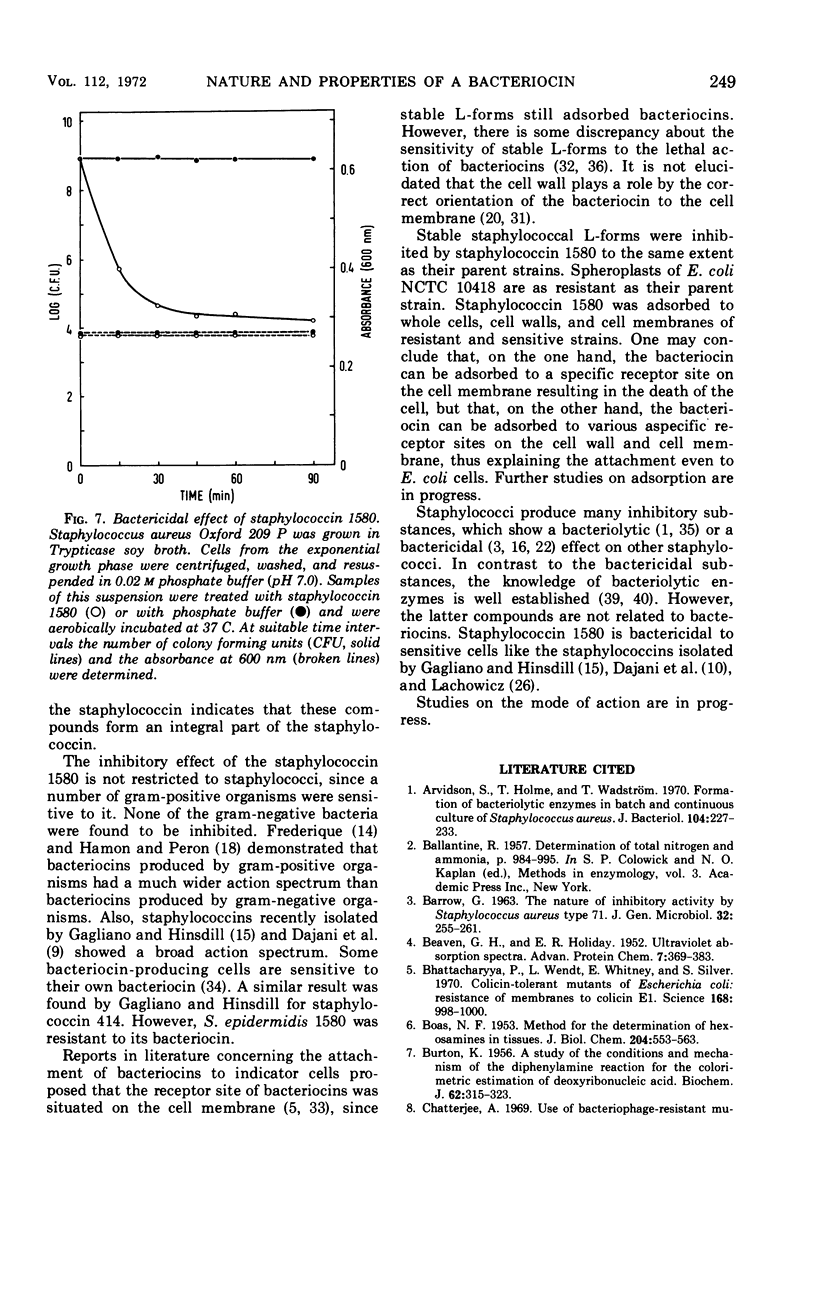
Staphylococcin 1580, produced by Staphylococcus epidermidis 1580, consisted of 41.8% protein, 34% carbohydrate, and 21.9% lipid. In the protein fraction, the acidic amino acids, glutamic and aspartic acid, and the neutral amino acids, glycine and alanine, predominated. Neutral sugars consisted of glucose, galactose, and fucose in a molar ratio of 6:3:1. The purified bacteriocin was not inactivated by heating for 15 min at 120 C in the presence of 0.5% serum albumin and was stable in the pH range from 3.5 to 8.5. The compound was sensitive to the action of the proteolytic enzymes trypsin, Pronase, and chymotrypsin. All gram-negative bacteria tested were resistant; a large number of gram-positive bacteria were sensitive to staphylococcin 1580 action. Growth of stable staphylococcal L-forms was inhibited by the bacteriocin to the same extent as their parent strains. The staphylococcin was adsorbed to cell walls, cell membranes, and resistant cells. The effect of staphylococcin 1580 appeared to be bactericidal but not bacteriolytic.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidson S., Holme T., Wadström T. Formation of bacteriolytic enzymes in batch and continuous culture of Staphylococcus aureus. J Bacteriol. 1970 Oct;104(1):227–233. doi: 10.1128/jb.104.1.227-233.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARROW G. I. THE NATURE OF INHIBITORY ACTIVITY BY STAPHYLOCOCCUS AUREUS TYPE 71. J Gen Microbiol. 1963 Aug;32:255–261. doi: 10.1099/00221287-32-2-255. [DOI] [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya P., Wendt L., Whitney E., Silver S. Colicin-tolerant mutants of Escherichia coli: resistance of membranes to colicin E1. Science. 1970 May 22;168(3934):998–1000. doi: 10.1126/science.168.3934.998. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. N. Use of bacteriophage-resistant mutants to study the nature of the bacteriophage receptor site of Staphylococcus aureus. J Bacteriol. 1969 May;98(2):519–527. doi: 10.1128/jb.98.2.519-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- Dajani A. S., Gray E. D., Wannamaker L. W. Bactericidal substance from Staphylococcus aureus. Biological properties. J Exp Med. 1970 May 1;131(5):1004–1015. doi: 10.1084/jem.131.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani A. S., Wannamaker L. W. Demonstration of a bactericidal substance against beta-hemolytic streptococci in supernatant fluids of staphylococcal cultures. J Bacteriol. 1969 Mar;97(3):985–991. doi: 10.1128/jb.97.3.985-991.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandeu J. P. Chemical and immunological study of colicins e(1), k, a, and q. Infect Immun. 1971 Jan;3(1):1–9. doi: 10.1128/iai.3.1.1-9.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOEBEL W. F., BARRY G. T. Colicine K. II. The preparation and properties of a substance having colicine K activity. J Exp Med. 1958 Feb 1;107(2):185–209. doi: 10.1084/jem.107.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliano V. J., Hinsdill R. D. Characterization of a Staphylococcus aureus bacteriocin. J Bacteriol. 1970 Oct;104(1):117–125. doi: 10.1128/jb.104.1.117-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMON Y., PERON Y. QUELQUES REMARQUES SUR LES BACT'ERIOCINES PRODUITES PAR LES MICROBES GRAM-POSITIFS. C R Hebd Seances Acad Sci. 1963 Jul 29;257:1191–1193. [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Purification and characterization of colicin E2 and colicin E3. J Biol Chem. 1967 Nov 25;242(22):5360–5368. [PubMed] [Google Scholar]
- Hill C., Holland I. B. Genetic basis of colicin E susceptibility in Escherichia coli. I. Isolation and properties of refractory mutants and the preliminary mapping of their mutations. J Bacteriol. 1967 Sep;94(3):677–686. doi: 10.1128/jb.94.3.677-686.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C., Wiseman G. M. Antibacterial substances from staphylococci. Can J Microbiol. 1967 Aug;13(8):947–955. doi: 10.1139/m67-127. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D., de Windt F. Production and purification of a Staphylococcus epidermidis bacteriocin. J Bacteriol. 1972 Oct;112(1):235–242. doi: 10.1128/jb.112.1.235-242.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lachowicz T., Walczak Z. Purification and properties of staphylococcin A. Arch Immunol Ther Exp (Warsz) 1968;16(6):855–863. [PubMed] [Google Scholar]
- Mitsui E., Mizuno D. Stabilization of colicin E2 by bovine serum albumin. J Bacteriol. 1969 Nov;100(2):1136–1137. doi: 10.1128/jb.100.2.1136-1137.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOMURA M. MECHANISM OF ACTION OF COLICINES. Proc Natl Acad Sci U S A. 1964 Dec;52:1514–1521. doi: 10.1073/pnas.52.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYAN F. J., FRIED P., MUKAI F. A colicin produced by cells that are sensitive to it. Biochim Biophys Acta. 1955 Sep;18(1):131–131. doi: 10.1016/0006-3002(55)90016-0. [DOI] [PubMed] [Google Scholar]
- Rolfe B., Onodera K. Demonstration of missing membrane proteins in a colicin-tolerant mutant of E. coli K12. Biochem Biophys Res Commun. 1971 Aug 20;44(4):767–773. doi: 10.1016/0006-291x(71)90776-5. [DOI] [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. PURIFICATION AND PROPERTIES OF LYSOSTAPHIN--A LYTIC AGENT FOR STAPHYLOCOCCUS AUREUS. Biochim Biophys Acta. 1965 Feb 15;97:242–250. doi: 10.1016/0304-4165(65)90088-7. [DOI] [PubMed] [Google Scholar]
- Smit J. A., de Klerk H. C., Coetzee J. N. Properties of a Proteus morganii bacteriocin. J Gen Microbiol. 1968 Nov;54(1):67–75. doi: 10.1099/00221287-54-1-67. [DOI] [PubMed] [Google Scholar]
- Starbuck W. C., Mauritzen C. M., McClimans C., Busch H. A computer program for the calculation of amino acid analysis data. Anal Biochem. 1967 Sep;20(3):439–462. doi: 10.1016/0003-2697(67)90289-8. [DOI] [PubMed] [Google Scholar]
- Trayer H. R., Buckley C. E., 3rd Molecular properties of lysostaphin, a bacteriolytic agent specific for Staphylococcus aureus. J Biol Chem. 1970 Sep 25;245(18):4842–4846. [PubMed] [Google Scholar]
- WEBB J. M., LEVY H. B. New developments in the chemical determination of nucleic acids. Methods Biochem Anal. 1958;6:1–30. doi: 10.1002/9780470110225.ch1. [DOI] [PubMed] [Google Scholar]
- de Klerk H. C., Smit J. A. Properties of a Lactobacillus fermenti bacteriocin. J Gen Microbiol. 1967 Aug;48(2):309–316. doi: 10.1099/00221287-48-2-309. [DOI] [PubMed] [Google Scholar]