Abstract
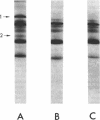
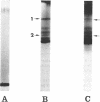
Major quantitative, but not qualitative, differences in the various species of proteins in purified membranes from Streptococcus pyogenes and its stabilized L-form have been demonstrated by acidic and alkaline disc gel electrophoresis with and without urea. The fact that no significant differences in the amino acid content or composition between these two membranes could be demonstrated emphasizes that these results are probably due to changes in the relative amounts of the various species of proteins in this subcellular component. The possibility of these protein changes in the L-form membrane being related to its inability to synthesize a rigid cell wall is discussed. Finally, phage-associated lysin, routinely used for removal of the group A streptococcal cell wall, does not appear to affect the protein profile or amino acid composition of the membrane either metabolically or nonmetabolically.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen M., Panos C. Cell wall polysaccharide biosynthesis by membrane fragments from Streptococcus pyogenes and stabilized L-form. J Bacteriol. 1971 May;106(2):347–355. doi: 10.1128/jb.106.2.347-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. Discussion. Mycoplasma and L forms. Ann N Y Acad Sci. 1967 Jul 28;143(1):813–823. doi: 10.1111/j.1749-6632.1967.tb27729.x. [DOI] [PubMed] [Google Scholar]
- LIU T. Y., GOTSCHLICH E. C. The chemical composition of pneumococcal C-polysaccharide. J Biol Chem. 1963 Jun;238:1928–1934. [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C. Muramic acid phosphate as a component of the mucopeptide of Gram-positive bacteria. J Biol Chem. 1967 Feb 10;242(3):471–476. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Panos C., Cohen M. Cell wall inhibition in a stable streptococcal L-form. Biochim Biophys Acta. 1966 Mar 28;117(1):98–106. doi: 10.1016/0304-4165(66)90156-5. [DOI] [PubMed] [Google Scholar]
- Panos C., Cohen M., Fagan G. Lipid alterations after cell wall inhibition. Fatty acid content of Streptococcus pyogenes and derived L-form. Biochemistry. 1966 May;5(5):1461–1468. doi: 10.1021/bi00869a003. [DOI] [PubMed] [Google Scholar]
- Razin S., Rottem S. Identification of Mycoplasma and other microorganisms by polyacrylamide-gel electrophoresis of cell proteins. J Bacteriol. 1967 Dec;94(6):1807–1810. doi: 10.1128/jb.94.6.1807-1810.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Shafer Z. Incorporation of cholesterol by membranes of bacterial L-phase variants with an appendix on the determination of the L-phase parentage by the electrophoretic patterns of cell proteins. J Gen Microbiol. 1969 Nov;58(3):327–339. doi: 10.1099/00221287-58-3-327. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Electrophoretic patterns of membrane proteins of Mycoplasma. J Bacteriol. 1967 Aug;94(2):359–364. doi: 10.1128/jb.94.2.359-364.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRISTRAM G. R., SMITH R. H. THE AMINO ACID COMPOSITION OF SOME PURIFIED PROTEINS. Adv Protein Chem. 1963;18:227–318. doi: 10.1016/s0065-3233(08)60270-3. [DOI] [PubMed] [Google Scholar]
- Theodore T. S., King J. R., Cole R. M. Identification of L forms by polyacrylamide-gel electrophoresis. J Bacteriol. 1969 Feb;97(2):495–499. doi: 10.1128/jb.97.2.495-499.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum G., Markman R. A rapid technique for distinguishing enzymatically active proteins in the cell"envelope" of Escherichia coli B. Biochim Biophys Acta. 1966 Jul 27;124(1):207–209. doi: 10.1016/0304-4165(66)90335-7. [DOI] [PubMed] [Google Scholar]