Abstract
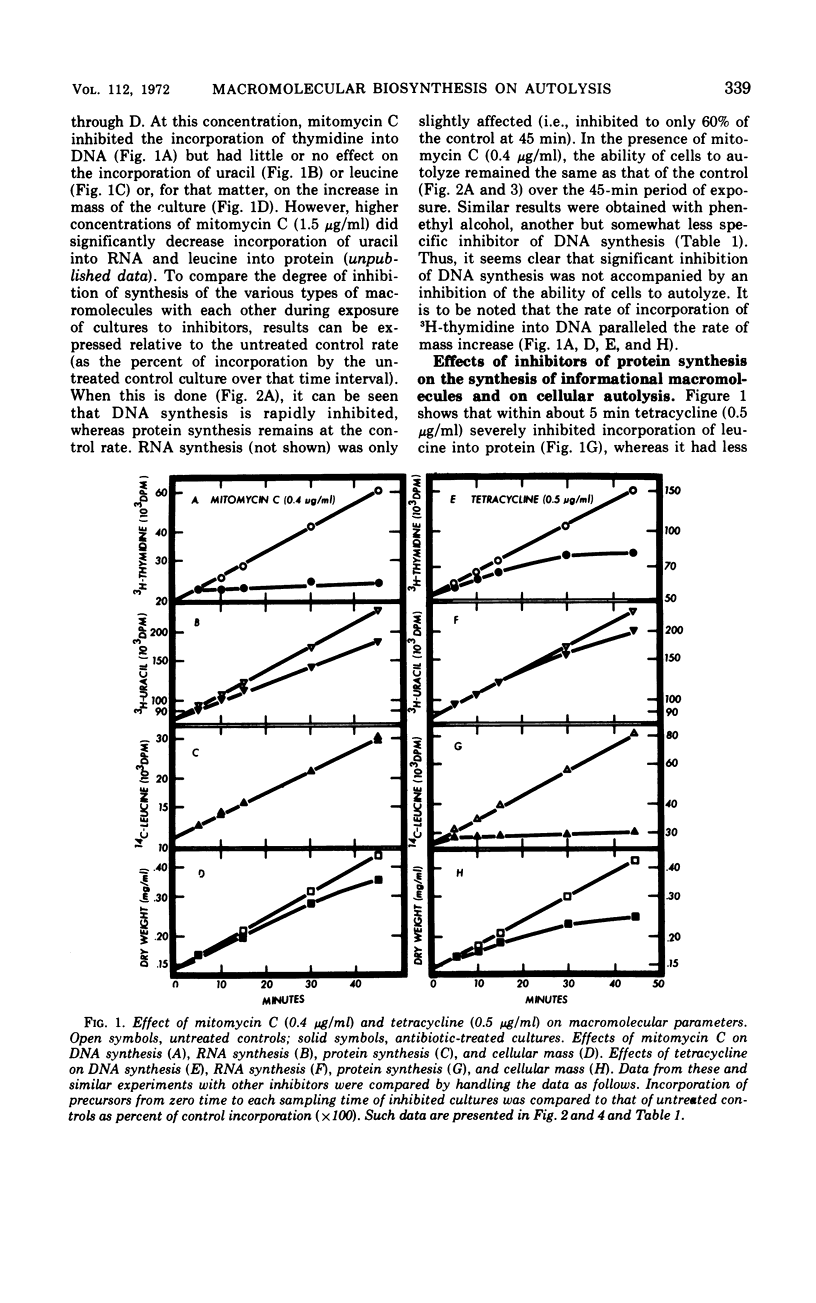
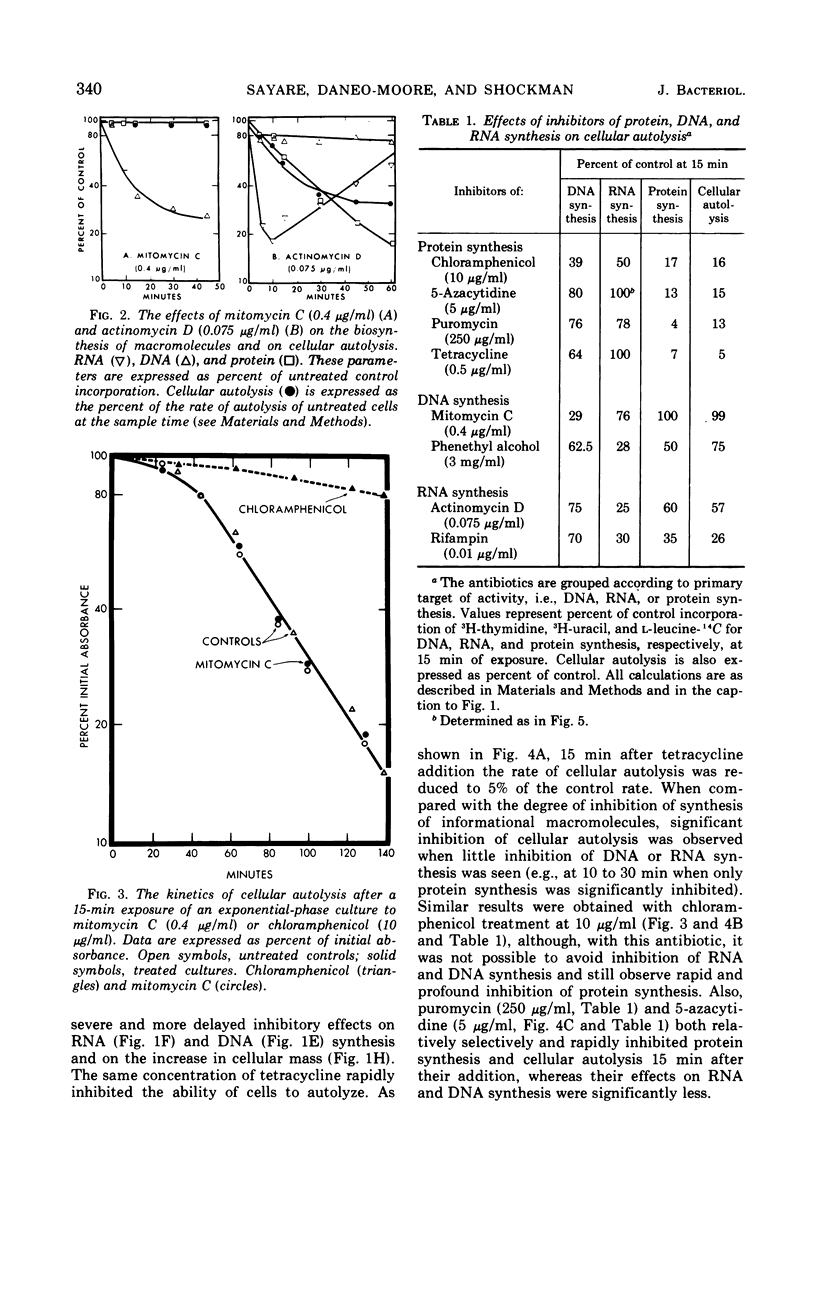
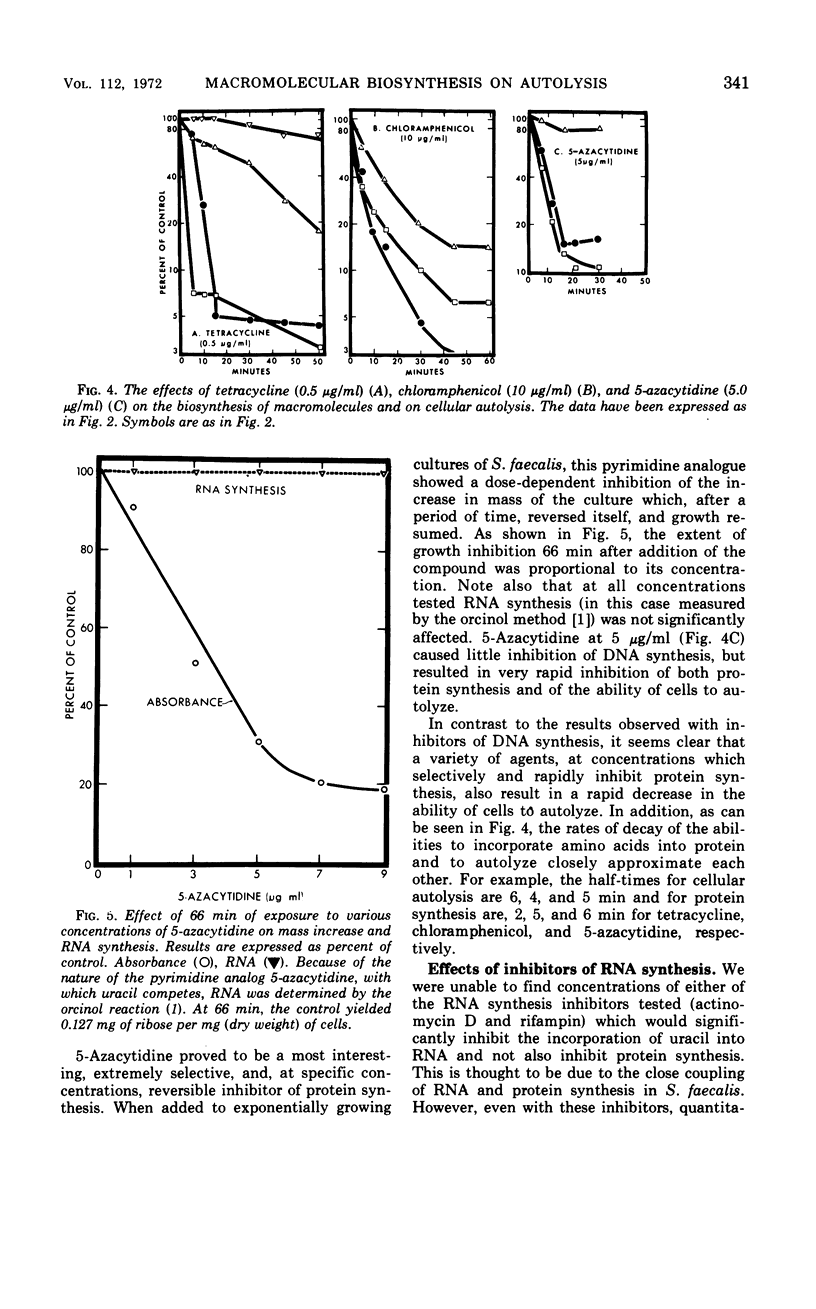
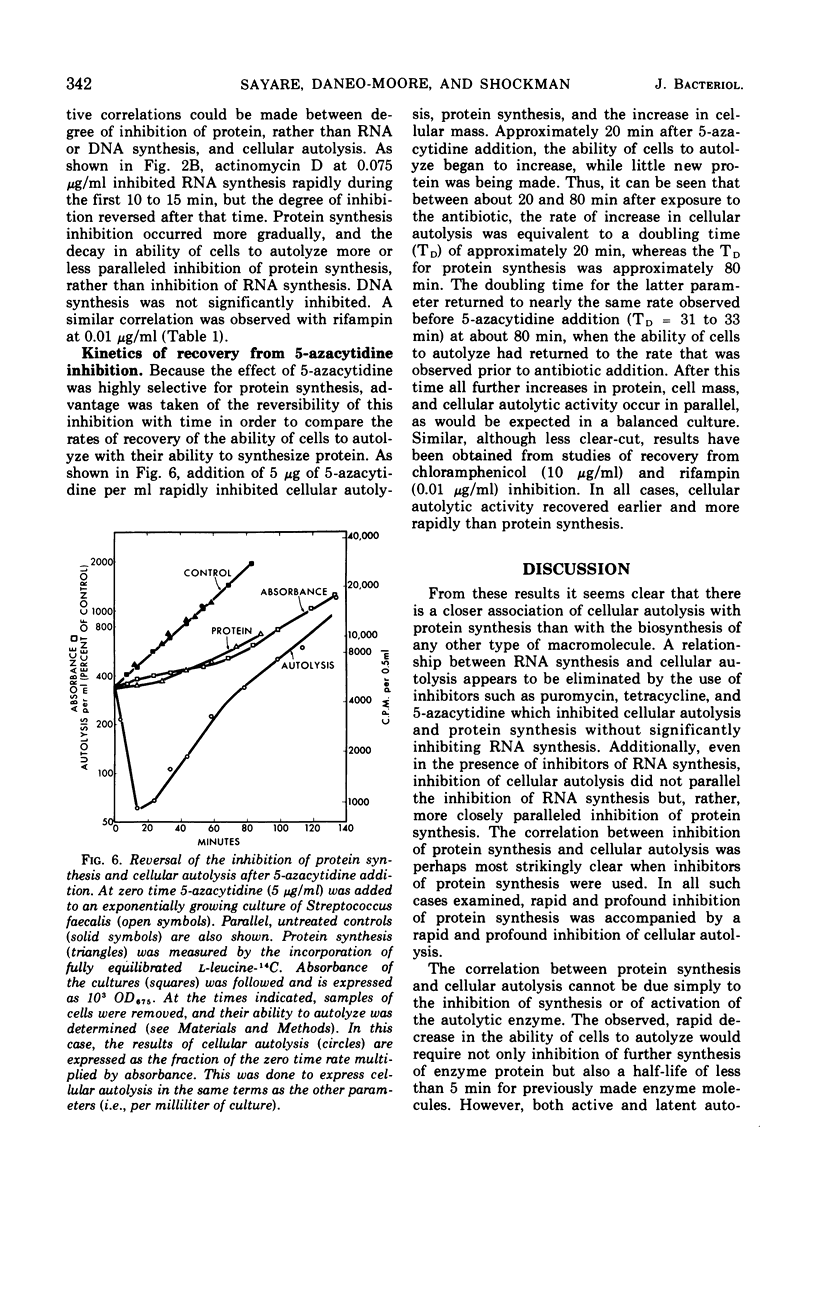
The addition of several different antibiotics to growing cultures of Streptococcus faecalis, ATCC 9790, was found to inhibit autolysis of cells in sodium phosphate buffer. When added to exponential-phase cultures, mitomycin C (0.4 μg/ml) or phenethyl alcohol (3 mg/ml) inhibited deoxyribonucleic acid synthesis, but did not appreciably affect the rate of cellular autolysis. Addition of chloramphenicol (10 μg/ml), tetracycline (0.5 μg/ml), puromycin (25 μg/ml), or 5-azacytidine (5 μg/ml) to exponential-phase cultures inhibited protein synthesis and profoundly decreased the rate of cellular autolysis. Actinomycin D (0.075 μg/ml) and rifampin (0.01 μg/ml), both inhibitors of ribonucleic acid (RNA) synthesis, also reduced the rate of cellular autolysis. However, the inhibitory effect of actinomycin D and rifampin on cellular autolysis was more closely correlated with their concomitant secondary inhibition of protein synthesis than with the more severe inhibition of RNA synthesis. The dose-dependent inhibition of protein synthesis by 5-azacytidine was quickly diluted out of a growing culture. Reversal of inhibition was accompanied by a disproportionately rapid increase in the ability of cells to autolyze. Thus, inhibition of the ability of cells to autolyze can be most closely related to inhibition of protein synthesis. Furthermore, the rapidity of the response of cellular autolysis to inhibitors of protein synthesis suggests that regulation is exerted at the level of autolytic enzyme activity and not enzyme synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daneo-Moore L., Higgins M. L. Morphokinetic reaction of Streptococcus faecalis (ATCC 9790) cells to the specific inhibition of macromolecular synthesis: nucleoid condensation on the inhibition of protein synthesis. J Bacteriol. 1972 Mar;109(3):1210–1220. doi: 10.1128/jb.109.3.1210-1220.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Autolysin(s) of Bacillus subtilis as dechaining enzyme. J Bacteriol. 1970 Aug;103(2):494–499. doi: 10.1128/jb.103.2.494-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- Paulton R. J. Nuclear and cell division in filamentous bacteria. Nat New Biol. 1971 Jun 30;231(26):271–274. doi: 10.1038/newbio231271a0. [DOI] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the latent form and the active form of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Nov;100(2):617–624. doi: 10.1128/jb.100.2.617-624.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the location of autolysin, cell wall synthesis, and the development of resistance to cellular autolysis in Streptococcus faecalis after inhibition of protein synthesis. J Bacteriol. 1970 Aug;103(2):457–466. doi: 10.1128/jb.103.2.457-466.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. S., Shockman G. D., Daneo-Moore L. Balanced macromolecular biosynthesis in "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):710–717. doi: 10.1128/jb.105.3.710-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]


