Abstract
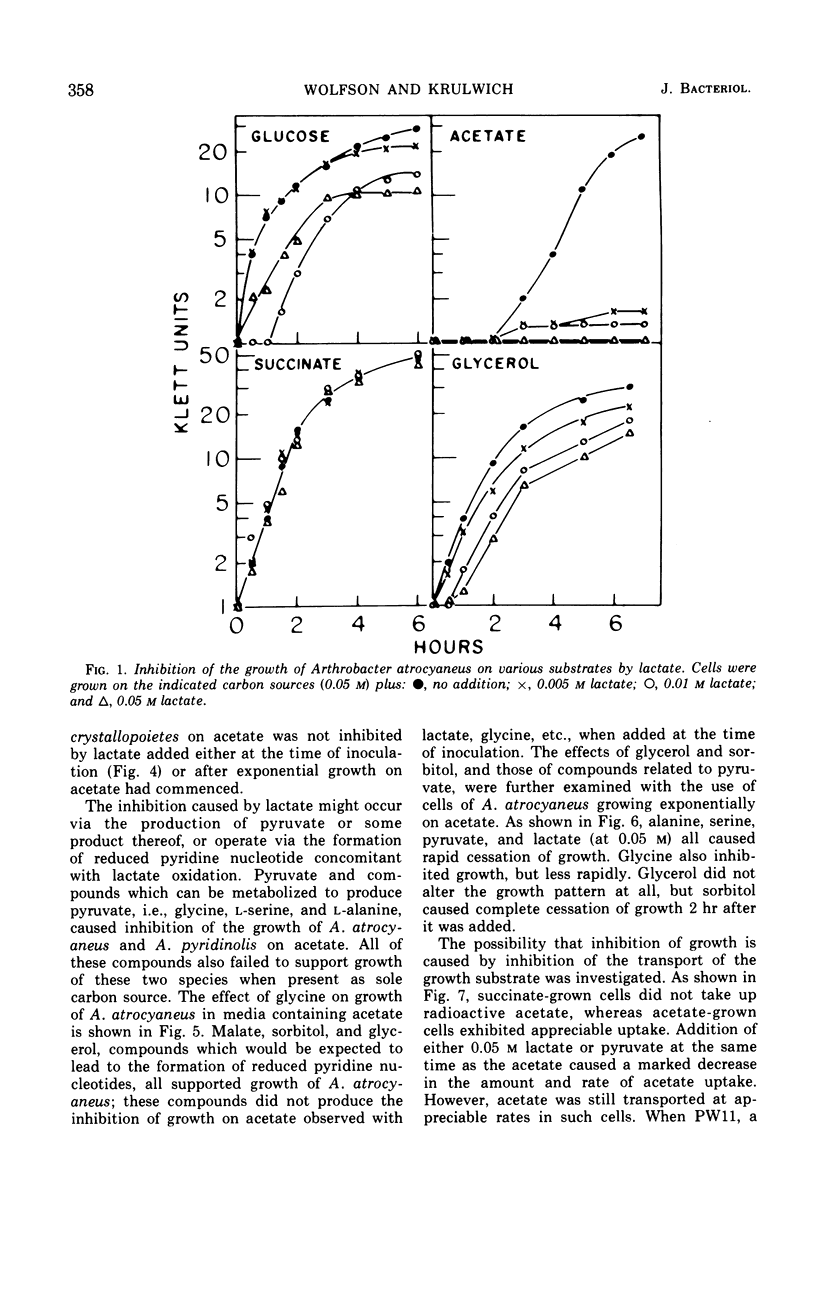
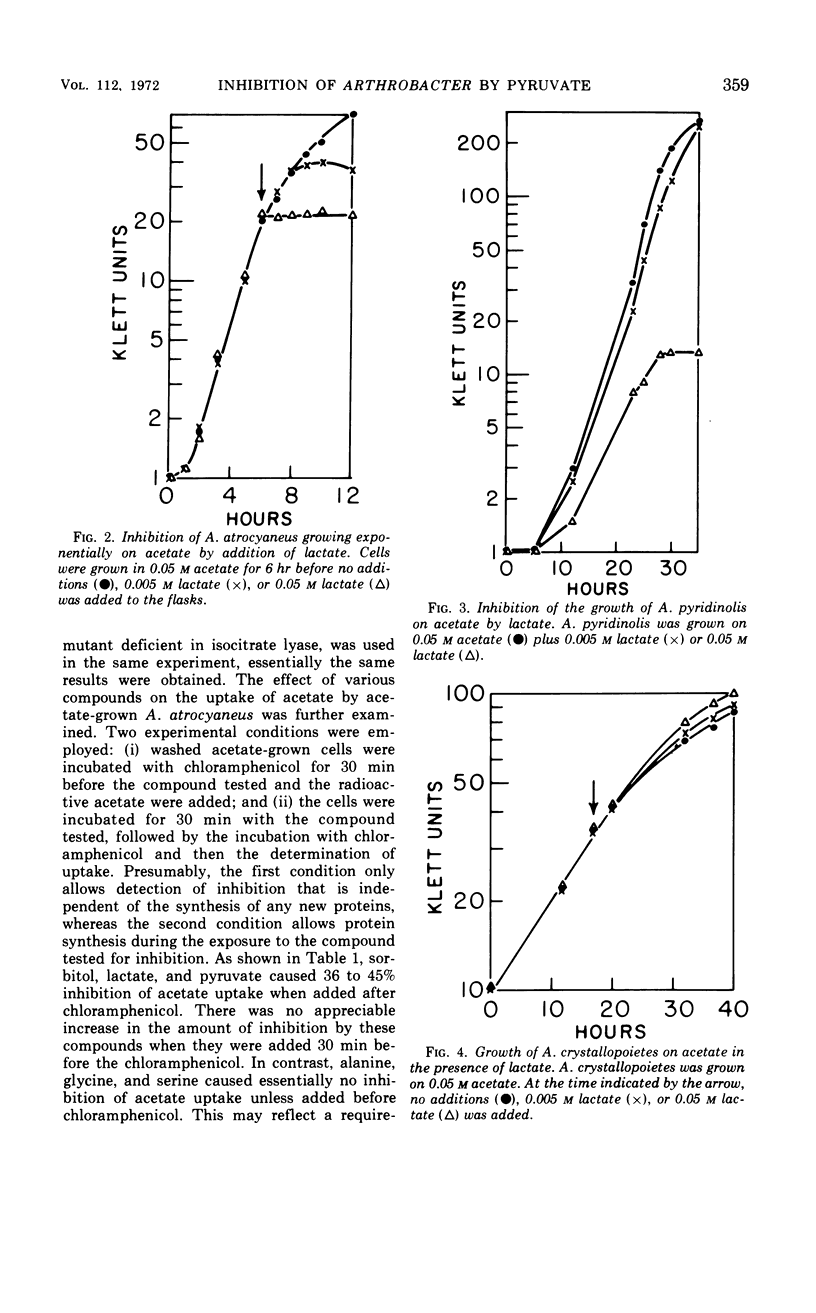
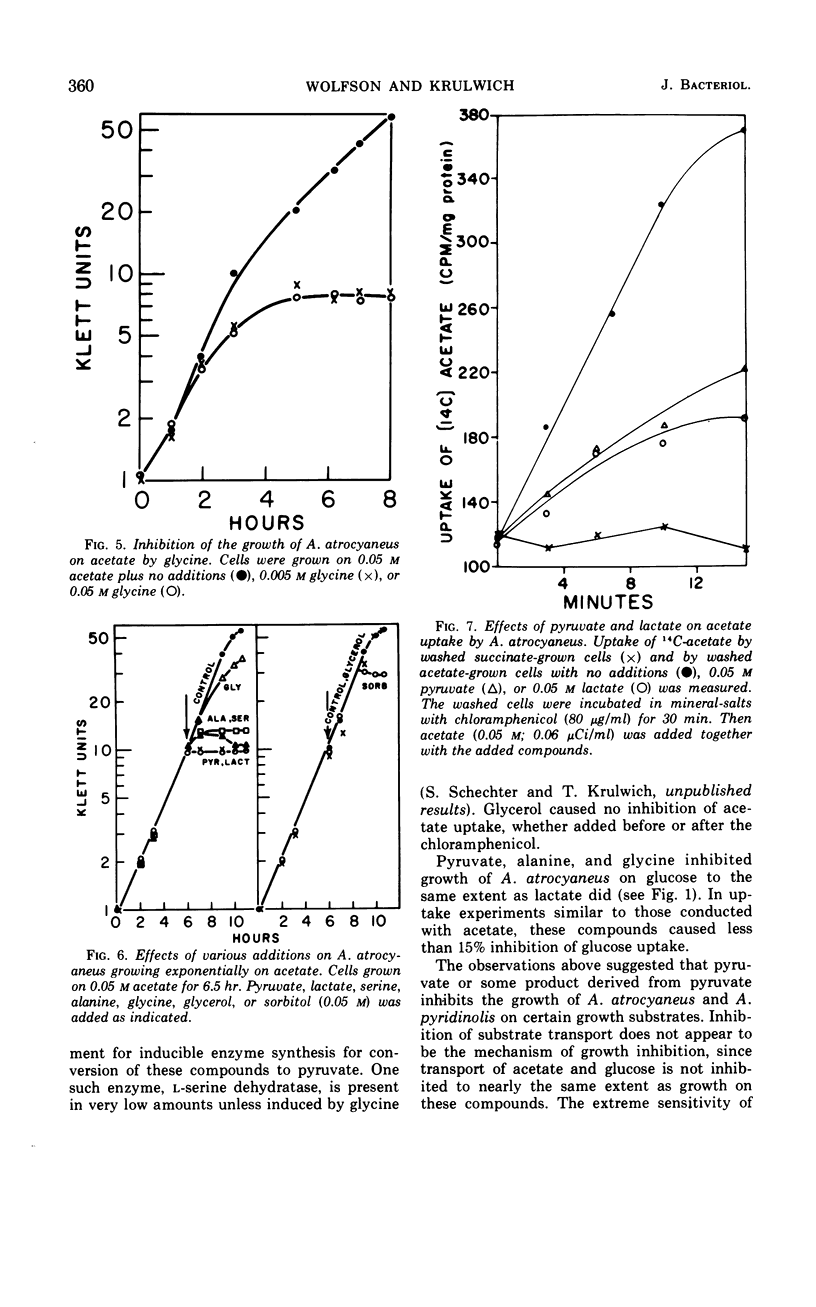
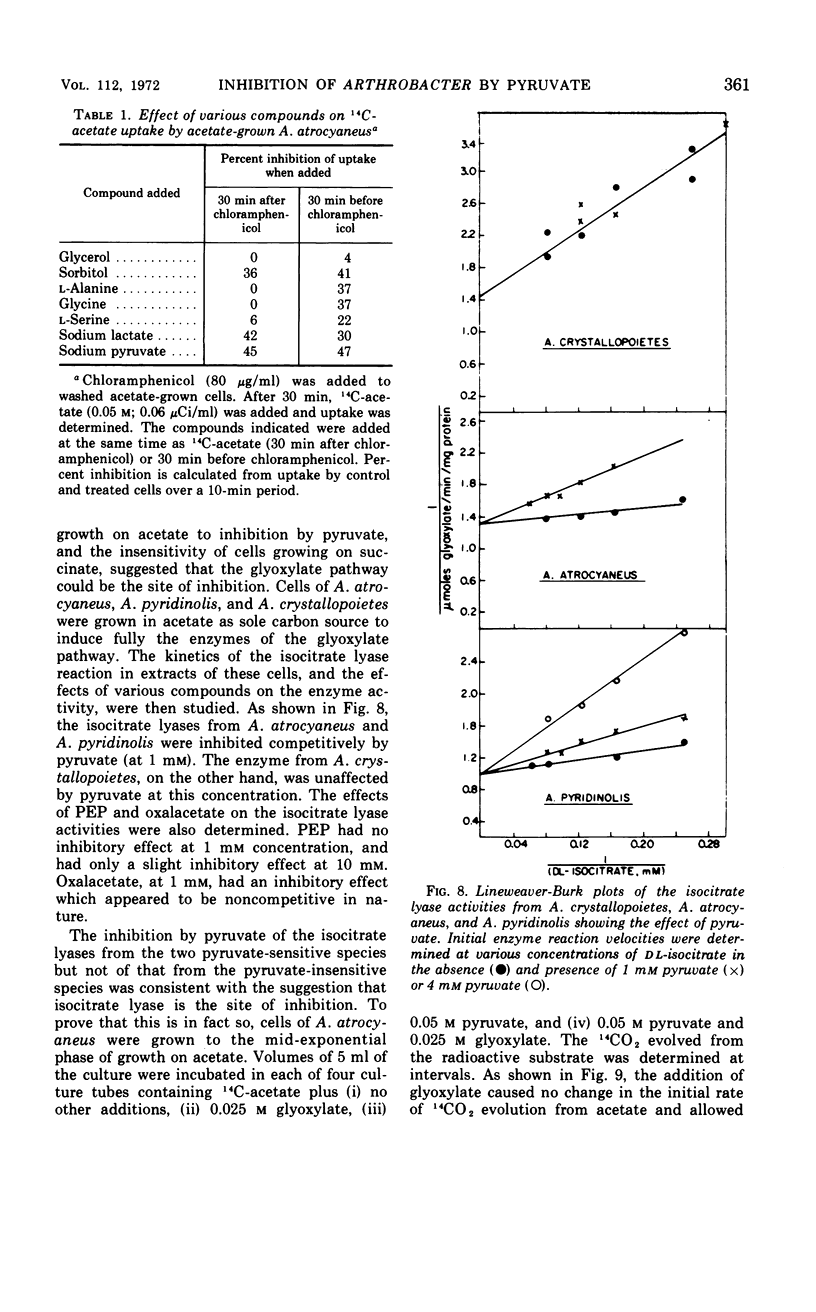
Growth of Arthrobacter atrocyaneus and A. pyridinolis on certain growth substrates was found to be inhibited by pyruvate and compounds which can be converted to pyruvate. Growth of A. atrocyaneus on acetate, for example, was completely inhibited by 5 mm pyruvate; growth of this organism on glucose was less sensitive and growth on succinate was insensitive to inhibition by pyruvate. Growth of a third Arthrobacter species, A. crystallopoietes, on acetate and other substrates was not inhibited by pyruvate. The site of pyruvate inhibition was shown to be the isocitrate lyase reaction. Glyoxylate, which affords a bypass of this reaction, restored the ability of A. atrocyaneus to evolve 14CO2 from acetate in the presence of pyruvate. The isocitrate lyases from A. atrocyaneus and A. pyridinolis were competitively inhibited by concentrations of pyruvate as low as 1 mm, whereas the enzyme from A. crystallopoietes was unaffected by this concentration of pyruvate. Comparable levels of phosphoenolpyruvate did not inhibit the isocitrate lyases from any of the species. A mutant strain of A. atrocyaneus, PW11, which is deficient in isocitrate lyase activity, grew on glucose at a reduced rate that was comparable to the rate of growth of the wild-type strain on glucose plus lactate. Addition of lactate to PW11 did not further reduce its rate of growth on glucose. Thus, the glyoxylate pathway appears to be used as an anaplerotic pathway during growth of A. atrocyaneus on glucose. Two other considerations suggest that A. atrocyaneus and A. pyridinolis, but not A. crystallopoietes, may be deficient in the ability to convert pyruvate to 4-carbon acids. First, the former two species accumulate intracellular pyruvate from exogenous l-alanine to a much greater extent than does A. crystallopoietes. Moreover, A. atrocyaneus and A. pyridinolis are incapable of growth on lactate as sole source of carbon whereas A. crystallopoietes can grow on lactate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John P. C., Syrett P. J. The inhibition, by intermediary metabolites, of isocitrate lyase from Chlorella pyrenoidosa. Biochem J. 1968 Dec;110(3):481–484. doi: 10.1042/bj1100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C. Alteration of glucose metabolism of Arthrobacter crystallopoietes by compounds which induce sphere to rod morphogenesis. J Bacteriol. 1969 Feb;97(2):526–534. doi: 10.1128/jb.97.2.526-534.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. I. Cell wall composition and polysaccharides of the peptidoglycan. J Bacteriol. 1967 Sep;94(3):734–740. doi: 10.1128/jb.94.3.734-740.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- ZAGALLO A. C., WANG C. H. Comparative carbohydrate catabolism in Arthrobacter. J Gen Microbiol. 1962 Nov;29:389–401. doi: 10.1099/00221287-29-3-389. [DOI] [PubMed] [Google Scholar]


