Abstract
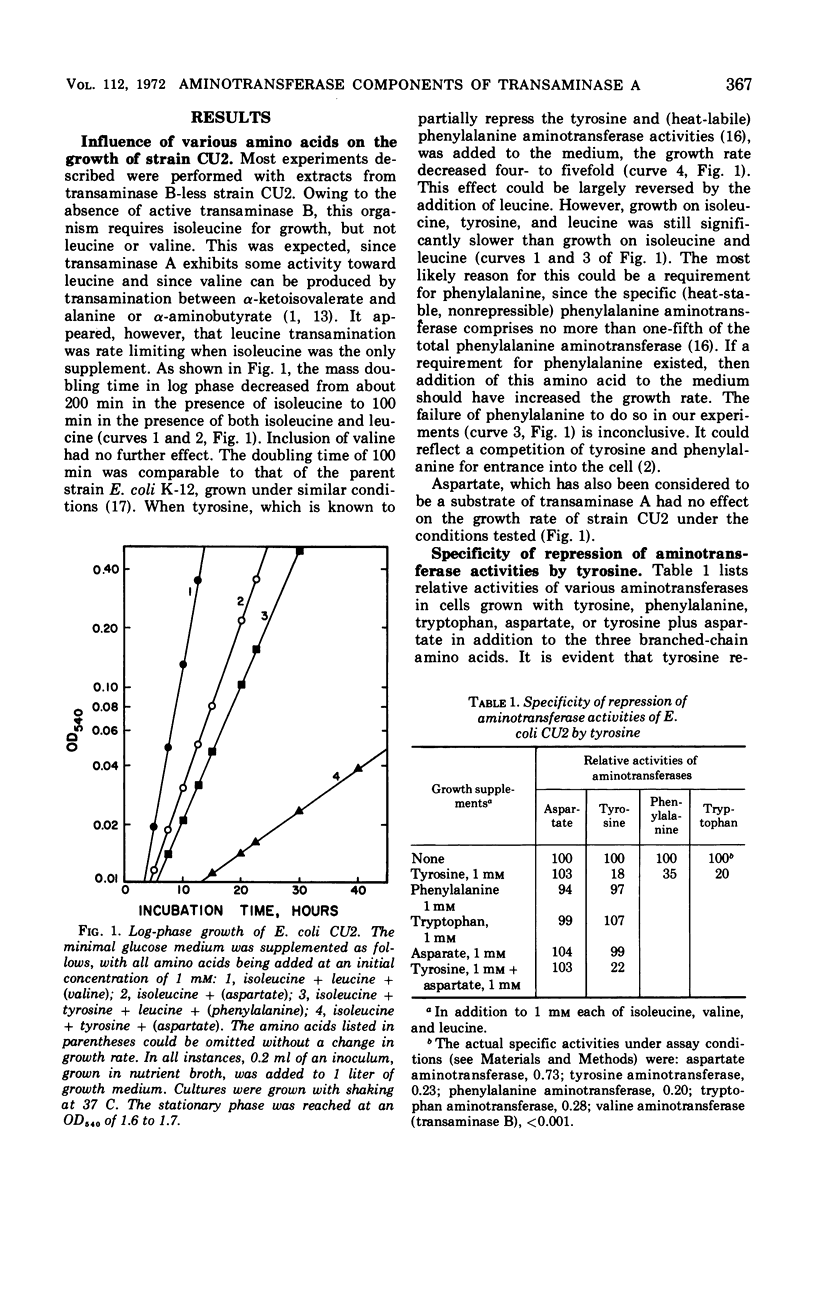
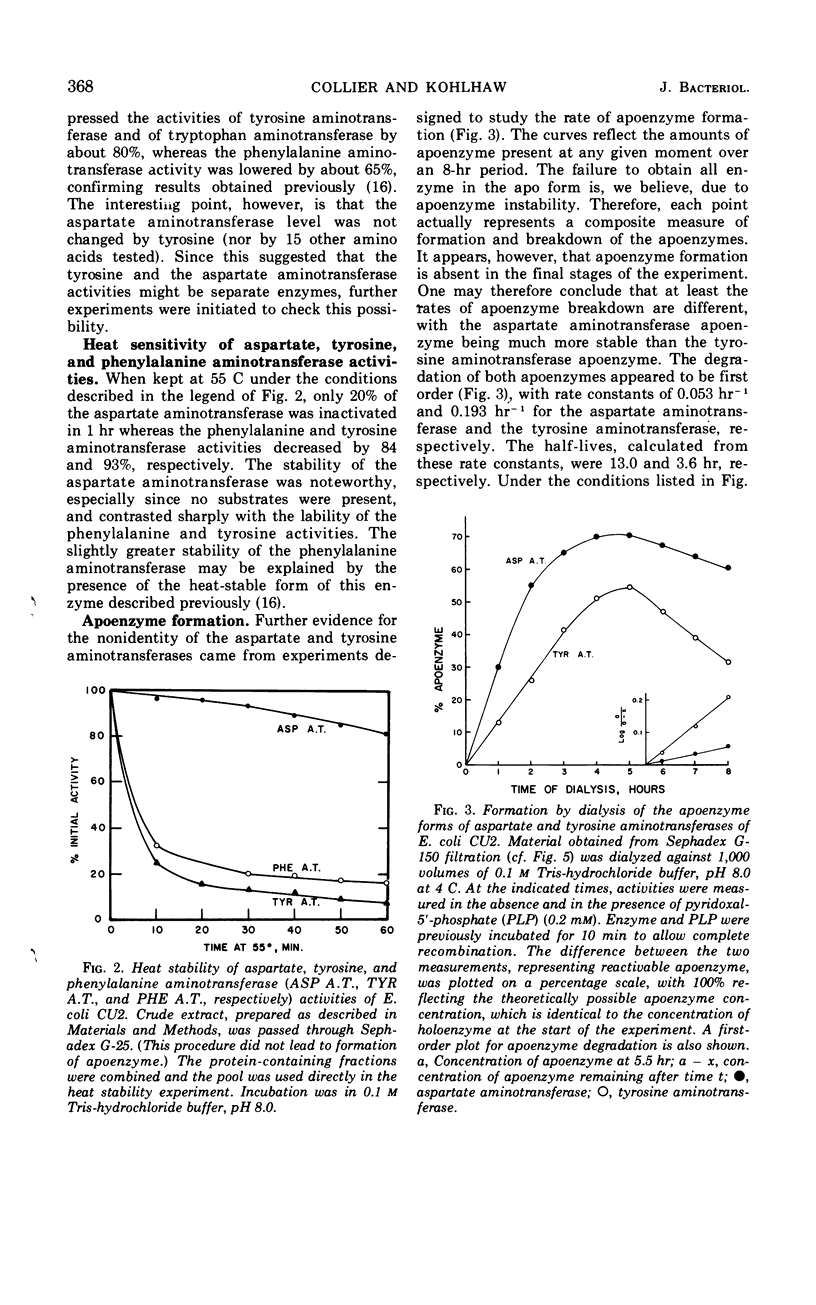
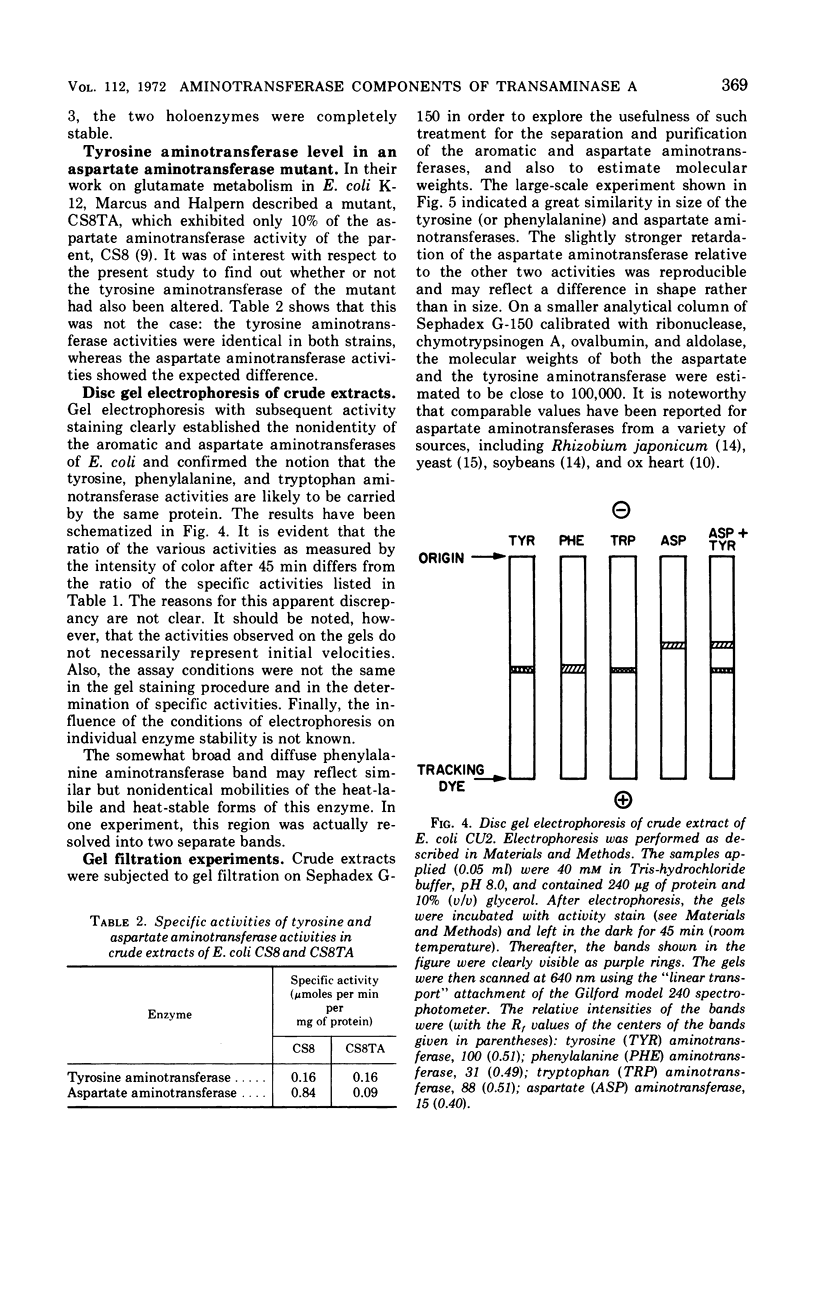
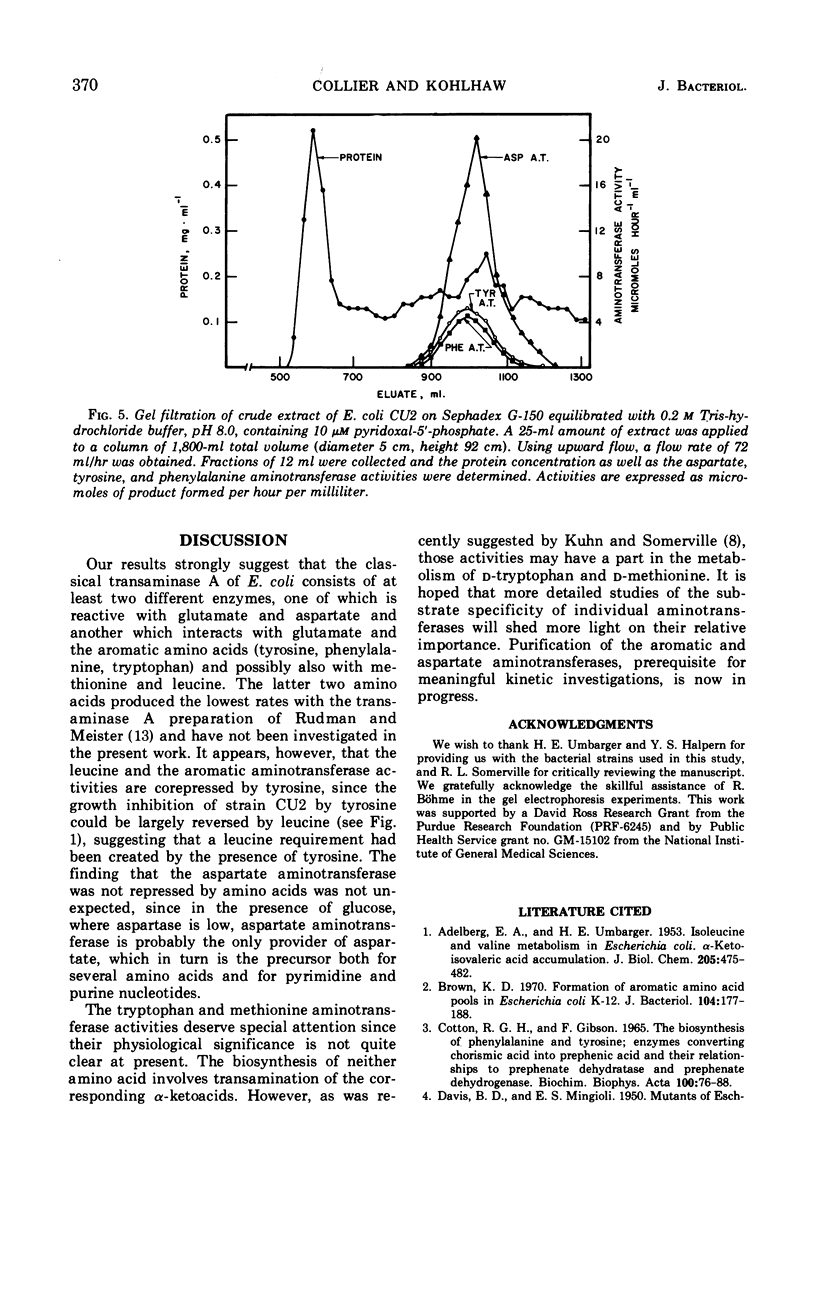
Tyrosine, added to the growth medium of a strain of Escherichia coli K-12 lacking transaminase B, repressed the tyrosine, phenylalanine, and tryptophan aminotransferase activities while leaving the aspartate aminotransferase activity unchanged. This suggested that the aspartate and the aromatic aminotransferase activities, previously believed to reside in the same protein, viz. transaminase A, are actually nonidentical. Further experiments showed that, upon incubation at 55 C, the aspartate aminotransferase of crude extracts was almost completely stable, whereas the tyrosine and phenylalanine activities were rapidly inactivated. Apoenzyme formation was faster, and apoenzyme degradation proceeded more slowly with aspartate aminotransferase than with tyrosine aminotransferase. Electrophoresis in polyacrylamide gels separated the aminotransferases. A more rapidly moving band contained tyrosine, phenylalanine, and tryptophan aminotransferases, and a slower band contained aspartate aminotransferase. A mutant of E. coli K-12 with low levels of aspartate aminotransferase exhibited unchanged levels of tyrosine aminotransferase. Thus, transaminase A appears to be made up of at least two proteins: one of broad specificity whose synthesis is repressed by tyrosine and another, specific for aspartate, which is not subject to repression by amino acids. The apparent molecular weights of both the aspartate and the aromatic aminotransferases, determined by gel filtration, were about 100,000.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. V. alpha-Ketoisovaleric acid accumulation. J Biol Chem. 1953 Nov;205(1):475–482. [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dixon H. B., Severin E. S. Dissociation of the prosthetic group of aspartate aminotransferase. Biochem J. 1968 Nov;110(2):18P–19P. doi: 10.1042/bj1100018p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM K. H., TCHEN T. T. Putrescine--alpha-ketoglutarate trans-aminase in E. coli. Biochem Biophys Res Commun. 1962 Sep 25;9:99–102. doi: 10.1016/0006-291x(62)90095-5. [DOI] [PubMed] [Google Scholar]
- Kuhn J., Somerville R. L. Mutant strains of Escherichia coli K12 that use D-amino acids. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2484–2487. doi: 10.1073/pnas.68.10.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M., Halpern Y. S. The metabolic pathway of glutamate in Escherichia coli K-12. Biochim Biophys Acta. 1969 Apr 1;177(2):314–320. doi: 10.1016/0304-4165(69)90141-x. [DOI] [PubMed] [Google Scholar]
- Marino G., Greco A. M., Scardi V., Zito R. Purification and general properties of aspartate aminotransferase of ox heart. Biochem J. 1966 Jun;99(3):589–594. doi: 10.1042/bj0990589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERKOFSKY B., GILVARG C. N-Succinyl-L-diaminopimelic-glutamic transaminase. J Biol Chem. 1961 May;236:1432–1438. [PubMed] [Google Scholar]
- RUDMAN D., MEISTER A. Transamination in Escherichia coli. J Biol Chem. 1953 Feb;200(2):591–604. [PubMed] [Google Scholar]
- SCHREIBER G., ECKSTEIN M., MAASS G., HOLZER H. DIE PHYSIKALISCH-CHEMISCHEN EIGENSCHAFTEN VON APOASPARTATAMINOTRANSFERASE AUS BIERHEFE. Biochem Z. 1964 Jul 8;340:21–34. [PubMed] [Google Scholar]
- SILBERT D. F., JORGENSEN S. E., LIN E. C. Repression of transaminase A by tyrosine in Escherichia coli. Biochim Biophys Acta. 1963 Jun 11;73:232–240. doi: 10.1016/0006-3002(63)90307-x. [DOI] [PubMed] [Google Scholar]
- TEMPLE R. J., UMBARGER H. E., MAGASANIK B. THE EFFECT OF L-VALINE ON ENZYME SYNTHESIS IN ESCHERICHIA COLI K-12. J Biol Chem. 1965 Mar;240:1219–1224. [PubMed] [Google Scholar]
- UMBARGER H. E., MUELLER J. H. Isoleucine and valine metabolism of Escherichia coli. I. Growth studies on amino acid-deficient mutants. J Biol Chem. 1951 Mar;189(1):277–285. [PubMed] [Google Scholar]
- UMBARGER H. E., UMBARGER M. A., SIU P. M. BIOSYNTHESIS OF SERINE IN ESCHERICHIA COLI AND SALMONELLA TYPHIMURIUM. J Bacteriol. 1963 Jun;85:1431–1439. doi: 10.1128/jb.85.6.1431-1439.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Regulator gene controlling enzymes concerned in tyrosine biosynthesis in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1234–1241. doi: 10.1128/jb.97.3.1234-1241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


