Abstract
Impaired insulin secretion is a characteristic of non-insulin-dependent diabetes mellitus (NIDDM). One possible therapeutic agent for NIDDM is the insulinotropic hormone glucagon-like peptide 1 (GLP-1). GLP-1 stimulates insulin secretion through several mechanisms including activation of protein kinase A (PKA). We now demonstrate that the subcellular targeting of PKA through association with A-kinase-anchoring proteins (AKAPs) facilitates GLP-1-mediated insulin secretion. Disruption of PKA anchoring by the introduction of anchoring inhibitor peptides or expression of soluble AKAP fragments blocks GLP-1 action in primary islets and cAMP-responsive insulin secretion in clonal beta cells (RINm5F). Displacement of PKA also prevented cAMP-mediated elevation of intracellular calcium suggesting that localized PKA phosphorylation events augment calcium flux.
Subcellular targeting of protein kinases and phosphatases is an efficient mechanism to selectively regulate cellular events by ensuring that phosphorylation only occurs at precise intracellular sites (1–3). Accumulating evidence suggests that the subcellular location of the cAMP-dependent protein kinase (PKA) is a determinant in the preferential phosphorylation of certain PKA substrates (4, 5). Compartmentalization of the PKA holoenzyme is conferred by the association of the regulatory subunit dimer (RII) with a family of A-kinase-anchoring proteins (AKAPs) (6, 7). Anchoring not only places the kinase close to preferred substrates but also positions the PKA holoenzyme at sites where it can optimally respond to fluctuations in the second messenger cAMP (1–3). This higher order of spatial organization may also coordinate effector-mediated events that integrate second messenger signals (8–11). For example, insulin secretion from pancreatic beta cells requires the coordinate action of metabolites, hormones, and neurotransmitters (12, 13). A recently identified hormone, glucagon-like peptide 1 (GLP-1), potentiates glucose-mediated insulin secretion through activation of PKA (14–16). In this report we demonstrate that the correct subcellular location of PKA is a determinant in the cAMP signaling pathway in response to GLP-1 to induce insulin secretion from pancreatic islets and related cell lines.
METHOD
RII Overlay.
The presence of AKAPs in primary rat pancreas was detected by a solid phase RII overlay as previously described (17, 18). Fifty micrograms of total pancreas protein was separated by electrophoresis on an SDS/10% polyacrylamide gel and electrotransferred to nitrocellulose. Filters were pretreated with either 3 μM Ht31 peptide or 3 μM Ht31P peptide and then incubated with 32P-labeled RIIα overnight at room temperature. RII binding proteins were detected by autoradiography.
Preparation of Primary Islets and Transfected RINm5F Cells.
Normal rat pancreas was isolated and infused with Hanks’ buffer before dissection. Pancreatic islets were isolated by collagenase digestion and plated on Falcon tissue culture dishes. Islets were maintained in culture for up to 5 days in RPMI 1640 containing 5 mM glucose and supplemented with streptomycin (100 μg/ml) and penicillin (100 units/ml). RINm5F cells at passage 5–10 were transfected with the mammalian expression vectors pcDNA3, pcHt31, or pcHt31P by the Lipofectin method (Life Technologies, Grand Island, NY). Clonal cell lines were selected for resistance to the antibiotic G418 (0.8 mg/ml), and stable cell lines with 100% of the cells expressing the appropriate protein were used. Cells were maintained in low glucose DMEM, with 10% fetal calf serum and 0.8 mg/ml G418. Expression of Ht31 and Ht31P was monitored by immunoblot. Cells passaged greater than 20 times after transfection began to lose expression and were not used in experiments.
Peptide Delivery.
Biotinylated Ht31, Ht31P, and PKI-(5–25) peptides were introduced into islets by a modification to the method of Welsh et al. (19). Dissociated primary cultures of rat pancreatic islets were incubated with Lipofectamine (10 μg/well) for 90 min at 37°C. Residual Lipofectamine was removed by washing with Krebs-Ringer Hepes buffer [KRBH buffer (10 mM Hepes, pH 7.4/0.1% BSA/130 mM NaCl/5.2 mM KCl/1.3 mM KH2PO4/1.6 mM MgCl2/2.8 mM CaCl2/20 mM NaHCO3/2.8 mM glucose)]. Fifty-sixty percent of islet cells showed uptake of peptides as detected immunochemically with streptavidin-conjugated secondary antibodies (data not shown).
Insulin Secretion Assay.
Insulin secretion was measured by incubating islets with KRBH containing 16.7 mM glucose (high glucose KRBH) or 1 μM GLP-1 (7–37-amide) for 30 min at 37°C. Media were collected and centrifuged at 15,000 × g for 10 min. The supernatant was stored at −20°C before determining insulin content by radioimmunoassay with rat insulin as a standard (Incstar, Stillwater, MN). RINm5F cells and anchoring inhibitor expressing cell lines were plated at 1 × 105 cells per 35-mm well. After 24–48 h in culture, the cells were rinsed in low glucose KRBH and incubated for 30 min with high glucose KRBH. Cyclic AMP-mediated insulin secretion was induced with 1 mM dibutyryl-cAMP for 30 min. Cell-soluble cAMP analogs were preferred over GLP-1 for these experiments because they maximize insulin secretion in the RINm5F cells. The media were collected and spun at 15,000 × g for 10 min.
Cyclic AMP and PKA Assays.
Pancreatic beta islets were incubated with 1 μM GLP-1 for 30 min at 37°C to stimulate cAMP production. The islets were rinsed with PBS, lysed with 0.5 M perchloric acid, and neutralized with 2 M NH4OH. Samples were centrifuged to remove cell debris, and the supernatant cAMP levels were measured by radioimmunoassay (DuPont/NEN). Samples used to measure PKA activity were prepared in a similar manner except cell lysis was performed in ice-cold 50 mM Tris, pH 8.0/150 mM NaCl/1% Nonidet P-40/2 μg/ml pepstatin A/leupeptin/1 mM 4-(2-aminoethyl)benzene-sulfonyl fluoride/10 μM 3-isobutylmethylxanthine. PKA activity was measured by the filter paper method of Corbin and Riemann (20) by using the Kemptide (LRRASLG) as a peptide substrate.
Confocal Microscopy.
RINm5F cells were grown on coverslips for 24–48 h. After rinsing with PBS cells were fixed with 3.7% formaldehyde in PBS at room temperature for 5 min and permeabilized with acetone at −20°C for 1 min. Cells were rehydrated and blocked in PBS/0.1% BSA. Cells were incubated with primary antibodies at room temperature for 1 h with affinity-purified rabbit Ht31 antisera (1:500) and affinity-purified goat anti-RII antisera (1:1000). After extensive washing (3× PBS/0.1% BSA) fluorescein-conjugated donkey anti-goat (1:100 dilution) and Texas red-conjugated donkey anti-rabbit (1:100) secondary antibodies were applied for 1 h at room temperature. Coverslips were washed 3 times in PBS/0.1% BSA and mounted with Slow Fade Antifade (Molecular Probes). Confocal analysis was performed on a Leitz Fluovert FU confocal photomicroscope with a 63/1.4 N.A. OEL PL APO lens. No staining was detected with secondary antibodies alone.
Intracellular Calcium Measurement.
After 3–5 days in culture, RINm5F cells were rinsed in low glucose KRBH and loaded with 2 μM fura-2 acetoxymethyl ester in dimethyl sulfoxide at 37°C for 30 min. The cells were mounted in a perifusion chamber held at 37°C on a Zeiss Axiophot inverted microscope. Cells were allowed to equilibrate for 10 min in low glucose KRBH. Dual wavelength excitation at 340/380 nm was detected at an emission signal of 510 nm every 4 s by a photomultiplier. Excitation lasted 0.1 s, and 16 frames were averaged for each measurement. A minimum of 15 cells per field was analyzed with a ×40 objective. Measurements were obtained throughout the basal infusion and perifusion of agonist for a minimum of 20 min.
RESULTS
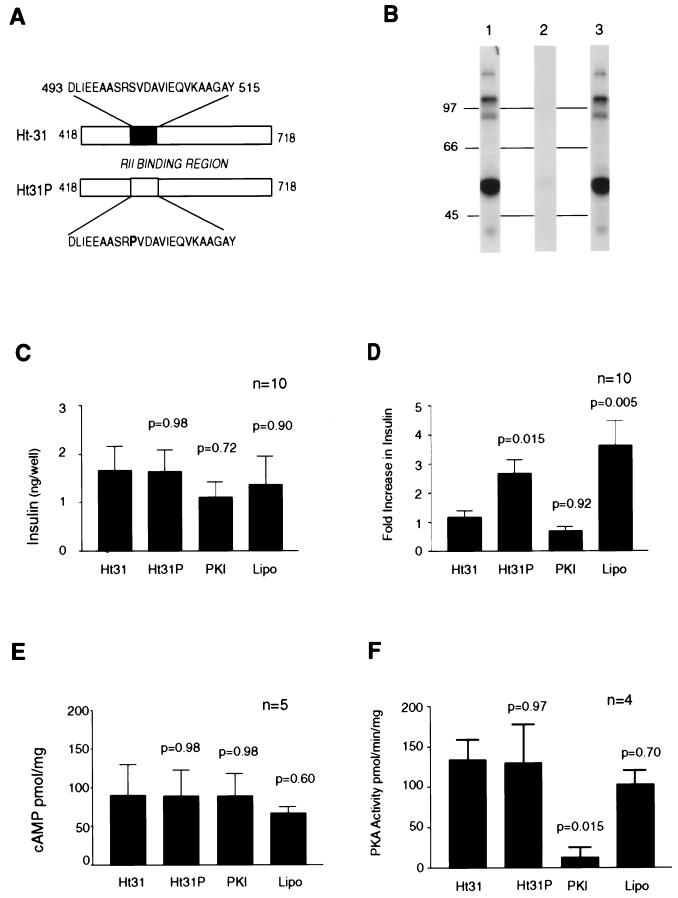
To determine whether PKA anchoring is involved in insulin secretion we used peptide-based reagents that are competitive inhibitors of RII–AKAP interaction (17, 18) to disrupt PKA anchoring inside primary cultures of rat islets. Treatment with the Ht31-(493–515) peptide (Fig. 1A) blocked RII binding to pancreatic AKAPs as assessed by a solid-phase overlay assay (Fig. 1B). A control peptide (Ht31P) was unable to block RII–AKAP interaction (Fig. 1 A and B). Lipofectamine was used as a delivery reagent to introduce Ht31, Ht31P control, or a PKA inhibitor peptide PKI-(5–24) into primary rat islets. Concomitant changes in insulin secretion were monitored by radioimmunoassay. Glucose promotes insulin secretion from islets through a predominately PKA-independent pathway (21, 22). The response of islets treated with any of the bioactive peptides to glucose was not significantly different (Fig. 1C). Nontreated islets had similar glucose-mediated insulin secretion of 4.5 ng/well ± 0.57, n = 5. The islets were then treated with insulinotropic hormone GLP-1 that activates PKA and increases intracellular calcium (23–25). Administration of 1 μM GLP-1-(7–37) further increased insulin secretion 3.5- ± 0.5-fold (n = 10) in control cells treated with Lipofectamine alone (Fig. 1D). Application of the PKA inhibitor peptide [PKI-(5–24)] blocked the GLP-1 effect (0.7 ± 0.15), confirming a role for the kinase in this process (Fig. 1D). Interestingly, the GLP-1 effect was blocked [0.9- ± 0.1-fold (n = 10)] in islets treated with the Ht31-anchoring inhibitor peptide whereas the control peptide (Ht31P) had no effect [2.8- ± 0.3-fold increase (n = 10)] (Fig. 1D). Similar results were obtained when cell-soluble myristoylated derivatives of these peptides were used in the absence of Lipofectamine (data not shown). Additional controls showed that none of the peptides affected cAMP production in response to GLP-1 (Fig. 1E). Likewise, Ht31, Ht31P, or Lipofectamine did not inhibit C subunit activity toward the heptapeptide substrate Kemptide (Fig. 1F). These results suggest that disruption of PKA–AKAP targeting attenuates GLP-1-stimulated insulin secretion in pancreatic beta islets.
Figure 1.
Disrupting PKA anchoring inhibits GLP-1-mediated insulin secretion in primary islets. (A) Schematic representation of the anchoring inhibitor peptides and fragments used in this study. The Ht31 and Ht31P reagents were derived from the human thyroid anchoring protein (18). The shaded box represents the putative RII-binding site on Ht31, sequence residues 493–515. Amino acids are indicated by the one-letter code. (B) Detection of AKAPs in rat pancreas. RII-binding proteins were detected by a solid-phase overlay procedure (lane 1). RII binding was blocked when filters were incubated in the presence of Ht31 peptide (lane 2) but not in the presence of the control peptide Ht31P (lane 3). Molecular mass markers (kDa) are indicated. (C and D) Peptides were introduced into primary cultures of pancreatic islets to assess the role of PKA anchoring on insulin secretion. (C) Glucose-mediated insulin secretion in islets treated with peptides or a Lipofectamine control (indicated below each bar) was measured in the culture media by radioimmunoassay. Data from 10 experiments are shown. (D) Changes in GLP-1-mediated insulin secretion were measured by similar methods and are presented as the fold increase over glucose-mediated insulin secretion. Data from 10 experiments are indicated. (E) Peptide-treated pancreatic islet extracts were assayed for GLP-1-induced cAMP production (pmol/mg) by radioimmunoassay. Data from five individual experiments are shown. (F) PKA activity in peptide-treated pancreatic islet extracts was measured by the filter paper assay by using Kemptide (LRRASLG) as a peptide substrate. Data from four individual experiments are indicated. (C–F) Two-tailed P values comparing the mean of Ht31 to the other treatments are indicated above the appropriate bar.
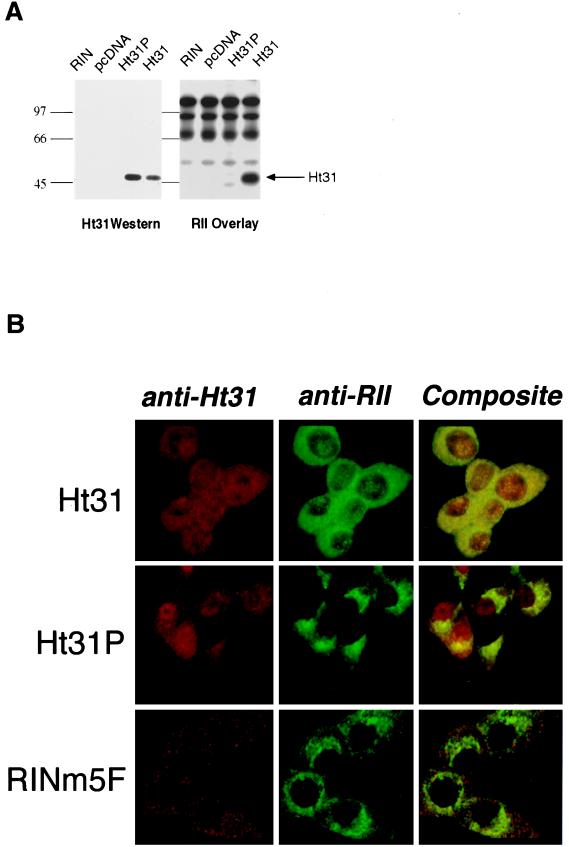
To evaluate more precisely the role of PKA anchoring in regulating insulin secretion a clonal rat beta cell line, RINm5F, was transfected with plasmids encoding a soluble Ht31 fragment (residues 418–718) or with a mutant form, Ht31P, which was unable to bind RII (Fig. 1A). Expression of both recombinant AKAP fragments was detected by immunoblot (Fig. 2A Left), but only the Ht31 fragment bound RII as assessed by overlay (Fig. 2A Right). Immunochemical analysis demonstrated the diffuse expression of the Ht31 and Ht31P proteins in the appropriate cells whereas only background staining was seen in the wild-type RINm5F cells (Fig. 2B). The intracellular location of RII was concentrated at perinuclear regions in cells expressing the Ht31P or RINm5F cells (Fig. 2B) similar to the RII distribution in other cells and tissues (6, 17, 18) including rat islet cells (data not shown). In contrast, RII was more evenly distributed throughout the cytoplasm in cells expressing the Ht31 fragment (Fig. 2B Top Center panel). This redistribution of RII by expression of the anchoring inhibitor protein confirms the in vitro disruption of RII–AKAP interactions in islets and RINm5F cells and is consistent with the role of these compounds to alter the subcellular location of the type II PKA holoenzyme (17, 18).
Figure 2.
Expression of recombinant anchoring inhibitor proteins displace PKA. (A) Expression of Ht31 (418–718) or control fragment Ht31P (418–718) in RINm5F cells was detected by immunoblot (Left) with affinity-purified rabbit polyclonal antibodies (1:500). The RII binding properties of the recombinant AKAP fragments were assessed by RII overlay (Right). Sample sources are indicated above each lane. Molecular mass markers (kDa) are indicated. (B) The subcellular distribution of Ht31 and RII in RINm5F cells was detected immunochemically with a Leitz Fluovert confocal photomicroscope. The cell lines are indicated next to each row. Ht31 staining (Left column), RII staining (Center column), and composite images of both signals (Right column) are presented.
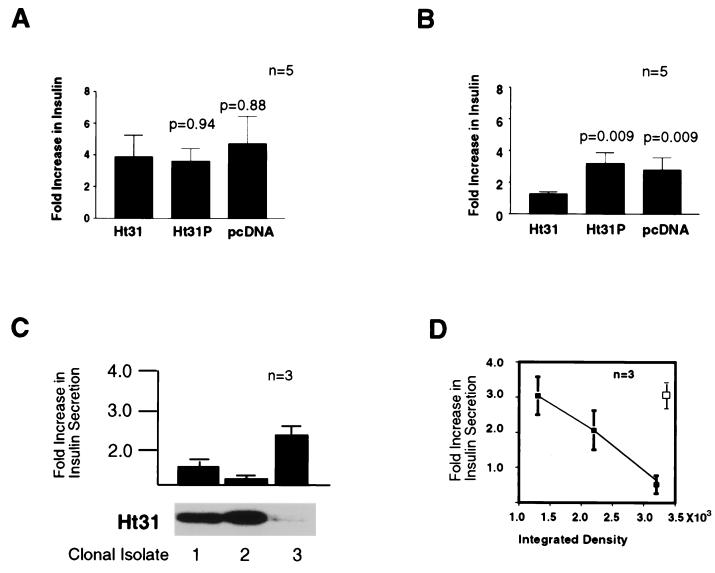
These clonal cell lines were used to confirm the finding of attenuated hormone-mediated insulin secretion in primary rat islets treated with the anchoring inhibitor peptides. Because RINm5F cells have reduced sensitivities to GLP-1 and glucose (26) we used dibutyryl-cAMP as an index of GLP-1 action and membrane depolarization with KCl as an indicator of PKA-independent insulin secretion. The response to 40 mM KCl was similar in all three groups (Fig. 3A), whereas cAMP-mediated insulin secretion was suppressed (1.2- ± 0.03-fold, n = 5) in cells expressing the active Ht31 fragment (Fig. 3C). Control cells expressing Ht31P or transfected with vector alone had cAMP-mediated insulin responses (Fig. 3C) similar to nontreated wild-type RINm5F cells (2.98- ± 0.8-fold above basal, n = 4). Suppression of cAMP-mediated insulin secretion was proportional (r = 0.99) to the level of Ht31 fragment expressed in RINm5F cells (Fig. 3D) as three independent clonal isolates exhibited variable levels of cAMP-responsive insulin secretion (Fig. 3 C and D). Only clonal isolates expressing moderate levels of Ht31 were used for the additional experiments. Collectively, these findings are consistent with our peptide-based studies in primary islets and confirm that PKA anchoring facilitates cAMP-mediated insulin secretion.
Figure 3.
Anchoring inhibitor expression blocks cAMP-mediated insulin secretion in beta cells. (A and B) RINm5F cells and clonal cell lines expressing the anchoring inhibitor proteins were monitored for (A) KCl-mediated insulin secretion and (B) cAMP-mediated insulin secretion by radioimmunoassay. Agonist-induced changes in insulin secretion are indicated as the fold increase over basal levels. Sample sources are indicated below each bar, and P values are for the mean ± SE (n = 5). (C) cAMP-mediated insulin secretion in three clonal RINm5F cell lines expressing different amounts of Ht31 determined by immunoblot (32) was measured by radioimmunoassay. Insulin secretion is indicated as the fold increase over basal levels. Data depict a representative of three separate experiments. (D) Increased Ht31 expression (closed squares) was inversely proportional to cAMP-mediated insulin secretion (r = 0.99) but not Ht31P expression (open square). AKAP fragment concentrations were estimated by densitometry. Data from three experiments are presented.
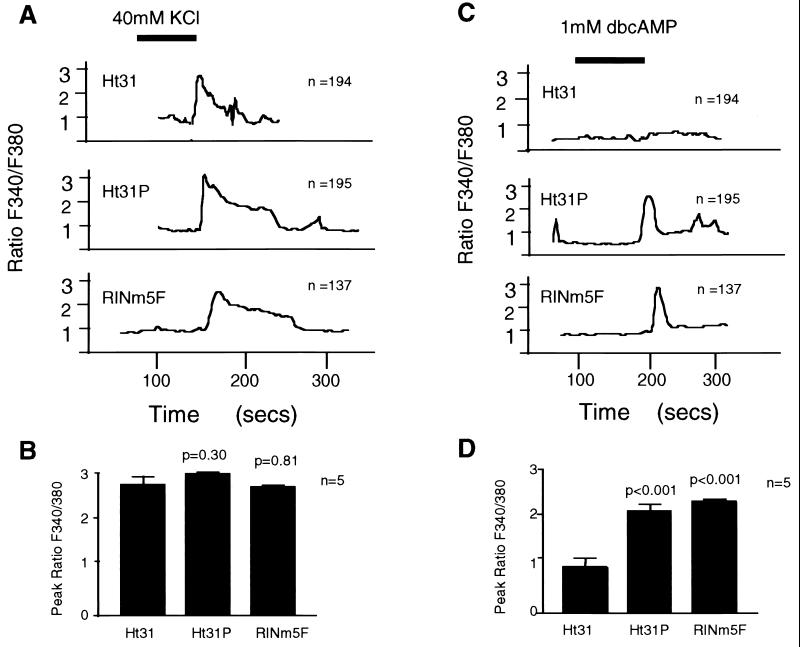
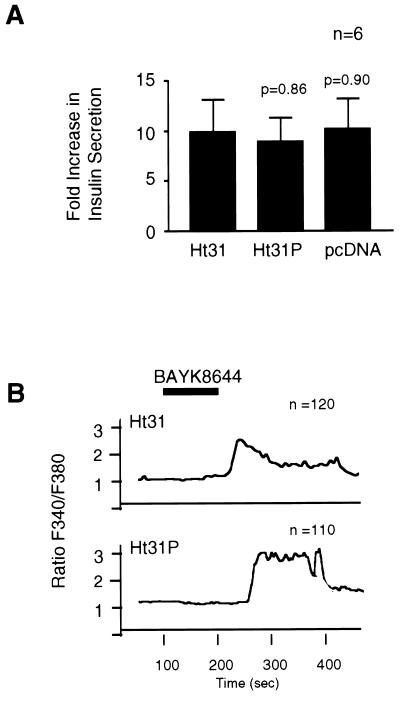
In an effort to identify how PKA anchoring facilitates cAMP-mediated insulin secretion, we monitored the intracellular calcium response of RINm5F cells expressing Ht31, Ht31P, or vector alone. Increased intracellular calcium levels were detected in response to depolarizing amounts of KCl in all cells (Fig. 4 A and B). However, the Ht31-expressing cells consistently failed (n = 120) to show an increase in intracellular calcium on application of dibutyryl-cAMP (Fig. 4 C and D). This suggests that anchored pools of PKA may facilitate cAMP-mediated phosphorylation events that modulate calcium fluxes from intracellular or extracellular sources. One site of PKA anchoring may be at or proximal to the L-type calcium channel as treatment with the agonist, BAYK8644, overcame the Ht31 effect on hormone-mediated insulin secretion (Fig. 5A) and calcium mobilization (Fig. 5B).
Figure 4.
Disrupting PKA anchoring blocks rise in intracellular calcium in RINm5F cells. The effect of PKA anchoring on changes in intracellular calcium levels in RINm5F cells and cell lines expressing AKAP fragments was assessed by single cell microfluorimetry on a Zeiss Axiophot scope with a ×40 objective attached to a photomultiplier. Changes in intracellular calcium (F340/F380 ratio) were detected by dual wavelength excitation at F340nm/F380nm with emission at 510 nm in response to (A) 40 mM KCl and (C) 1 mM dibutyryl-cAMP. Recordings were obtained every 4 s in a minimum of 15 cells per field. A representative response is shown for each experimental group, and the total number of cells evaluated is indicated in each panel. The cell source is indicated for each sample group. The time of agonist infusion is indicated by the solid bar. The peak F340/F380 ratio (from five individual experiments) is graphically depicted for the response to (B) 40 mM KCl and (D) 1 mM dibutyryl-cAMP. Two-tailed P values comparing the mean of Ht31 to the other cell lines is indicated above the appropriate bar.
Figure 5.
L-type calcium channel agonist partially restores insulin secretion and rise in intracellular calcium. RINm5F cell lines expressing AKAP fragments were treated with the L-type calcium channel agonist, BAYK8644 (33). (A) Depicts changes in insulin secretion on application of BAYK8644 (1 μM). Data are presented as the fold increase over basal levels. Data from six individual experiments are presented as the mean ± SE. The cell source is indicated below each bar. (B) Changes in intracellular calcium levels (340/380 ratio) on application of BAYK8644 were assessed by single cell microfluorimetry. Representative curves from cells expressing Ht31 and Ht31P are presented, and the number of cells analyzed is indicated. The time of agonist infusion is indicated by the solid bar.
DISCUSSION
Insulin secretion is regulated by a variety of second messenger-mediated signaling events that alter the dynamic balance in kinase and phosphatase activity in pancreatic beta cells (12). For example, activation of PKA by the hormone GLP-1 enhances protein phosphorylation and favors exocytosis of insulin secretory granules (14–16). In this study we demonstrate that the subcellular targeting of PKA by a family of targeting proteins called AKAPs is an additional mechanism in the regulation of hormone-mediated insulin secretion. By using specific anchoring inhibitor peptides or proteins in rat islets and clonal beta cells we determined the effect of AKAP-targeted PKA on hormone-mediated insulin secretion. We found that PKA anchoring was required for hormone-mediated insulin secretion whereas glucose-mediated insulin secretion was not significantly affected. The predominate effect on hormone-mediated insulin secretion is consistent with previous findings that PKA activity is not required for primary glucose-mediated insulin secretion (21, 22). Our findings indicate that the specific subcellular location of PKA is also a requirement for hormone-mediated insulin secretion.
We confirmed that this effect was related to the subcellular location of the kinase by measuring upstream components of the signaling cascade. There was no effect of the anchoring inhibitor peptides on the kinase activity or second messenger formation. In addition, the location of PKA in beta cells expressing the anchoring inhibitor proteins was diffuse throughout the cytoplasm whereas the control cells had a typical perinuclear concentration of PKA. This is consistent with the hypothesis that anchoring inhibitor compounds can disrupt AKAP targeting of PKA to specific subcellular locations (2, 6, 7). Previous studies have confirmed that these peptides are valuable reagents to disrupt the location of PKA as demonstrated by their ability to uncouple cAMP-responsive events such as the modulation of ion channels (4, 5). Despite the utility of these peptides and convincing evidence that the proline derivatives of these compounds are ineffective in the disruption of PKA anchoring we cannot rule out the possibility that the active anchoring inhibitor reagents are producing some secondary effects in situ.
Although the main pool of visualized RII is perinuclear previous work has established that small pools of targeted PKA are located at other subcellular sites and are critical for the regulation of specific cellular events (2, 4, 5, 7). In particular, the subcellular targeting of PKA by AKAPs has been shown to be required for the modulation of several channels (4, 5, 27, 28). Recent data in pancreatic beta cells have suggested that the L-type Ca2+ channel is a site for PKA phosphorylation (29); therefore the L-type Ca2+ channel may be one site for anchored PKA in pancreatic beta cells. Consistent with this hypothesis was the loss of elevation in intracellular calcium in response to cAMP but not to depolarization with KCl. This is supported by the reversal of the anchoring inhibitory effect on calcium and insulin secretion by the L-type channel agonist, BAYK8644. The hypothesis that PKA anchoring is modifying beta cell calcium channel activity is supported by evidence that PKA anchoring augments Ca2+ channel modulation (27, 28) and recent data showing that the beta cell L-type Ca2+ channel is phosphorylated by the kinase in RINm5F cells (29). The L-type calcium channel is one likely site for PKA targeting although other subcellular anchoring sites may exist. This concept of multiple pools of targeted PKA is supported by the data that beta cells express multiple AKAPs and PKA phosphorylates several substrates including the GLP-1 receptor and the glucose transporter GLUT2 (30, 31). We postulate that discrete pools of AKAP-targeted PKA are localized at several sites in beta cells and assist in integrating multiple signal transduction pathways. Additional work is in progress that will allow us to identify the specific beta cell sites for PKA targeting and dissect out the role of each on the coordinated insulin secretion.
Our results provide the first evidence that subcellular anchoring of PKA through association with AKAPs is a critical determinant in the coordination of a hormone-activated signaling pathway in intact cells. It is likely that alterations in the subcellular location of PKA or other signaling enzymes in beta cells could result in abnormalities in the regulation of insulin secretion. Therefore, understanding how pools of anchored PKA orchestrate signaling events may provide valuable insight into the mechanism of insulin secretion from pancreatic beta cells and ultimately the pathogenesis of beta cell dysfunction in non-insulin-dependent diabetes mellitus.
Acknowledgments
We thank Dr. John Rabkin and members of the Pancreatic Transplant Laboratory for the rat islet preps, J. Engstrom for assistance with confocal microscopy, and our colleagues at the Vollum Institute for critical evaluation of the manuscript. This work was supported in part by National Institutes of Health Grants DK02353 (to L.B.L.) and DK44239 (to J.D.S.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: PKA, cAMP-dependent protein kinase; AKAP, A-kinase-anchoring protein; GLP-1, glucagon-like peptide 1; KRBH buffer, Krebs-Ringer Hepes buffer.
References
- 1.Mochly-Rosen D. Science. 1995;268:247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- 2.Faux M C, Scott J D. Trends Biochem Sci. 1996;21:312–315. [PubMed] [Google Scholar]
- 3.Hubbard M, Cohen P. Trends Biochem Sci. 1993;18:172–177. doi: 10.1016/0968-0004(93)90109-z. [DOI] [PubMed] [Google Scholar]
- 4.Rosenmund C, Carr D W, Bergeson S E, Nilaver G, Scott J D, Westbrook G L. Nature (London) 1994;368:853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- 5.Johnson B D, Scheuer T, Catterall W A. Proc Natl Acad Sci USA. 1994;91:11492–11496. doi: 10.1073/pnas.91.24.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin C S. Biochim Biophys Acta. 1994;1224:467–479. [PubMed] [Google Scholar]
- 7.Dell’Acqua M L, Scott J D. J Biol Chem. 1997;272:12881–12884. doi: 10.1074/jbc.272.20.12881. [DOI] [PubMed] [Google Scholar]
- 8.Coghlan V M, Perrino B A, Howard M, Langeberg L K, Hicks J B, Gallatin W M, Scott J D. Science. 1995;267:108–112. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 9.Klauck T M, Faux M C, Labudda K, Langeberg L K, Jaken S, Scott J D. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 10.Faux M C, Scott J D. Cell. 1996;70:8–12. [Google Scholar]
- 11.Faux M C, Scott J D. J Biol Chem. 1997;272:17038–17044. doi: 10.1074/jbc.272.27.17038. [DOI] [PubMed] [Google Scholar]
- 12.Ammala C, Eliason L, Bokvist K, Berggren P O, Honkanen R E, Sjoholm A, Rorsman P. Proc Natl Acad Sci USA. 1994;91:4343–4347. doi: 10.1073/pnas.91.10.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjoholm A, Honkanen R E, Berggren P O. Endocrinology. 1995;136:3391–3397. doi: 10.1210/endo.136.8.7628374. [DOI] [PubMed] [Google Scholar]
- 14.Drucker D J, Philippe J, Mojsov S, Chick W L, Habener J F. Proc Natl Acad Sci USA. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorens B. Proc Natl Acad Sci USA. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaekura K, Kakei M, Yada T. Diabetes. 1996;45:295–301. doi: 10.2337/diab.45.3.295. [DOI] [PubMed] [Google Scholar]
- 17.Carr D W, Stofko-Hahn R E, Fraser I D C, Bishop S M, Acott T S, Brennan R G, Scott J D. J Biol Chem. 1991;266:14188–14192. [PubMed] [Google Scholar]
- 18.Carr D W, Hausken Z E, Fraser L D C, Stofko-Hahn R E, Scott J D. J Biol Chem. 1992;267:13376–13382. [PubMed] [Google Scholar]
- 19.Welsh N, Hallberg A, Sandier S, Helierstrom C. Diabetes. 1988;37:1123–1128. doi: 10.2337/diab.37.8.1123. [DOI] [PubMed] [Google Scholar]
- 20.Corbin J D, Reimann E M. Methods Enzymol. 1974;38:287–294. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- 21.Persaud S J, Jones P M, Howell S L. Biochem Biophys Res Commun. 1990;173:833–839. doi: 10.1016/s0006-291x(05)80862-9. [DOI] [PubMed] [Google Scholar]
- 22.Lu M, Wheeler M B, Leng X-H, Boyd A E. Endocrinology. 1993;32:94–100. doi: 10.1210/endo.132.1.8380389. [DOI] [PubMed] [Google Scholar]
- 23.Yada T, Itoh K, Nakata M. Endocrinology. 1993;133:1685–1691. doi: 10.1210/endo.133.4.8404610. [DOI] [PubMed] [Google Scholar]
- 24.Gromada J, Rorsman P, Dissing S, Wulff B S. FEBS Lett. 1995;373:182–186. doi: 10.1016/0014-5793(95)01070-u. [DOI] [PubMed] [Google Scholar]
- 25.Cullinan C A, Brady E J, Saperstein R, Leibowitz M D. Cell Calcium. 1994;15:391–400. doi: 10.1016/0143-4160(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe Y, Kawai K, Ohashi S, Yokota C, Suzuki S, Yamashita K. J Endocrinol. 1994;140:45–52. doi: 10.1677/joe.0.1400045. [DOI] [PubMed] [Google Scholar]
- 27.Johnson B D, Brousal J P, Peterson B Z, Gallombardo P A, Hockerman G H, Lai Y, Scheuer T, Catterall W A. J Neurosci. 1997;17:1243–1255. doi: 10.1523/JNEUROSCI.17-04-01243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray P C, Tibbs V C, Catterall W A, Murphy B J. J Biol Chem. 1997;272:6297–6302. doi: 10.1074/jbc.272.10.6297. [DOI] [PubMed] [Google Scholar]
- 29.Safayhi H, Haase H, Kramer U, Bihlmayer A, Roenfeldt M, Ammon H P T, Froschmayr M, Cassidy T N, Morano I, Ahlijanian M K, Striessnig J. Mol Endocrinol. 1997;11:619–629. doi: 10.1210/mend.11.5.9922. [DOI] [PubMed] [Google Scholar]
- 30.Thorens B, Deriaz N, Bosco D, DeVos A, Pipeleers D, Schuit F, Meda P, Porret A. J Biol Chem. 1996;271:8075–8081. doi: 10.1074/jbc.271.14.8075. [DOI] [PubMed] [Google Scholar]
- 31.Widmann C, Dolci W, Thorens B. Mol Endocrinol. 1996;10:62–75. doi: 10.1210/mend.10.1.8838146. [DOI] [PubMed] [Google Scholar]
- 32.Hausken Z E, Coghlan V M, Schafer Hastings C A, Reimann E M, Scott J D. J Biol Chem. 1994;269:24245–24251. [PubMed] [Google Scholar]
- 33.Larsson-Nyren G, Sehim J. Biochem J. 1996;314:167–173. doi: 10.1042/bj3140167. [DOI] [PMC free article] [PubMed] [Google Scholar]