Abstract
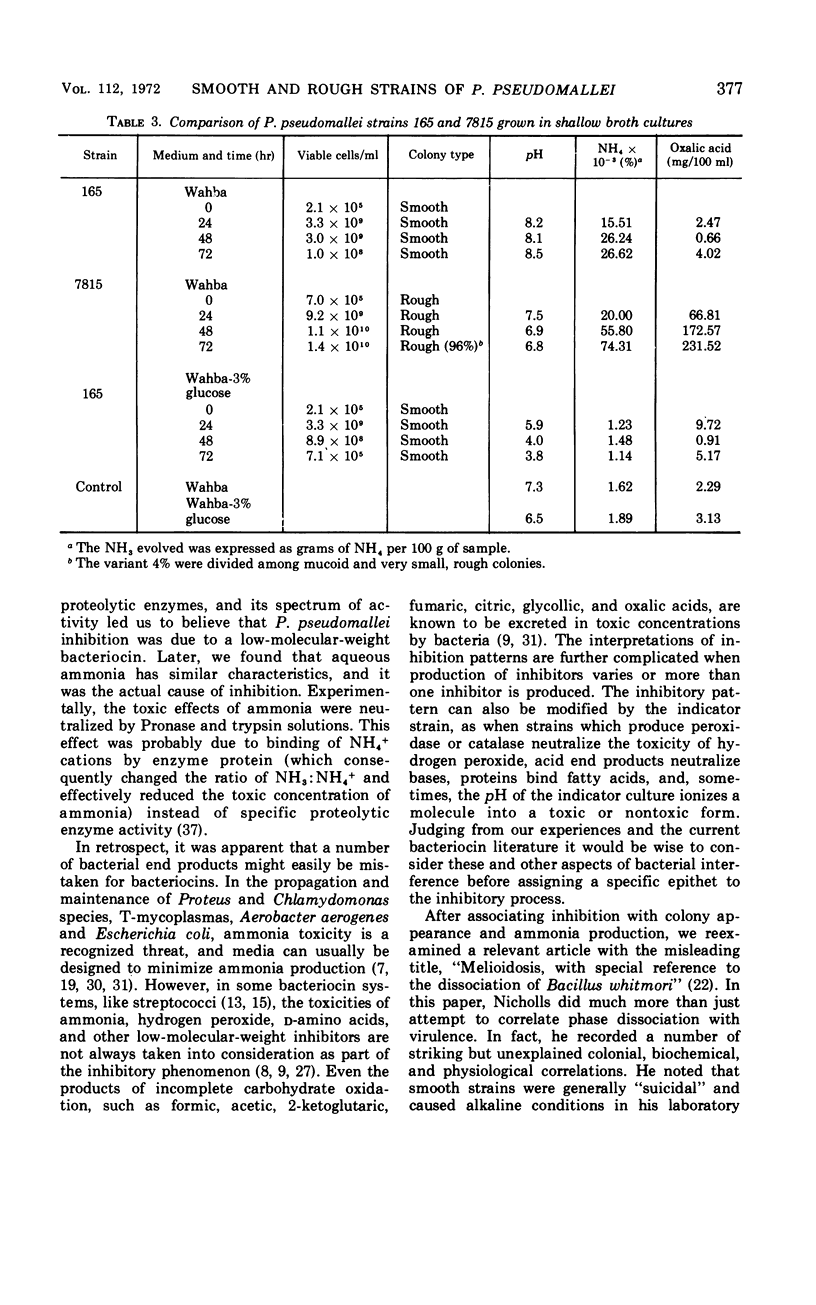
The colonial morphology of some strains of Pseudomonas pseudomallei was correlated with certain biochemical and physiological traits. After 3 days of growth on Wahba or heart infusion agars, smooth-colony strains generated toxic amounts of ammonia. Under the same conditions, the rough strains simultaneously produced oxalic acid which decreased the inhibitory concentration of ammonia. The ammonia-ammonium concentrations in smooth cultures exhibited certain bacteriocin-like characteristics. An unusually stable, smooth strain (strain 165) was chosen to compare and emphasize any differences with typical, rough strain 7815. Three-day-old smooth cultures grown on Wahba agar containing 3% (w/v) glycerol demonstrated ammonia toxicity. The substitution of glucose for glycerol completely obviated this toxicity. In highly aerated Wahba broth containing glucose, the amount of ammonia found in strain 165 smooth cultures and the amount of oxalic acid found in strain 7815 rough cultures were greatly reduced. In Difco nitrate broth smooth strain 165 did not form gas, and it reduced nitrate to nitrite only. Strain 7815 produced a gas and reduced both nitrate and nitrite.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTENBERN R. A., WILLIAMS D. R., KELSH J. M., MAUZY W. L. Metabolism and population changes in Brucella abortus. II. Terminal oxidation and oxygen tension in population changes. J Bacteriol. 1957 Jun;73(6):697–702. doi: 10.1128/jb.73.6.697-702.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERK R. S. NUTRITIONAL STUDIES ON THE "AUTO-PLAQUE" PHENOMENON IN PSEUDOMONAS AERUGINOSA. J Bacteriol. 1963 Oct;86:728–734. doi: 10.1128/jb.86.4.728-734.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles A. M. Effect of growth substrate on enzymes of the citric and glyoxylic acid cycles in Thiobacillus novellus. Can J Microbiol. 1971 May;17(5):617–624. doi: 10.1139/m71-101. [DOI] [PubMed] [Google Scholar]
- DARRELL J. H., WAHBA A. H. PYOCINE-TYPING OF HOSPITAL STRAINS OF PSEUDOMONAS PYOCYANEA. J Clin Pathol. 1964 May;17:236–242. doi: 10.1136/jcp.17.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODLOW R. J., BRAUN W., MIKA L. A. The role of D-alanine in the growth and variation of Brucella abortus. Arch Biochem. 1951 Feb;30(2):402–406. [PubMed] [Google Scholar]
- Hentges D. J. Influence of pH on the inhibitory activity of formic and acetic acids for Shigella. J Bacteriol. 1967 Jun;93(6):2029–2030. doi: 10.1128/jb.93.6.2029-2030.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMESON J. E. An antibiotic from Pfeifferella whitmori. J Hyg (Lond) 1949 Jun;47(2):142-5, pl. doi: 10.1017/s002217240001439x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Influence of carbon or nitrogen starvation on amino acid transport in Pseudomonas aeruginosa. J Bacteriol. 1969 Oct;100(1):276–282. doi: 10.1128/jb.100.1.276-282.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelstrup J., Gibbons R. J. Bacteriocins from human and rodent streptococci. Arch Oral Biol. 1969 Mar;14(3):251–258. doi: 10.1016/0003-9969(69)90227-1. [DOI] [PubMed] [Google Scholar]
- Kuttner A. G. Production of bacteriocines by group A streptococci with special reference to the nephritogenic types. J Exp Med. 1966 Sep 1;124(3):279–291. doi: 10.1084/jem.124.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE H. B., WOLOCHOW H. Occurrence of poly-beta-hydroxybutyrate in Pseudomonas pseudomallei. J Bacteriol. 1960 Feb;79:305–306. doi: 10.1128/jb.79.2.305-306.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- MacKelvie R. M., Campbell J. J., Gronlund A. F. Absence of storage products in cultures of Pseudomonas aeruginosa grown with excess carbon or nitrogen. Can J Microbiol. 1968 Jun;14(6):627–631. doi: 10.1139/m68-105. [DOI] [PubMed] [Google Scholar]
- OSMAN M. A. PYOCINE TYPING OF PSEUDOMONAS AERUGINOSA. J Clin Pathol. 1965 Mar;18:200–202. doi: 10.1136/jcp.18.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Shiio I. Regulation of the TCA and glyoxylate cycles in Brevibacterium flavum. I. Ingibition of isocitrate lyase and isocitrate dehydrogenase by organic acids related to the TCA and glyoxylate cycles. J Biochem. 1968 Sep;64(3):355–363. doi: 10.1093/oxfordjournals.jbchem.a128902. [DOI] [PubMed] [Google Scholar]
- ROSEBURY T., GALE D., TAYLOR D. F. An approach to the study of interactive phenomena among microorganisms indigenous to man. J Bacteriol. 1954 Feb;67(2):135–152. doi: 10.1128/jb.67.2.135-152.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Rogul M., Brendle J. J., Haapala D. K., Alexander A. D. Nucleic acid similarities among Pseudomonas pseudomallei, Pseudomonas multivorans, and Actinobacillus mallei. J Bacteriol. 1970 Mar;101(3):827–835. doi: 10.1128/jb.101.3.827-835.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDERS E., HUDDLESON I. F. The influence of environmental conditions on the growth and dissociation of Brucella abortus. Am J Vet Res. 1956 Apr;17(63):324–330. [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Occurrence of urease in T strains of Mycoplasma. J Bacteriol. 1967 May;93(5):1513–1520. doi: 10.1128/jb.93.5.1513-1520.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Strange R. E., Hunter J. R. Substrate-accelerated death' of nitrogen-limited bacteria. J Gen Microbiol. 1966 Aug;44(2):255–262. doi: 10.1099/00221287-44-2-255. [DOI] [PubMed] [Google Scholar]
- Tomov A. Ts. Antagonizm u bakterii iz roda Malleomyces. Zh Mikrobiol Epidemiol Immunobiol. 1970 Jun;47(6):105–108. [PubMed] [Google Scholar]
- WAHBA A. H. THE PRODUCTION AND INACTIVATION OF PYOCINES. J Hyg (Lond) 1963 Dec;61:431–441. doi: 10.1017/s0022172400021057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabransky R. J., Day F. E. Pyocine typing of clinical strains of Pseudomonas aeruginosa. Appl Microbiol. 1969 Feb;17(2):293–296. doi: 10.1128/am.17.2.293-296.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierdt C. H. Autolytic nature of iridescent lysis in Pseudomonas aeruginosa. Antonie Van Leeuwenhoek. 1971;37(3):319–337. doi: 10.1007/BF02218503. [DOI] [PubMed] [Google Scholar]


