Abstract
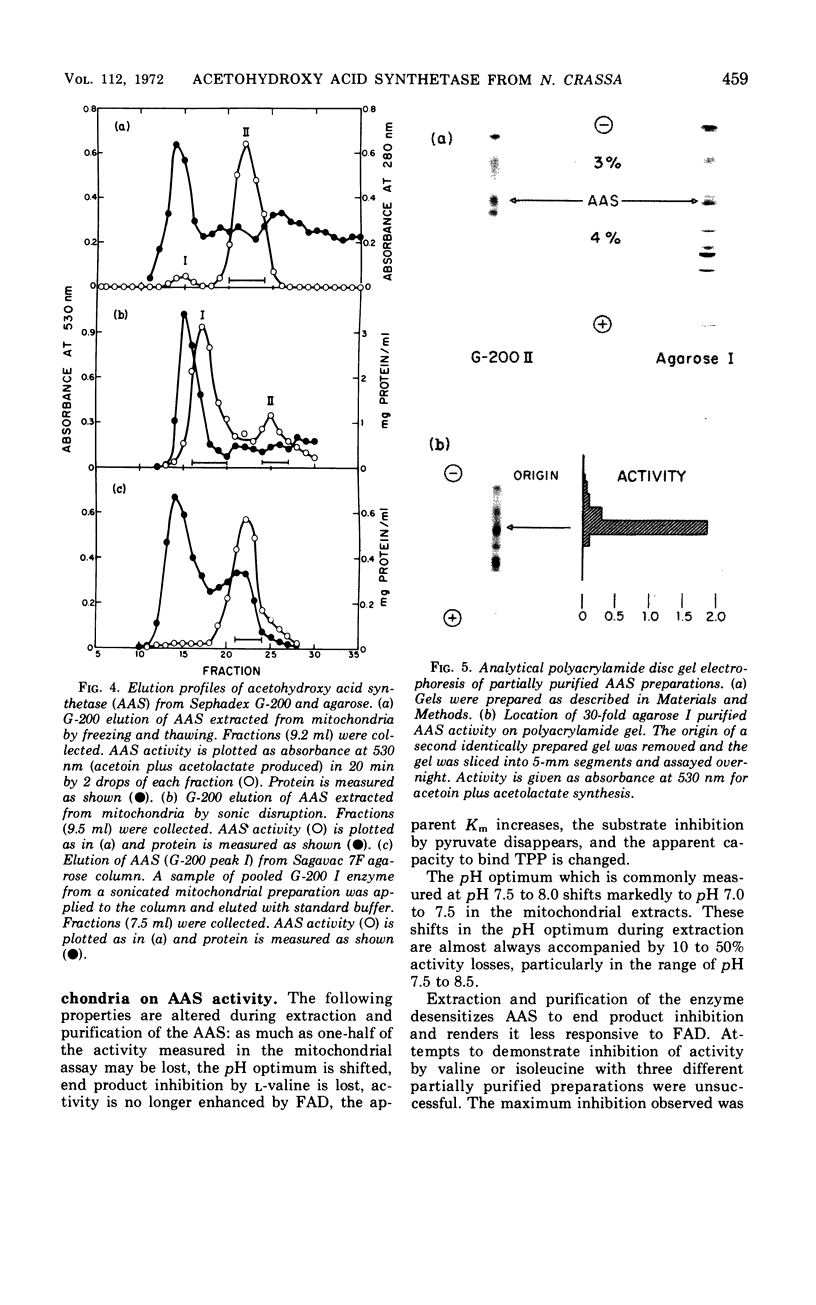
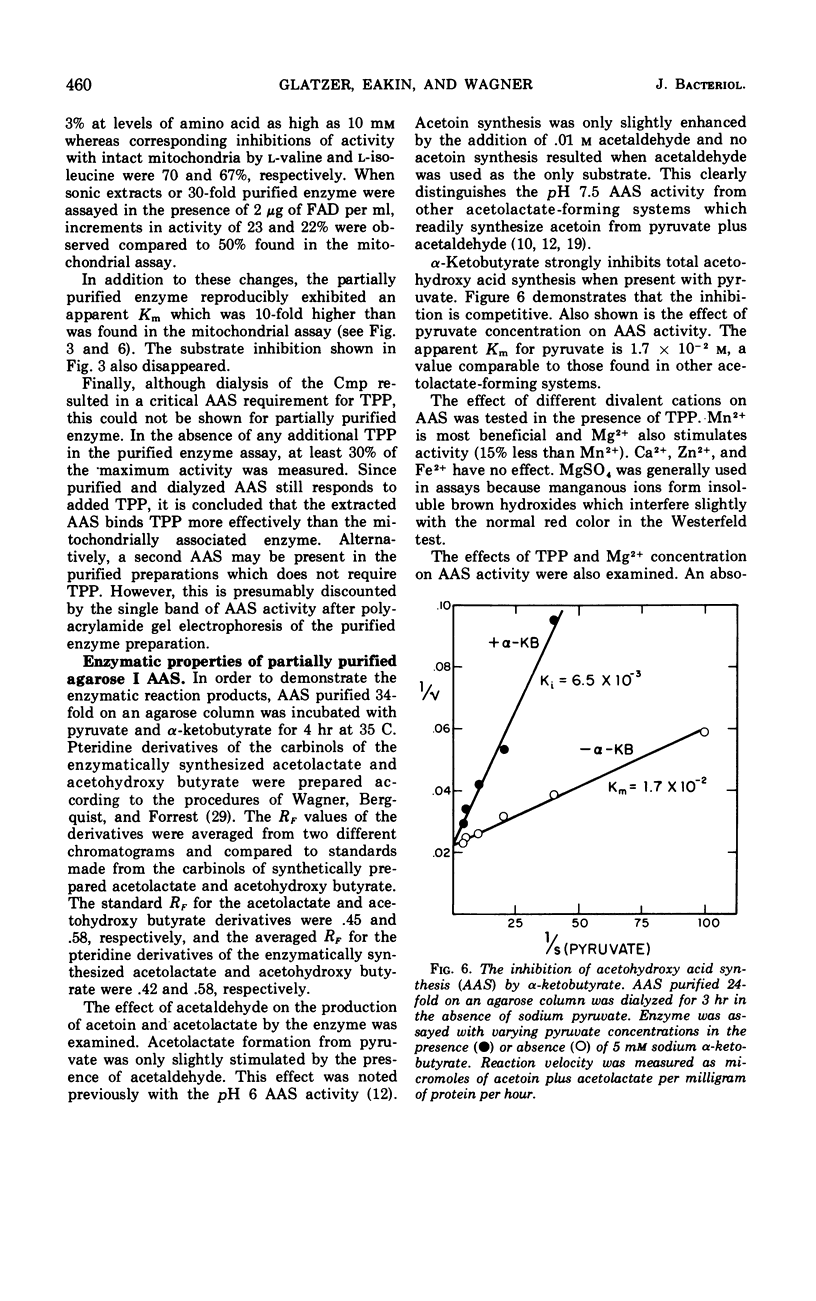
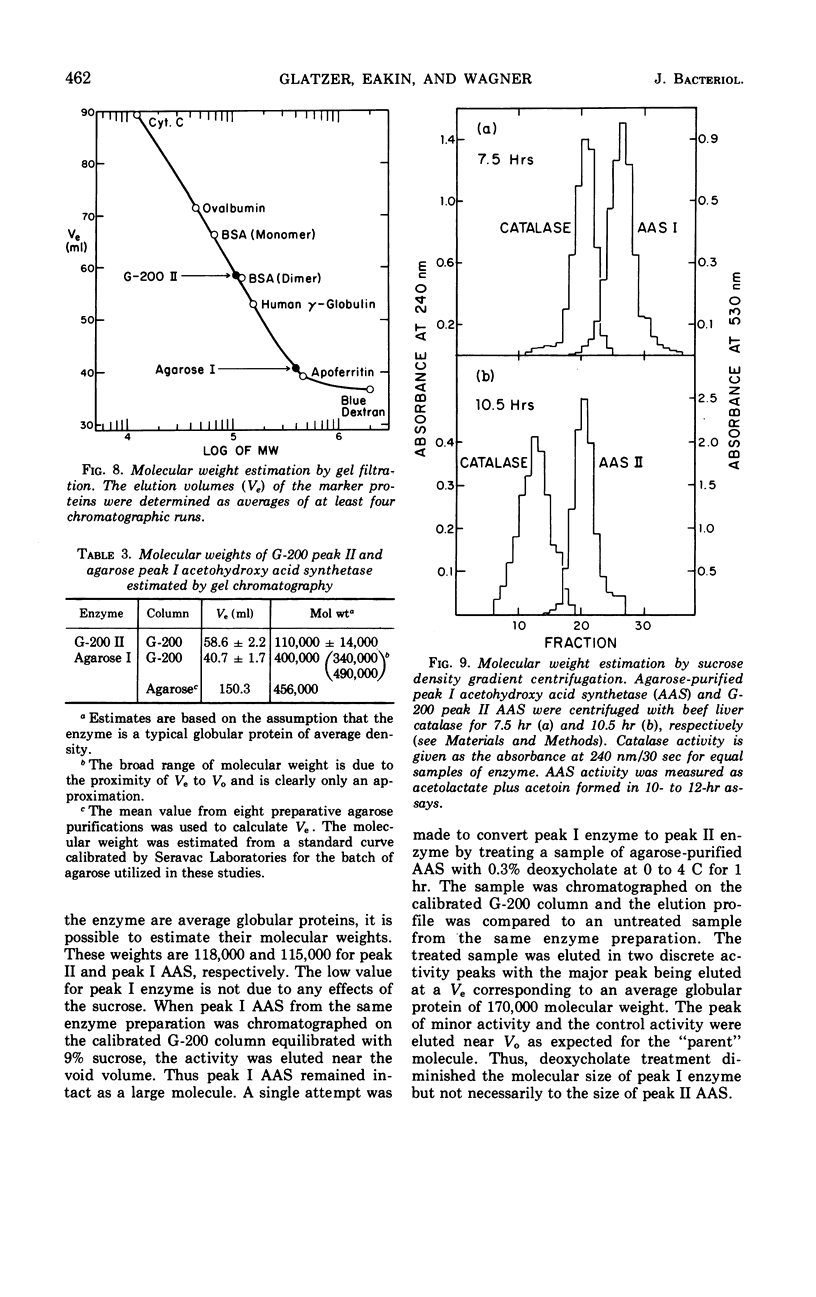
An acetohydroxy acid synthetase (AAS) has been found associated with the mitochondrial fraction of wild-type Neurospora crassa. It has a pH optimum of 7.5 and is presumed to be homologous to the pH 8.0 AAS that synthesizes the valine and isoleucine precursors in bacteria and yeast. The enzyme was characterized and purified 30- to 60-fold. The AAS activity of intact mitochondria requires thiamine pyrophosphate (TPP), Mn2+ or Mg2+, and flavine adenine dinucleotide (FAD), and is sensitive to end product inhibition by l-valine. This inhibition is pH-dependent and noncompetitive with respect to pyruvate. Activity is slightly repressed during exponential growth in the presence of valine, isoleucine, and leucine. Extraction of the AAS from the mitochondria has a profound influence on the following properties: pH optimum, sensitivity to l-valine, response to FAD, binding of TPP, apparent Km, and stability at 0 to 4 C. The catalytic properties of the partially purified enzyme are described. Two forms of the partially purified AAS can be isolated from preparative Sephadex G-200 chromatographic columns. Both forms are electrophoretically and antigenically similar but one form has an estimated molecular weight of 110,000 to 120,000 whereas the predominant form is a much larger and more buoyant molecule.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUERLE R. H., FRUENDLICH M., STORMER F. C., UMBARGER H. E. CONTROL OF ISOLEUCINE, VALINE AND LEUCINE BIOSYNTHESIS. II. ENDPRODUCT INHIBITION BY VALINE OF ACETOHYDROXY ACID SYNTHETASE IN SALMONELLA TYPHIMURIUM. Biochim Biophys Acta. 1964 Oct 23;92:142–149. [PubMed] [Google Scholar]
- Caroline D. F., Harding R. W., Kuwana H., Satyanarayana T., Wagner R. P. The iv-3 mutants of Neurospora crassa. II. Activity of acetohydroxy acid synthetase. Genetics. 1969 Jul;62(3):487–494. doi: 10.1093/genetics/62.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady W. E., Leiter E. H., Bergquist A., Wagner R. P. Separation of mitochondrial membranes of Neurospora crassa. II. Submitochondrial localization of the isoleucine-valine biosynthetic pathway. J Cell Biol. 1972 Apr;53(1):66–72. doi: 10.1083/jcb.53.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Desai I. D., Polglase W. J. Characterization and properties of acetohydroxy acid synthetase of streptomycin-dependent Escherichia coli. Biochim Biophys Acta. 1965 Oct 25;110(1):181–188. doi: 10.1016/s0926-6593(65)80107-2. [DOI] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Evidence for two distinct enzyme systems forming acetolactate in Aerobacter aerogenes. J Biol Chem. 1959 Dec;234:3067–3071. [PubMed] [Google Scholar]
- Hall D. O., Greenawalt J. W. The preparation and biochemical properties of mitochondria from Neurospora crassa. J Gen Microbiol. 1967 Sep;48(3):419–430. doi: 10.1099/00221287-48-3-419. [DOI] [PubMed] [Google Scholar]
- Harding R. W., Caroline D. F., Wagner R. P. The pyruvate dehydrogenase complex from the mitochondrial fraction of Neurospora crassa. Arch Biochem Biophys. 1970 Jun;138(2):653–661. doi: 10.1016/0003-9861(70)90393-0. [DOI] [PubMed] [Google Scholar]
- JUNI E., HEYM G. A. Acyloin condensation reactions of pyruvic oxidase. J Biol Chem. 1956 Jan;218(1):365–378. [PubMed] [Google Scholar]
- Kuwana H., Caroline D. F., Harding R. W., Wagner R. P. An acetohydroxy acid synthetase from Neurospora crassa. Arch Biochem Biophys. 1968 Oct;128(1):184–193. doi: 10.1016/0003-9861(68)90021-0. [DOI] [PubMed] [Google Scholar]
- Kuwana H., Wagner R. P. The iv-3 mutants of Neurospora crassa. I. Genetic and biochemical characteristics. Genetics. 1969 Jul;62(3):479–485. doi: 10.1093/genetics/62.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Magee P. T., Hereford L. M. Multivalent repression of isoleucine- valine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1969 Jun;98(3):857–862. doi: 10.1128/jb.98.3.857-862.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P. T., Robichon-Szulmajster H. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. 2. Identification and characterization of mutants lacking the acetohydroxyacid synthetase. Eur J Biochem. 1968 Feb;3(4):502–506. doi: 10.1111/j.1432-1033.1967.tb19559.x. [DOI] [PubMed] [Google Scholar]
- RADHAKRISHANAN A. N., SNELL E. E. Biosynthesis of valine and isoleucine. 2. Formation of alpha-acetolactate and alpha-aceto-alpha-hydroxybutyrate in Neurospora crassa and Escherichia coli. J Biol Chem. 1960 Aug;235:2316–2321. [PubMed] [Google Scholar]
- SATYANARAYANA T., RADHAKRISHNAN A. N. BIOSYNTHESIS OF VALINE AND ISOLEUCINE IN PLANTS. I. FORMATION OF ALPHA-ACETOLACTATE IN PHASEOLUS RADIATUS. Biochim Biophys Acta. 1963 Sep 3;77:121–132. doi: 10.1016/0006-3002(63)90474-8. [DOI] [PubMed] [Google Scholar]
- SINGER T. P., PENSKY J. Mechanism of acetoin synthesis by alpha-carboxylase. Biochim Biophys Acta. 1952 Sep;9(3):316–327. doi: 10.1016/0006-3002(52)90167-4. [DOI] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Isoleucine and valine metabolism in Escherichia coli. VIII. The formation of acetolactate. J Biol Chem. 1958 Nov;233(5):1156–1160. [PubMed] [Google Scholar]
- UMBARGER H. E. Feedback control by endproduct inhibition. Cold Spring Harb Symp Quant Biol. 1961;26:301–312. doi: 10.1101/sqb.1961.026.01.036. [DOI] [PubMed] [Google Scholar]
- Varga J. M., Horváth I. Studies on acetohydroxy acid synthetase in Pseudomonas aeruginosa. J Mol Biol. 1965 Sep;13(2):596–599. doi: 10.1016/s0022-2836(65)80121-8. [DOI] [PubMed] [Google Scholar]
- WAGNER R. P., BERGQUIST A., BARBEE T. THE SYNTHESIS IN VITRO OF VALINE AND ISOLEUCINE FROM PYRUVATE AND ALPHA-KETOBUTYRATE IN NEUROSPORA. Biochim Biophys Acta. 1965 May 4;100:444–450. doi: 10.1016/0304-4165(65)90014-0. [DOI] [PubMed] [Google Scholar]
- WAGNER R. P., BERGQUIST A., FORREST H. S. The accumulation of acetylmethylcarbinol and acetylethylcarbinol by a mutant of Neurospora crassa and its significance in the biosynthesis of isoleucine and valine. J Biol Chem. 1959 Jan;234(1):99–104. [PubMed] [Google Scholar]
- WAGNER R. P., BERGQUIST A. Synthesis of valine and isoleucine in the presence of a particulate cell fraction of Neurospora. Proc Natl Acad Sci U S A. 1963 Jun;49:892–897. doi: 10.1073/pnas.49.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]