Abstract
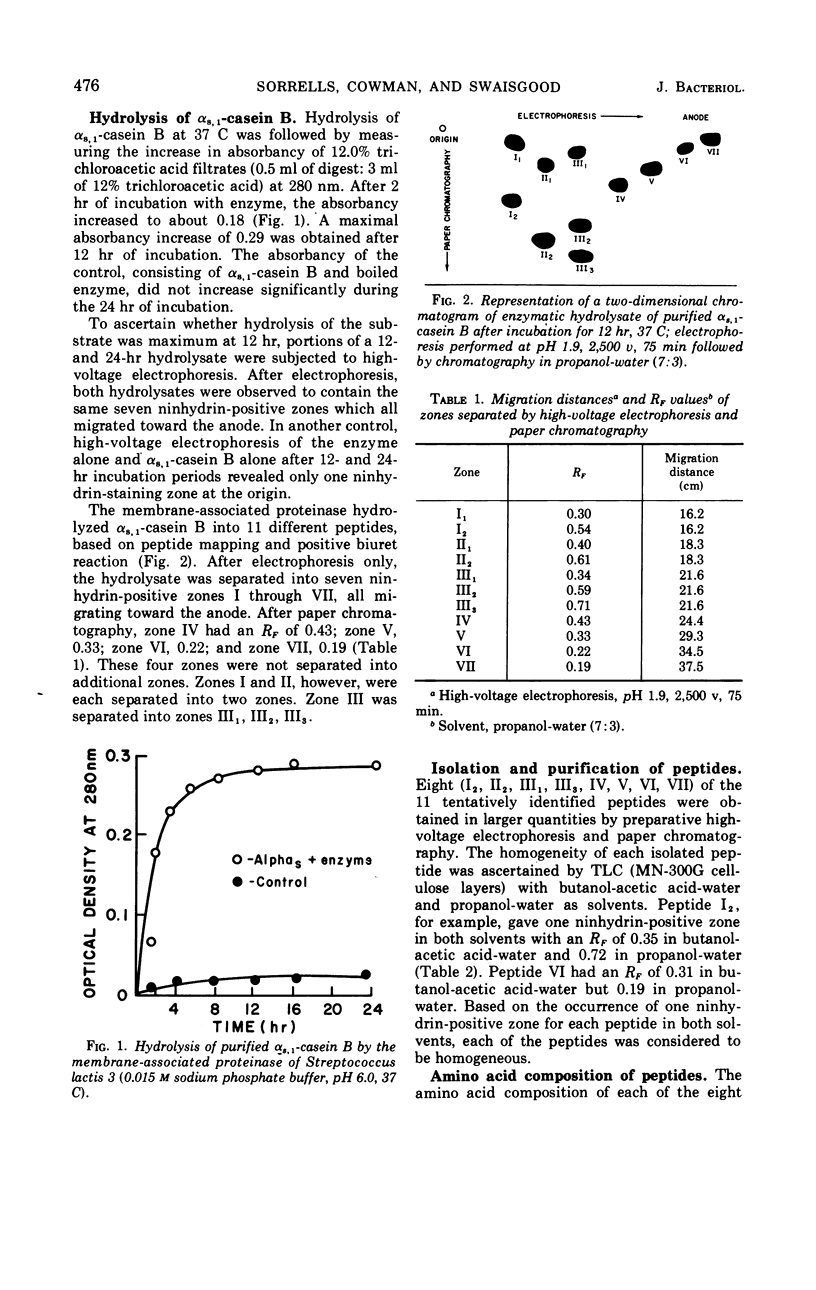
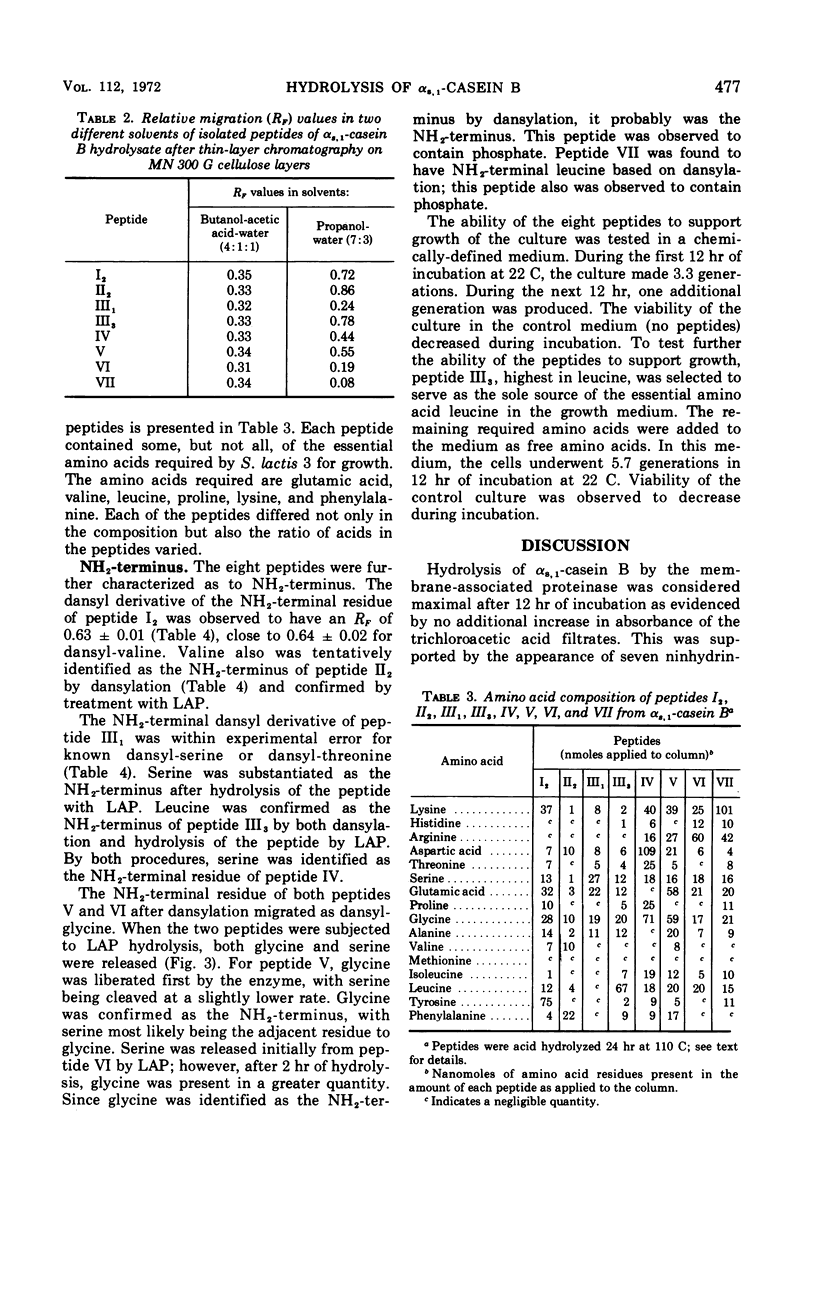
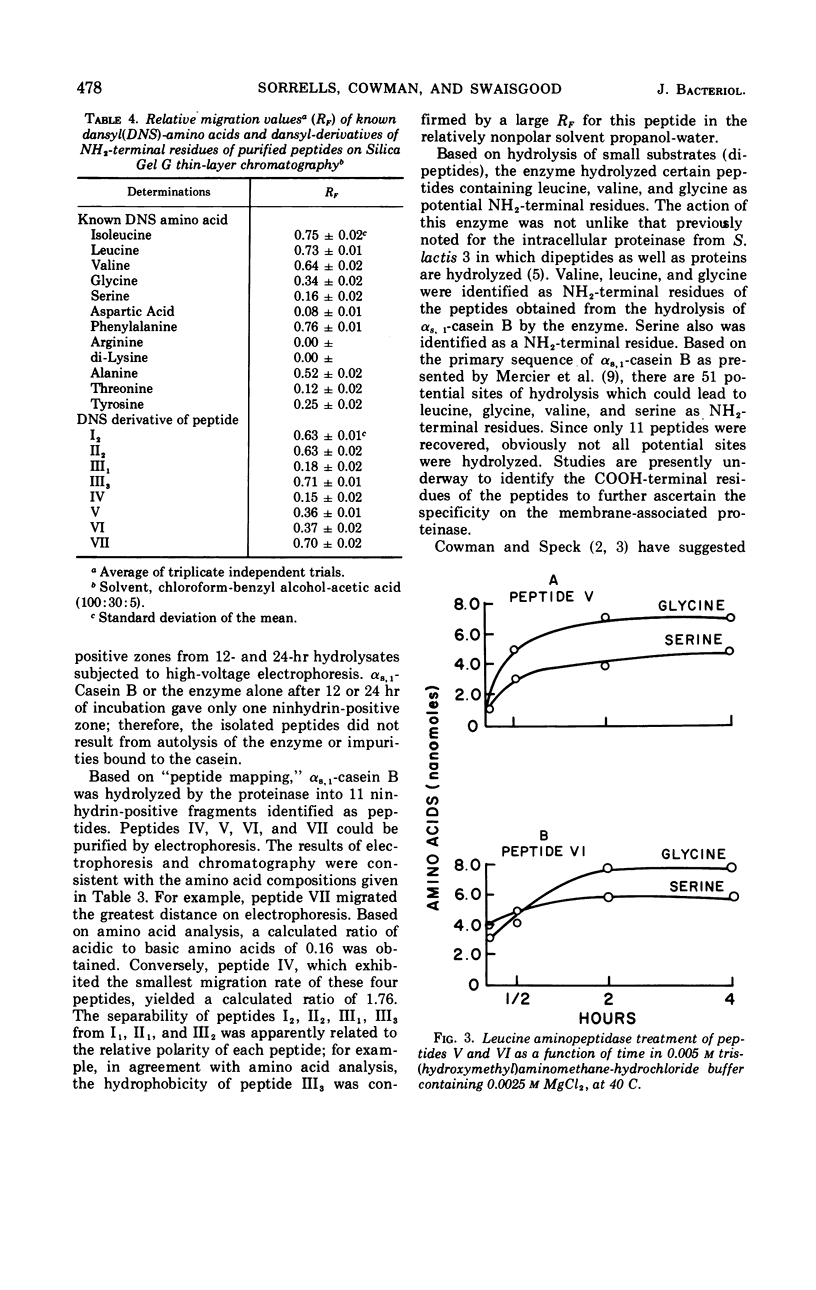
The membrane-associated proteinase of Streptococcus lactis strain 3 hydrolyzed αs, 1-casein B into 11 peptide fragments. Eight of the 11 peptides were purified and partially characterized. Each peptide contained several, but not all six, essential amino acids required for growth. The culture was able to utilize one peptide as the sole source for the essential amino acid leucine. Leucine, serine, valine, and glycine were found to be NH2-terminal residues. Two of the peptides were phosphopeptides. The data support the functional role of the membrane-associated proteinase as being involved in the initial breakdown of proteins to peptides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cowman R. A., Speck M. L. Low temperature as an environmental stress on microbial enzymes. Cryobiology. 1969 Mar-Apr;5(5):291–299. doi: 10.1016/s0011-2240(69)80457-8. [DOI] [PubMed] [Google Scholar]
- Cowman R. A., Swaisgood H. E., Speck M. L. Proteinase enzyme system of lactic streptococci. II. Role of membrane proteinase in cellular function. J Bacteriol. 1967 Oct;94(4):942–948. doi: 10.1128/jb.94.4.942-948.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman R. A., Yoshimura S., Swaisgood H. E. Proteinase enzyme system of lactic streptococci. 3. Substrate specificity of Streptococcus lactis intracellular proteinase. J Bacteriol. 1968 Jan;95(1):181–187. doi: 10.1128/jb.95.1.181-187.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- HILL R. L., SMITH E. L. Leucine aminopeptidase. VII. Action on long chain polypeptides and proteins. J Biol Chem. 1957 Oct;228(2):577–600. [PubMed] [Google Scholar]
- Mercier J. C., Grosclaude F., Ribadeau-Dumas B. Structure primaire de la caséine alpha S1 bovine. Séquence partielle. Eur J Biochem. 1970 Nov;16(3):453–460. doi: 10.1111/j.1432-1033.1970.tb01101.x. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Speck M. L. Identification of nutritional components in trypticase responsible for recovery of Escherichia coli injured by freezing. J Bacteriol. 1966 Mar;91(3):1098–1104. doi: 10.1128/jb.91.3.1098-1104.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff D. C., Cowman R. A. Influence of the growth medium on the proteinase system of Streptococcus lactis no. 3. J Dairy Sci. 1970 Sep;53(9):1286–1287. doi: 10.3168/jds.S0022-0302(70)86382-2. [DOI] [PubMed] [Google Scholar]


