Abstract
Two NADPH:cytochrome P450 oxidoreductases (CPRs) from parsley (Petroselinum crispum) were cloned, and the complete proteins were expressed and functionally identified in yeast. The two enzymes, designated CPR1 and CPR2, are 80% identical in amino acid sequence with one another and about 75% identical with CPRs from several other plant species. The mRNA accumulation patterns for CPR1 and CPR2 in fungal elicitor-treated or UV-irradiated cultured parsley cells and in developing or infected parsley plants were compared with those for cinnamate 4-hydroxylase (C4H), one of the most abundant CPR-dependent P450 enzymes in plants. All treatments strongly induced the mRNAs for C4H and CPR1 but not for CPR2, suggesting distinct metabolic roles of CPR1 and CPR2 and a functional relationship between CPR1 and C4H.
Keywords: cinnamate 4-hydroxylase, functional expression, phenylpropanoid metabolism, yeast
Cytochrome P450 enzymes are involved in a large variety of metabolic reactions. In plants, these reactions include various hydroxylation steps in secondary metabolism, such as the lignin, flavonoid, coumarin, and other phenylpropanoid biosynthetic pathways (1–3). All known plant cytochrome P450 monooxygenase reactions depend on the associated activity of an NADPH:cytochrome P450 oxidoreductase (CPR; EC 1.6.2.4) that catalyzes the transfer of electrons from NADPH via FAD and FMN to the prosthetic heme group of the P450 protein. Although for animal systems detailed characteristics including structural properties of CPRs have been reported (4), only few of these enzymes have been purified and cloned from plant sources (5–8).
Amino acid sequence comparison of all known CPRs revealed high degrees of similarity, particularly for several extended FAD, FMN, NADPH, and cytochrome P450 binding domains (9, 10). The combined occurrence and relative positions of these domains serve as characteristic features for identification of this class of enzymes. In accord with their membrane-associated functions, CPRs are localized in the endoplasmic reticulum and frequently have been used as a marker for this subcellular fraction (11). Although mammals and yeast contain only a single CPR (9, 12), two or even three isoforms each have been described for two plant species, Helianthus tuberosus (7) and Arabidopsis thaliana (8). However, the physiological relevance of the occurrence of multiple CPRs in these plants is unknown.
Our interest in CPRs initially arose from studies of the phenylpropanoid biosynthetic P450 monooxygenase, cinnamate 4-hydroxylase (C4H; EC 1.14.13.11), and its coregulation with phenylalanine ammonia-lyase (PAL; EC 4.3.1.5) and 4-coumarate:CoA ligase (4CL; EC 6.2.1.12) in cultured parsley cells (Petroselinum crispum). In this system, PAL, C4H, and 4CL are strongly, transiently, and coordinately induced by fungal elicitor or UV light and are regulated with the same high degree of coordination under all other conditions tested (13). Considering the functional dependence of C4H on CPR, we now asked whether CPR is included in this highly coordinated regulation and, if more than one isoform exists, whether or not they are similarly regulated. Although induction of CPR activity in cultured parsley cells has been demonstrated previously (14), these questions have not been addressed. Here we report that parsley contains at least two CPRs with expression patterns suggesting differential functional associations with C4H and other P450 enzymes.
MATERIALS AND METHODS
Maintenance and Treatment of Plant Material.
Cell suspension cultures of parsley (Petroselinum crispum) were propagated for 6 days as previously described (15) and then treated with 1 μg/ml synthetic Pep25 oligopeptide elicitor (16). Equivalent amounts of water were added to control cultures. Cells were harvested at the indicated times, frozen in liquid nitrogen, and stored at −80°C. Leaves from adult parsley plants were infiltrated either with water or with a crude elicitor preparation from Phytophthora sojae (17).
RNA Isolation, Blot Analysis, and in Situ Hybridization.
Total RNA for blot analysis and for reverse transcriptase (RT)-PCR was prepared according to a published procedure (18) with the following modifications. The final pellet was resuspended in water that had been treated with diethylpyrocarbonate and adjusted to 2 M LiCl and then was incubated for at least 10 h at 4°C. The pellet obtained by centrifugation was washed once with 70% ethanol, dried, and resuspended in water. All procedures for RNA blot analysis (19), growth and inoculation of parsley seedlings, tissue sectioning, and in situ RNA/RNA hybridization (20) have been reported. [35S]UTP-labeled sense and antisense riboprobes were generated from SmaI- and MunI-linearized CPR1 and CPR2 cDNAs by using T3 and T7 polymerases.
Cloning and Sequencing of cDNAs.
Total RNA from untreated controls and from 4-h elicitor-treated parsley cells was transcribed into cDNA by using SuperScriptReverse Transcriptase (GIBCO/BRL). Two degenerate primers for PCR were deduced from sequence alignment of published cDNAs: 5′-GA(GA) CCI ACI GA(CT) AA(CT) GCI GC-3′ and 5′-TC(AG) AA(ACT) TCI A(GA)(GA) TG(TGA) (GA)T(GA) CA-3′. The DNA was amplified in a 50-μl reaction containing 60 pmol each of the two primers, cDNA derived from 0.5 μg total RNA, 1.5 mM MgCl2, 200 μM dNTPs, 1.25 units Taq DNA polymerase, and PCR buffer (GIBCO/BRL). The mixture was overlaid with mineral oil and subjected to 35 cycles of PCR amplification by using 94°C/1 min for denaturation, 45°C/1 min for annealing, and 72°C/1 min for primer extension. The resulting products were separated on an agarose gel. DNA fragments of the expected size (about 500 bp) were isolated, subcloned into SmaI-cleaved pBS vector (Stratagene), and used to screen a previously generated cDNA library (21). The PCR products and cDNAs were sequenced by using a 377 Automated Sequencer (Applied Biosystems). The C4H cDNA has been described elsewhere (13).
Expression and Functional Identification in Yeast.
The complete cDNAs were cloned as XbaI/KpnI (CPR1) or XbaI/XhoI (CPR2) fragments into the yeast expression vector p416Gal1 (22). The yeast strain W3036 was transformed by using the modified Li+ acetate procedure (23) and propagated as follows (24). Single colonies were taken from SGI plates, grown for 24 h at 30°C in 50 ml medium containing 2% glucose, and used to inoculate (1:20) two flasks, each containing 2% glucose in 200 ml yeast extract/peptone/glucose (YPG) medium. After growth for 24 h, the cells were pelleted for 1 min at 1,500 × g, washed once with sterile water to remove residual glucose, resuspended in 200 ml fresh YPG medium, containing either 2% glucose or 2% galactose/1% raffinose, and incubated for another 12 h. Microsomes were prepared as described (25), except that ultracentrifugation (100,000 × g for 1 h at 4°C) was employed instead of precipitation with polyethylene glycol.
CPR and C4H Activity Measurements.
By using parsley microsomes (26), CPR activity was determined (27) in a reaction mixture (1 ml) containing 100 mM sodium phosphate buffer (pH 7.4), 1 mM KCN, 0.15 mM NADH or NADPH, and 1–7.5 μg microsomal protein. The reaction was started by adding 50 μM cytochrome c and monitored at 25°C by using an absorbance wavelength of 550 nm (ɛcytochrome c = 21 mM−1 cm−1). C4H activity was measured (26) by using [3-14C]cinnamate as substrate [53.9 mCi/mmol (1 Ci = 37 GBq), Isotopchim, Ganagobie, France).
Production of Antisera.
The 3′ portion of the CPR2 cDNA (1,080-bp HindIII fragment) was cloned into the pQE30 vector for transformation of SG13009 cells (Qiagen, Chatsworth, CA) containing the pUBS 520 plasmid (28). Overnight cultures were used for 1:25 inoculation of 50-ml cultures in Double Yeast Tryptone medium. These cultures were grown to an optical density of 0.8 at 600 nm, then treated with 2 mM isopropylthiogalactopyranoside and incubated for another 2 h at 37°C. The induced partial CPR2 protein was purified on Ni-NTA-Agarose according to the manufacturer’s protocol (Qiagen) by using 8 M urea in all buffers and injected in four portions of 200 μg each (1 mg/ml) within 3 months into rabbits (Eurogentec, Brussels). The antiserum from the final bleeding was used. An analogous procedure was employed to prepare the C4H antiserum.
Analytical Methods.
After electrophoresis under denaturing conditions in 10% polyacrylamide gels (29) and electroblotting onto poly(vinylidene difluoride) membranes (Millipore; ref. 30), protein–antibody complexes were detected by using HRP-conjugated goat anti(rabbit IgG) antibodies (BioGenes, Berlin) and the ECL Detection System (Amersham). Protein was determined by the BCA Assay (Sigma) by using BSA as standard.
RESULTS
Isolation and Functional Identification of Two Distinct CPRs.
Two conserved amino acid sequences from the FAD and FMN binding domains, respectively, were deduced from CPR proteins from Catharanthus roseus (5), Vigna radiata (6), and Vicia sativa (GenBank accession no. Z26252), and degenerate oligonucleotide primers for these regions were synthesized. These primers enabled the isolation of two distinct RT-PCR fragments of the expected length of ≈500 bp. The two fragments were about 78% identical in nucleotide sequence and exhibited a similar degree of identity with CPRs from other plant species.
Both fragments served as probes for screening of a cDNA library generated with pooled mRNA fractions from 0.5-, 1.5-, and 3-h elicitor-treated parsley cells (21). The resulting cDNAs, designated CPR1 and CPR2, are both about 2.5 kb in length and very probably contain the complete coding regions. Although the putative translational start codon of CPR2 is located 12 nt downstream from a stop codon, CPR1 contains two possible start codons and no preceding stop codon (see Fig. 1 for alternative N termini of the deduced protein). Because an acidic amino acid residue in the second position is probably required for retention of the protein in the endoplasmic reticulum (31), we assume that the start codon located farther downstream is the authentic one. On this basis, the relative molecular masses and isoelectric points of the deduced proteins were calculated to be 77,390 Da and 5.24 for CPR1, and 75,673 Da and 5.14 for CPR2.
Figure 1.
Comparison of deduced amino acid sequences for CPR1 and CPR2 from parsley (Pc, Petroselinum crispum) and a previously described CPR from Catharanthus roseus (5) by using the GAP program from the GCG package with the gap weight set at 3.0 and the gap length set at 0.1. Identical amino acids are marked in gray, and putative cytochrome P450 and cofactor binding domains (8, 9) are indicated by brackets.
The two proteins are 80% identical in amino acid sequence. They are compared in Fig. 1 with the enzyme from C. roseus, which shares 78% sequence identity with both of them. Except for highly divergent N termini, the degree of identity is similar throughout the entire proteins. Hydropathy plots (32) suggest that the first 55 aa of CPR1 and the first 31 aa of CPR2 form hydrophobic domains that may act as membrane anchors, in agreement with an earlier proposal for proteins associated with the endoplasmic reticulum (33). These domains end with a WRR sequence, i.e., with the characteristic WBB motif of all plant CPRs (where B represents a basic amino acid; ref. 34). Most characteristically, however, both CPR1 and CPR2 contain all established cofactor and cytochrome P450 binding domains (Fig. 1) whose combined occurrence is typical for CPRs (9, 10, 12, 35, 36). In contrast to a recently reported CPR from Arabidopsis thaliana (8), neither CPR1 nor CPR2 possesses an N-terminal serine/threonine-rich stretch of amino acids as a possible indication of plastid localization.
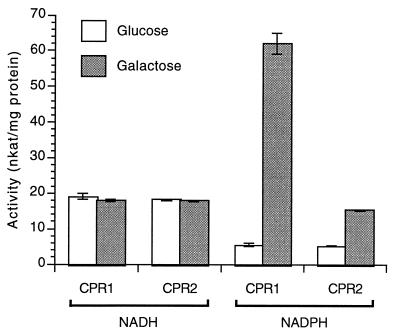
The high structural similarities of CPR1 and CPR2 with authentic CPRs, including the C. roseus CPR shown in Fig. 1, suggested similar functions. To verify this conclusion, both proteins were expressed in yeast and functionally assayed in microsomal extracts. As a prerequisite for the distinction of endogenous and ectopically expressed CPR activity, the two cDNAs were cloned into the galactose-inducible yeast expression vector p416Gal1, allowing controlled expression of the foreign gene by growth on either glucose (repression) or galactose (induction) (22). Fig. 2 shows that the transformed yeast cells contained about 10-fold (CPR1) and 3-fold (CPR2) higher CPR activity in the presence of NADPH when grown on galactose as compared with glucose. Because all CPR proteins are known to be strictly NADPH-dependent (24), a control experiment was performed by using NADH as reducing agent. In this case, the conversion rates were equal under all tested conditions (Fig. 2), further confirming that CPR1 and CPR2 are members of the widely occurring, structurally closely related CPR family.
Figure 2.
Total NADH- and NADPH-dependent CPR activities in microsomes isolated from yeast cells expressing either CPR1 or CPR2 from parsley and grown in the presence of either glucose or galactose as indicated. Mean values from three independent measurements using two independent clones each are given.
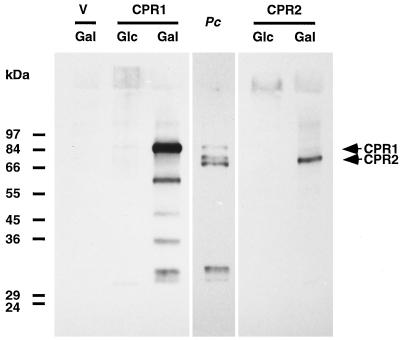
The expression of CPR1 and CPR2 in the transformed yeast cells was also demonstrated at the protein level. For this purpose, the C-terminal portion of CPR2 was selected for expression in Escherichia coli to raise a polyclonal antiserum in rabbits. Analogous attempts to express the complete CPR1 or CPR2 proteins were unsuccessful. The antiserum obtained was employed in Western blot analysis of the same microsomal extracts from transformed yeast cells as used above for CPR activity measurements. Fig. 3 shows that the yeast cells grown on galactose as an inducing agent contained appreciable amounts of immunoreactive proteins. A control lane containing a microsomal extract from parsley cells that had been treated for 20 h with elicitor demonstrated the presence of three proteins in the expected size range of around 70–84 kDa as well as some small putative degradation products. The two uppermost bands corresponded to the major protein bands detected in the CPR1 and CPR2 expressing yeast cells, respectively. The third band could be either a degradation product or an additional, as yet undetected, CPR. The detection of both CPR1 and CPR2 by the CPR2 antiserum is a result of a considerable degree of cross-reactivity (data not shown). The lower bands in the CPR1/Gal lane are again considered to be degradation products, as frequently observed with CPRs (37). Sizes somewhat larger than estimated for the putative undegraded CPR1 and CPR2 proteins may be a result of glycosylation, likewise a common feature of CPRs (38).
Figure 3.
Immunoblot analysis of microsomal proteins isolated from elicitor-treated parsley cells (Pc) or from yeast cells transformed either with the empty vector (V) or with the same vector containing CPR1 or CPR2 cDNA as indicated. Yeast cells were grown under either inducing (Gal) or repressing (Glc) conditions. Each lane contains 5 μg of protein. CPR2 antiserum was used at a dilution of 1:2,000. Positions of size markers are indicated.
mRNA Accumulation Patterns.
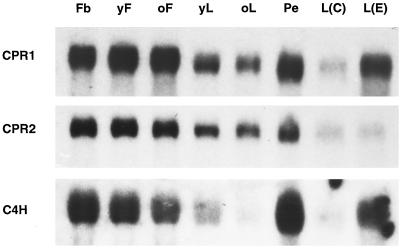
Because our interest in CPRs arose from studies of the regulation of general phenylpropanoid metabolism, with particular emphasis on the central CPR-dependent C4H reaction, C4H mRNA served as a reference throughout the following RNA blot analyses. In a first set of experiments, RNA samples from various aerial parts of parsley plants were tested for the relative abundance of CPR1, CPR2, and C4H mRNAs. Fig. 4 shows that all three mRNA levels were high in most of the tissues analyzed, although the relative proportions varied to some extent. The most clear-cut differential behavior of CPR1 and CPR2 mRNAs was observed in 8-h elicitor-treated leaves, where the CPR1 and C4H mRNAs were strongly induced from a low background, in contrast to a complete lack of CPR2 mRNA induction at this time point. Furthermore, these data confirmed the low degree of cross-hybridization between CPR1 and CPR2, which had been observed in several independent experiments (data not shown).
Figure 4.
Comparison of total extractable CPR1, CPR2, and C4H mRNA levels in various aerial parts of parsley plants. Blots from gels loaded with 15 μg RNA per lane were hybridized with the respective cDNAs and analyzed by autoradiography. Exposure time was 4 days. Fb, flower bud; yF, young flower; oF, old flower; yL, young leaf; oL, old leaf; Pe, pedicel; L(C), control leaf infiltrated with water; L(E), leaf infiltrated with elicitor.
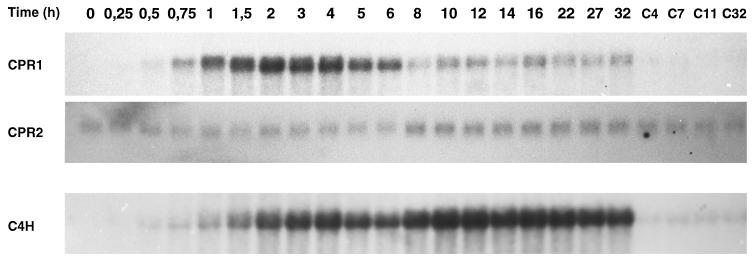
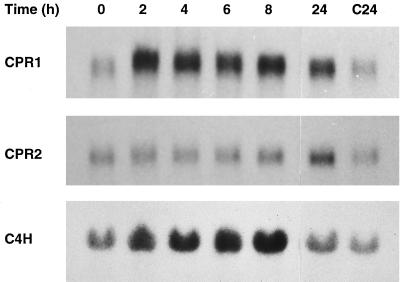
This differential response to elicitor was analyzed in more detail in cell suspension cultures of parsley. Fig. 5 shows the time courses of CPR1, CPR2, and C4H mRNA accumulation during the first 32 h after the addition of elicitor to cultured parsley cells. Again, the CPR1 and C4H mRNAs were strongly induced. However, although rapid and transient changes in the CPR1 mRNA level coincided to a large extent, though not fully, with a first peak of C4H mRNA accumulation, CPR2 mRNA was induced several hours later and much more weakly, to some extent concomitant with the second, major and more long-lasting peak of C4H mRNA accumulation.
Figure 5.
Relative timing of CPR1, CPR2, and C4H mRNA induction in elicitor-treated parsley cells. Total RNA was isolated at the indicated times after addition of the oligopeptide elicitor (C = control treatment with water). Blots from gels loaded with 10 μg total RNA per lane were hybridized with the respective cDNAs and analyzed by autoradiography. Exposure times were 8 h for CPR1 and C4H, and 4 days for CPR2.
In all cases analyzed so far, the induction of a given mRNA in elicitor-treated leaves or cultured cells of parsley has always mimicked an analogous response of parsley leaves at fungal infection sites. To see whether this also applies to CPR1 and CPR2, we measured the two mRNA levels by in situ hybridization in parsley leaf buds at an early time point postinoculation with Phytophthora sojae, a soybean pathogenic fungus to which parsley has previously been shown to react with a strong non-host-resistance response (39). An infection stage roughly equivalent to the 2-h time point in Fig. 5 was estimated to occur around 6 h postinoculation with fungal zoospores, allowing for several hours of fungal germination and formation of infection structures.
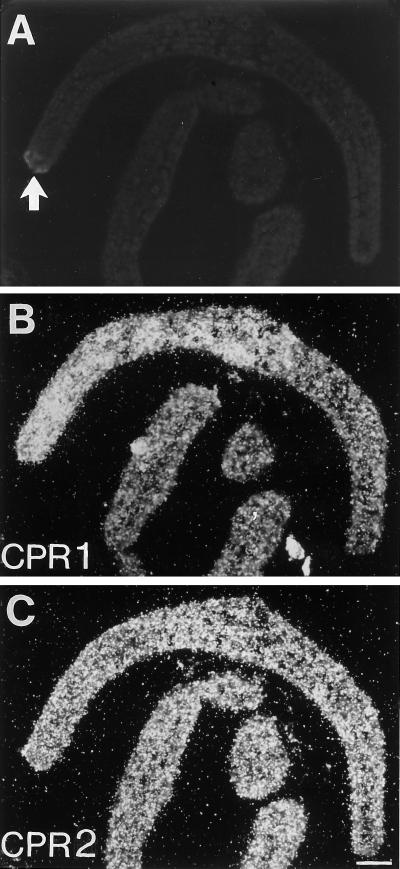
Fig. 6 demonstrates that, indeed, a small infection site at this early stage (Fig. 6A) was the apparent focal point of strong CPR1 mRNA accumulation (Fig. 6B), whereas a somewhat higher background level of CPR2 mRNA in a neighboring section remained unaltered (Fig. 6C). In both cases, immediately adjacent sections were used for control hybridizations with the respective sense RNA probes that gave low signal intensities throughout. The infection site was too small to obtain an additional section for C4H mRNA hybridization. However, results almost identical to those shown in Fig. 6B were recently obtained for C4H mRNA in a separate set of experiments (E.K., unpublished results) and have been reported for PAL and 4CL mRNA induction at similar times postinoculation (20).
Figure 6.
In situ localization of CPR1 and CPR2 mRNAs in parsley leaf buds 6 h postinoculation with Phytophthora sojae. Adjacent cross-sections were hybridized with 35S-labeled CPR1 (B) and CPR2 (C) antisense riboprobes. The fungal infection site was visualized by autofluorescence of the necrotic spot under UV/blue light of 365 nm (A, arrow) by using the same specimen as shown in C. Magnification bar represents 100 μm.
Of the various kinds of stress to which phenylpropanoid metabolism responds in parsley, including C4H mRNA induction (13), UV light has been extensively investigated (40) and therefore was included in this study. Fig. 7 shows the effects of irradiation with UV-containing white light on the CPR1, CPR2, and C4H mRNA levels in cultured parsley cells. Although the changes relative to control cells (0- and C24-h time points) were considerably smaller than those observed above in Fig. 5, a clear-cut differential response was again obvious. The CPR1 and C4H mRNAs were rapidly and concomitantly induced severalfold above the initial level, whereas CPR2 mRNA remained unaffected (Fig. 7).
Figure 7.
Relative timing of CPR1, CPR2, and C4H mRNA induction in cultured parsley cells irradiated with UV-containing white light. See legend of Fig. 5 for further details. Exposure times were 3 days for CPR1 and C4H, and 5 days for CPR2.
Elicitor-Induced CPR Protein and Enzyme Activity.
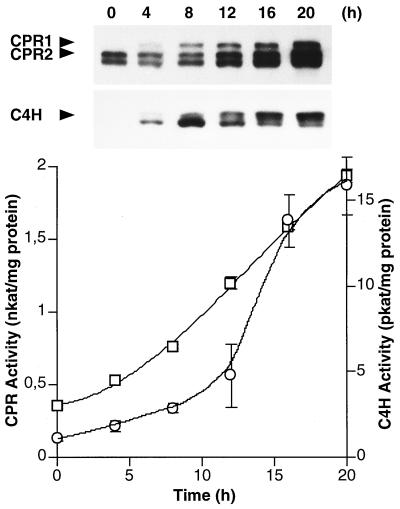
Finally, in view of the complex patterns of CPR1, CPR2, and C4H mRNA induction by elicitor, we further analyzed this response at the protein and enzyme activity levels. Although the distinction of CPR1 and CPR2 on immunoblots was not quantitative and their catalytic activities were indistinguishable in the crude protein extracts used, qualitatively clear-cut results were obtained (Fig. 8). The CPR1 and C4H proteins accumulated earlier than CPR2 protein and remained high far beyond the transient peak of CPR1 mRNA induction (Fig. 5), coincident with a rapid, prolonged increase in C4H activity. Total CPR activity increased essentially as expected from the combined CPR1 and CPR2 protein accumulation patterns.
Figure 8.
Relative timing of CPR1, CPR2, and C4H protein accumulation, as compared with increases in total CPR (□) and C4H (○) enzyme activities, in elicitor-treated parsley cells. Changes in protein amounts were estimated by immunoblotting by using the CPR2 and C4H antisera at dilutions of 1:2,000 and 1:1,000, respectively. See Fig. 3 for protein identification.
DISCUSSION
We have demonstrated the existence of at least two CPRs in parsley. Both isoforms exhibit about the same degree of sequence similarity to one another and to several CPRs from other plant species, suggesting that both of them possess similar catalytic activities. However, although the presence of single CPRs in some plant species, as well as in yeast and all animals tested so far, indicates for these cases full exchangeability among a multitude of biochemical functions, our present data argue for a considerable degree of distinction in the metabolic roles of CPR1 and CPR2 in parsley. Although each isoform may be involved in a large variety of cytochrome P450-related reactions, as suggested by the similarity in abundance of both mRNAs in various plant tissues, the two specific conditions tested, i.e., the defense responses to pathogens and UV light, clearly indicate different roles at least in stress metabolism. By contrast, the only previous study in which two distinct CPR mRNAs have been analyzed with respect to regulatory properties, by using wounded Helianthus tuberosus tubers, gave little or no indication of a differential response (7).
A particularly striking observation in elicitor-treated parsley cells was the initial coordination in the induction of the CPR1 and C4H mRNAs, which ceased after several hours, in contrast to both the concomitant accumulation of the encoded proteins (Fig. 8) and the highly coordinated PAL, C4H, and 4CL mRNA induction (13). One plausible explanation of this seeming discrepancy would be a corresponding difference in the half-lives of CPR1 and the other three proteins. Thus, the protein degradation rates may contribute substantially to an overall coordination of the induced changes in the four functionally related enzyme activities, PAL, C4H, CPR, and 4CL. However, even if CPR1 were less tightly included in the coordination, our data suggest that the CPR component of this sequence of reactions is CPR1 and not CPR2.
The response to pathogen attack or elicitor treatment in parsley affects numerous metabolic activities, including several branches of phenylpropanoid metabolism. Among the strongly induced pathways are those leading to the accumulation of a large variety of soluble and wall-bound cinnamate-derived esters, amides, aldehydes, and alcohols, and of structurally closely related furanocoumarins. By contrast, a comparatively small number of flavonoid glycosides appear to be the only UV light-induced compounds, generated via the concomitant induction of general phenylpropanoid metabolism and the flavone and flavonol branch pathways (40). The single known overlap in secondary metabolism between the responses to pathogens and UV light in parsley is the short sequence of reactions constituting general phenylpropanoid metabolism, including C4H. Within this metabolic overlap, C4H is the only enzyme that probably is encoded by a single gene (ref. 13; E.K. and E. Logemann, unpublished results). Hence, it is likely that the concomitant induction of CPR1 and C4H by fungal elicitor, infection, or UV light reflects the functional interaction of CPR1 with the same C4H protein in all cases. In addition, however, CPR1 may well be associated with various other elicitor- or infection-induced monooxygenase reactions that are involved in the formation of the large variety of accumulating hydroxylated and methoxylated phenylpropanoid derivatives.
Among the most prominent phenolic products accumulating during a comparatively late stage of infection are variously hydroxylated and methoxylated furanocoumarins (41–43). This stage coincides with the second peak of C4H mRNA induction in elicitor-treated parsley cells (Fig. 5) and, more pertinent to our present results, with the induction of CPR2 mRNA. Therefore, it is possible that CPR2 is functionally associated with furanocoumarin biosynthesis, either exclusively or in addition to other functions. Furthermore, it is still unclear whether CPR1 and CPR2 are the only CPRs existing in parsley. Preliminary results of DNA blot experiments using 5′ and 3′ probes from the CPR1 and CPR2 cDNAs (data not shown) indicate either rather complex structures of the corresponding genes or the occurrence of an additional member(s) of the parsley CPR gene family. The appearance of a so far unexplained, immunologically cross-reacting protein in Fig. 3 may point in the latter direction.
In any case, the observed differential regulation indicating different metabolic roles of CPR1 and CPR2 is particularly interesting in view of both the occurrence of only a small number of CPRs relative to the large variety of CPR-dependent reactions and the reported mutual exchangeability of various CPRs in reconstitution experiments (34). Considering the localization within membranes, an important key to an explanation may lie in the combined roles of the subcellular compartmentation, the lipid-associated dynamics, and the molecular details of the interaction between CPRs and P450 enzymes.
Acknowledgments
We are grateful for generous help and supply of materials from Olaf Batz, Nils Johnson, Elke Logemann, and Susanne Reinold, and thank Paul Rushton and Imre E. Somssich for valuable comments on the manuscript.
ABBREVIATIONS
- C4H
cinnamate 4-hydroxylase
- 4CL
4-coumarate:CoA ligase
- CPR
NADPH:cytochrome P450 oxidoreductase
- PAL
phenylalanine ammonia-lyase
Footnotes
References
- 1.Bolwell G P, Bozak K, Zimmerlin A. Phytochemistry. 1994;37:1491–1506. doi: 10.1016/s0031-9422(00)89567-9. [DOI] [PubMed] [Google Scholar]
- 2.Durst F, O’Keefe D P. In: Plant Cytochromes P450: An Overview in Drug Metabolism and Drug Interactions. Durst F, O’Keefe D P, editors. London: Freund Publishing House; 1995. pp. 171–183. [DOI] [PubMed] [Google Scholar]
- 3.Schuler M A. Crit Rev Plant Sci. 1996;15:235–284. [Google Scholar]
- 4.Kim J J, Roberts D L, Djordjevic S, Wang M, Shea T M, Masters B S. Methods Enzymol. 1996;272:368–377. doi: 10.1016/s0076-6879(96)72042-6. [DOI] [PubMed] [Google Scholar]
- 5.Meijer A H, Cardoso M I L, Voskuilen J T, Waal A D, Verpoorte R, Hoge J H C. Plant J. 1993;4:47–60. doi: 10.1046/j.1365-313x.1993.04010047.x. [DOI] [PubMed] [Google Scholar]
- 6.Shet M S, Sathasivan K, Arlotto M A, Mehdy M C, Estabrook R W. Proc Natl Acad Sci USA. 1993;90:2890–2894. doi: 10.1073/pnas.90.7.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lesot A, Hasenfratz M-P, Batard Y, Durst F, Benveniste I. Plant Physiol Biochem. 1995;33:751–757. [Google Scholar]
- 8.Urban P, Mignotte C, Kazmeier M, Delorme F, Pompon D. J Biol Chem. 1997;272:19176–19186. doi: 10.1074/jbc.272.31.19176. [DOI] [PubMed] [Google Scholar]
- 9.Porter T D, Kasper C B. Proc Natl Acad Sci USA. 1985;82:973–977. doi: 10.1073/pnas.82.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porter T D, Kasper C B. Biochemistry. 1986;25:1682–1687. doi: 10.1021/bi00355a036. [DOI] [PubMed] [Google Scholar]
- 11.Benveniste I, Salaun J P, Durst F. Phytochemistry. 1978;17:359–363. [Google Scholar]
- 12.Yabusaki Y, Murikimi H, Ohkawa H. J Biochem. 1988;103:1004–1010. doi: 10.1093/oxfordjournals.jbchem.a122370. [DOI] [PubMed] [Google Scholar]
- 13.Logemann E, Parniske M, Hahlbrock K. Proc Natl Acad Sci USA. 1995;92:5905–5909. doi: 10.1073/pnas.92.13.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagmann M-L, Heller W, Grisebach H. Eur J Biochem. 1983;134:547–554. doi: 10.1111/j.1432-1033.1983.tb07601.x. [DOI] [PubMed] [Google Scholar]
- 15.Kombrink E, Hahlbrock K. Plant Physiol. 1986;81:216–221. doi: 10.1104/pp.81.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nürnberger T, Nennstiel D, Jabs T, Sacks W R, Hahlbrock K, Scheel D. Cell. 1994;78:449–460. doi: 10.1016/0092-8674(94)90423-5. [DOI] [PubMed] [Google Scholar]
- 17.Kirsch C, Takamiya-Wik M, Reinold S, Hahlbrock K, Somssich I. Proc Natl Acad Sci USA. 1997;94:2079–2084. doi: 10.1073/pnas.94.5.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logemann J, Schell J, Willmitzer L. Anal Biochem. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- 19.Weißhaar B, Armstrong G A, Block A, de Costa e Silva O, Hahlbrock K. EMBO J. 1991;10:1777–1786. doi: 10.1002/j.1460-2075.1991.tb07702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinold S, Hahlbrock K. Plant Physiol. 1996;112:131–140. doi: 10.1104/pp.112.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korfhage U, Trezzini G F, Meier I, Hahlbrock K, Somssich I E. Plant Cell. 1994;6:695–708. doi: 10.1105/tpc.6.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domberg D, Müller R, Funk M. Nucleic Acids Res. 1994;22:5767–5768. doi: 10.1093/nar/22.25.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiser C, Michaelis S, Mitchell A. Methods in Yeast Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1990. [Google Scholar]
- 24.Truan G, Cullin C, Reisdorf P, Urban P, Pompon D. Gene. 1993;125:49–55. doi: 10.1016/0378-1119(93)90744-n. [DOI] [PubMed] [Google Scholar]
- 25.Urban P, Werck-Reichhart D, Teutsch H G, Durst F, Regnier S, Kazmaier M, Pompon D. Eur J Biochem. 1994;222:843–850. doi: 10.1111/j.1432-1033.1994.tb18931.x. [DOI] [PubMed] [Google Scholar]
- 26.Vetter H P, Mangold U, Schroeder G, Marner F J, Reichhart D W, Schroeder J. Plant Physiol. 1992;102:998–1007. doi: 10.1104/pp.100.2.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madyastha K M, Coscia C J. J Biol Chem. 1979;254:2419–2427. [PubMed] [Google Scholar]
- 28.Brinkmann U, Martes R E, Buckel P. Gene. 1989;85:109–114. doi: 10.1016/0378-1119(89)90470-8. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beltzer J P, Fiedler K, Fuhrer C, Geffen I, Handschin C, Wessels H P, Spiess M. J Biol Chem. 1991;266:972–978. [PubMed] [Google Scholar]
- 32.Kyte J, Doolittle R F. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 33.Black S D, Williams J S, Williams C H J, Coon M J. Biochem Biophys Res Commun. 1979;91:1528–1531. doi: 10.1016/0006-291x(79)91238-5. [DOI] [PubMed] [Google Scholar]
- 34.Durst F, Nelson D R. In: Diversity and Evolution of Plant Cytochromes P450 and P450 Reductases in Drug Metabolism and Drug Interactions. Durst F, O’Keefe D P, editors. London: Freund Publishing House; 1995. pp. 189–206. [DOI] [PubMed] [Google Scholar]
- 35.Porter T D, Wilson T E, Kasper C B. Arch Biochem Biophys. 1987;254:353–367. doi: 10.1016/0003-9861(87)90111-1. [DOI] [PubMed] [Google Scholar]
- 36.Sutter T R, Sangard D, Loper J C. J Biol Chem. 1990;265:16428–16436. [PubMed] [Google Scholar]
- 37.Benveniste I, Gabriac B, Durst F. Biochem J. 1986;235:365–373. doi: 10.1042/bj2350365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benveniste I, Lesot A, Hasenfratz M-P, Kochs G, Durst F. Biochem Biophys Res Commun. 1991;177:105–112. doi: 10.1016/0006-291x(91)91954-b. [DOI] [PubMed] [Google Scholar]
- 39.Schmelzer E, Krüger-Lebus S, Hahlbrock K. Plant Cell. 1989;1:993–1001. doi: 10.1105/tpc.1.10.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hahlbrock K, Scheel D. Annu Rev Plant Physiol Mol Biol. 1989;40:347–369. [Google Scholar]
- 41.Tietjen K G, Hunkler D, Matern U. Eur J Biochem. 1983;131:401–407. doi: 10.1111/j.1432-1033.1983.tb07277.x. [DOI] [PubMed] [Google Scholar]
- 42.Hauffe K D, Hahlbrock K, Scheel D. Z Naturf. 1986;41c:228–239. [Google Scholar]
- 43.Jahnen W, Hahlbrock K. Planta. 1988;173:197–204. doi: 10.1007/BF00403011. [DOI] [PubMed] [Google Scholar]