Abstract
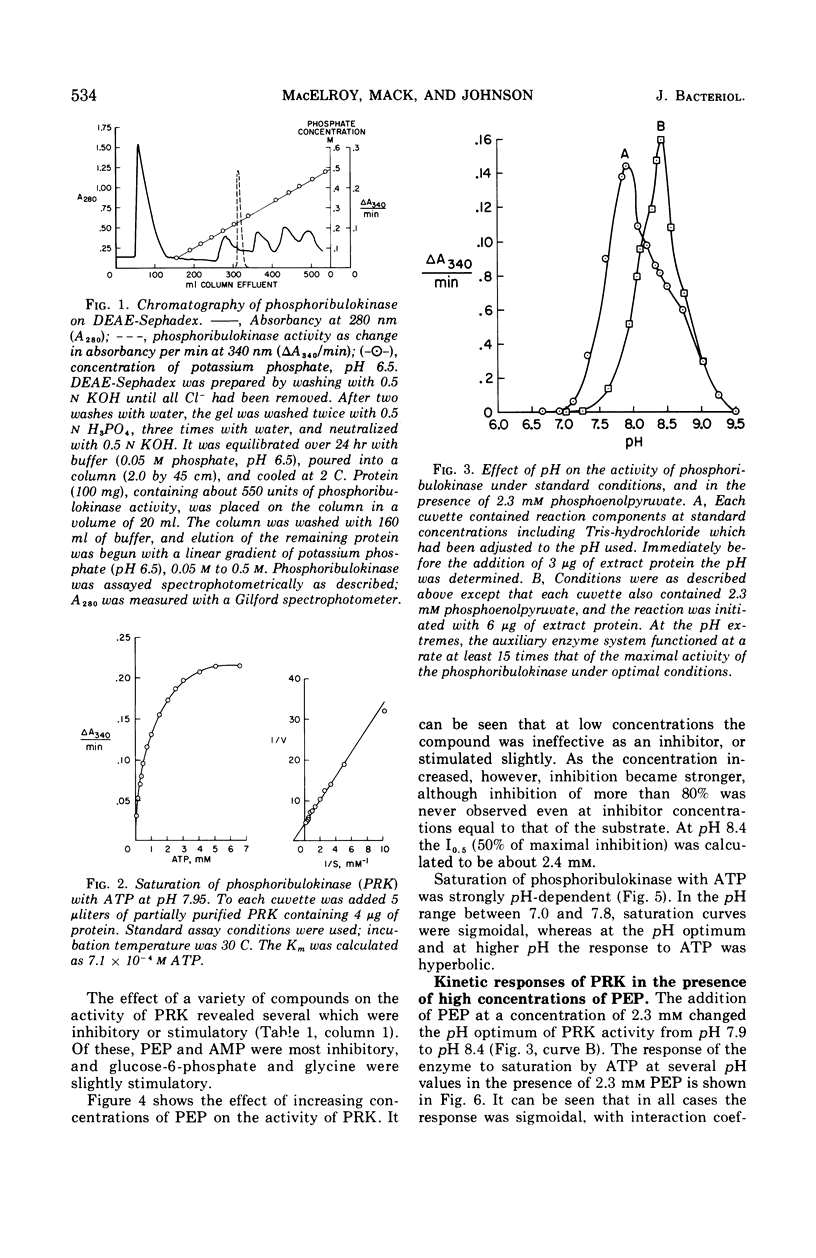
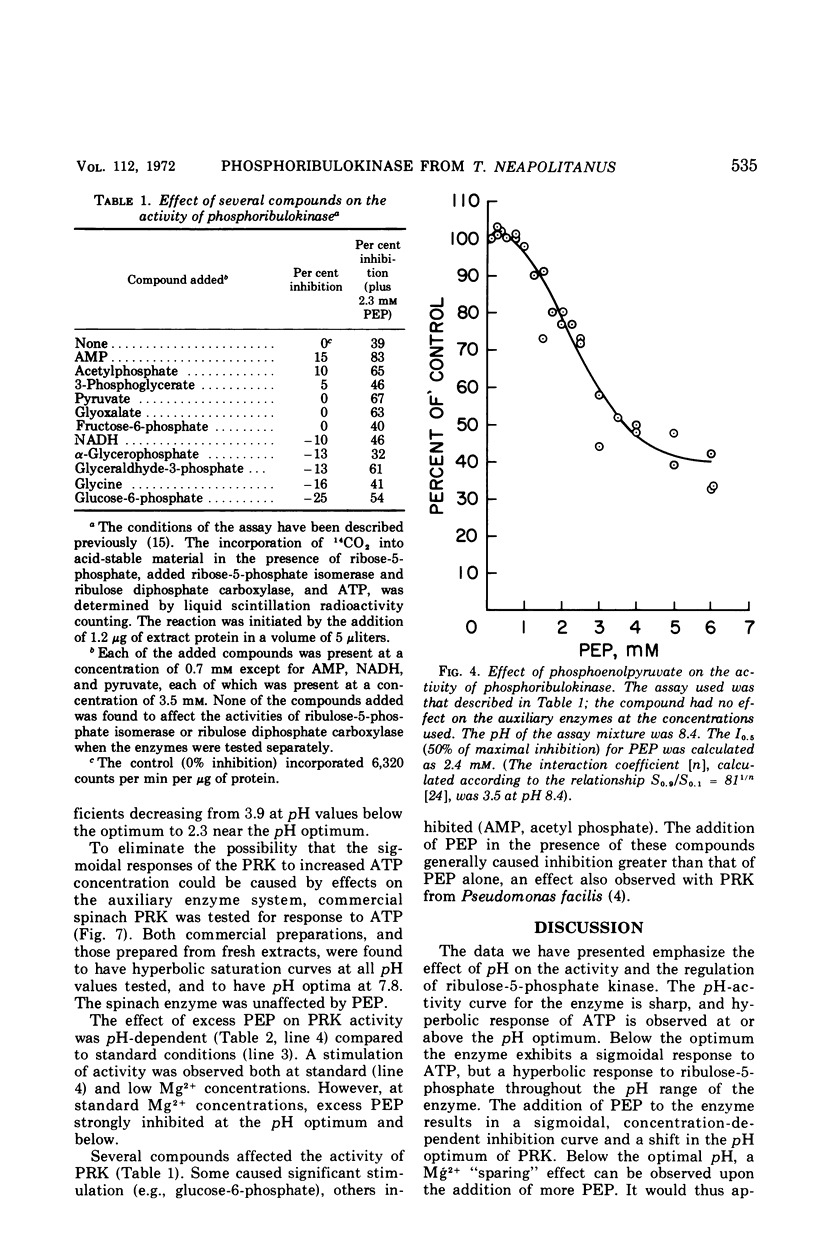
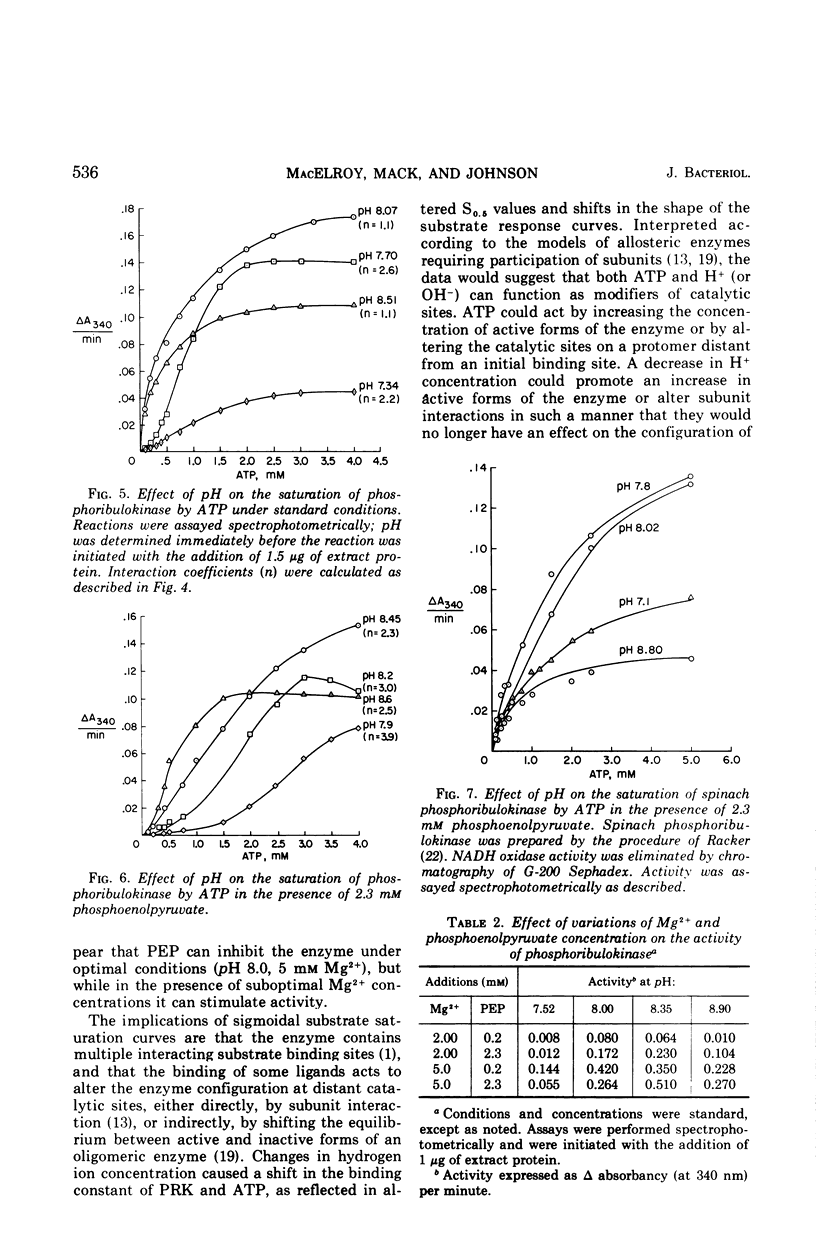
Partially purified preparations of ribulose-5-phosphate kinase (specific activity, 50 to 125 μmoles per min per mg of protein) were employed in a series of kinetic experiments in the presence of several concentrations of H+, Mg2+, adenosine triphosphate (ATP), and phosphoenolpyruvate (PEP). The pH optimum of the enzyme was found to be 7.9; at this pH and above, response of the enzyme to variations in ATP concentration was hyperbolic, exhibiting a Km of 7 × 10−4m ATP. At pH values below the optimum the response to ATP was sigmoidal, as it was throughout the entire pH range in the presence of PEP at a concentration greater than 5 × 10−4m. In the presence of PEP the pH optimum shifted to pH 8.4. In contrast, phosphoribulokinase from spinach exhibited hyperbolic responses throughout its pH range with no inhibition caused by PEP. Thiobacillus neapolitanus phosphoribulokinase was inhibited by PEP in a sigmoidal manner; however, in the presence of suboptimal concentrations of Mg2+ the addition of PEP caused significant stimulation of activity. It is postulated that the enzyme consists of interacting subunits with several sites on the enzyme for binding ATP and with several separate sites binding PEP. It is suggested that PEP functions as a regulator of CO2 fixation when the organism is under conditions of unlimited concentrations of substrate and CO2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. Regulation of enzyme function. Annu Rev Microbiol. 1969;23:47–68. doi: 10.1146/annurev.mi.23.100169.000403. [DOI] [PubMed] [Google Scholar]
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Ballard R. W., MacElroy R. D. Phosphoenolpyruvate, a new inhibitor of phosphoribulokinase in pseudomonas facilis. Biochem Biophys Res Commun. 1971 Aug 6;44(3):614–618. doi: 10.1016/s0006-291x(71)80127-4. [DOI] [PubMed] [Google Scholar]
- Cornish A. S., Johnson E. J. Regulation of pyruvate kinase from Thiobacillus neapolitanus. Arch Biochem Biophys. 1971 Feb;142(2):584–590. doi: 10.1016/0003-9861(71)90522-4. [DOI] [PubMed] [Google Scholar]
- Gale N. L., Beck J. V. Competitive inhibition of phosphoribulokinase by AMP. Biochem Biophys Res Commun. 1966 Sep 8;24(5):792–796. doi: 10.1016/0006-291x(66)90396-2. [DOI] [PubMed] [Google Scholar]
- Hart B. A., Gibson J. Ribulose-5-phosphate kinase from Chromatium sp. strain D. Arch Biochem Biophys. 1971 May;144(1):308–321. doi: 10.1016/0003-9861(71)90483-8. [DOI] [PubMed] [Google Scholar]
- Johnson E. J. Occurrence of the adenosine monophosphate inhibition of carbon dioxide fixation in photosynthetic and chemosynthetic autotrophs. Arch Biochem Biophys. 1966 Apr;114(1):178–183. doi: 10.1016/0003-9861(66)90319-5. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Neet K. E. The catalytic and regulatory properties of enzymes. Annu Rev Biochem. 1968;37:359–410. doi: 10.1146/annurev.bi.37.070168.002043. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILHAUD G., AUBERT J. P., MILLET J. Métabolisme du carbone dans la chimioautotrophie; cycle d'assimilation de l'anhydride carbonique. C R Hebd Seances Acad Sci. 1956 Jul 2;243(1):102–105. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- MacElroy R. D., Johnson E. J., Johnson M. K. Allosteric regulation of phosphoribulokinase activity. Biochem Biophys Res Commun. 1968 Mar 27;30(6):678–682. doi: 10.1016/0006-291x(68)90566-4. [DOI] [PubMed] [Google Scholar]
- MacElroy R. D., Johnson E. J., Johnson M. K. Characterization of ribulose diphosphate carboxylase and phosphoribulokinase from Thiobacillus thioparus and Thiobacillus neapolitanus. Arch Biochem Biophys. 1968 Sep 20;127(1):310–316. doi: 10.1016/0003-9861(68)90231-2. [DOI] [PubMed] [Google Scholar]
- MacElroy R. D., Johnson E. J., Johnson M. K. Control of ATP-dependent CO2 fixation in extracts of Hydrogenomonas facilis: NADH regulation of phosphoribulokinase. Arch Biochem Biophys. 1969 Apr;131(1):272–275. doi: 10.1016/0003-9861(69)90131-3. [DOI] [PubMed] [Google Scholar]
- PONTREMOLI S., MANGIAROTTI G. A simple method for the preparation of D-ribulose 5-phosphate. J Biol Chem. 1962 Mar;237:643–645. [PubMed] [Google Scholar]
- RACKER E. The reductive pentose phosphate cycle. I. Phosphoribulokinase and ribulose diphosphate carboxylase. Arch Biochem Biophys. 1957 Jul;69:300–310. doi: 10.1016/0003-9861(57)90496-4. [DOI] [PubMed] [Google Scholar]
- Rindt K. P., Ohmann E. NADH and AMP as allosteric effectors of ribulose-5-phosphate kinase in Rhodopseudomonas spheroides. Biochem Biophys Res Commun. 1969 Aug 7;36(3):357–364. doi: 10.1016/0006-291x(69)90572-5. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Jiménez de Asúa L., Carminatti H. Some kinetic properties of liver pyruvate kinase (type L). II. Effect of pH on its allosteric behavior. J Biol Chem. 1969 Jun 25;244(12):3142–3147. [PubMed] [Google Scholar]


