Abstract
Recent discovery of crania, dentitions, and postcrania of a primitive anthropoidean primate, Proteopithecus sylviae, at the late Eocene L-4l quarry in the Fayum, Egypt, provides evidence of a new taxonomic family of early African higher primates, the Proteopithecidae. This family could be part of the basal radiation that produced the New World platyrrhine primates, or it could be unrelated to any subsequent lineages. Although no larger than a small callitrichid or a dwarf lemur, this tiny primate already possessed many of the derived features of later anthropoids and was a diurnal and probably dimorphic species. In dental formula and other dental proportions, as well as in known postcranial features, Proteopithecus more nearly resembles platyrrhines than does any other Old World higher primate. The small size of the Proteopithecus cranium demonstrates that the defining cranial characteristics of Anthropoidea did not arise as a consequence of an increase in size during derivation from earlier prosimians.
The Oligocene deposits north of Lake Qarun, Fayum Province, Egypt have—during this century—produced a well known series of land mammals, birds, other vertebrates, trace fossils, and plants (see ref. 1 and references cited there). During the past decade an extensive new series of mammalian, bird, and fish fossils have been recovered from an older Fayum Eocene site, L-4l. A new fauna of primates, including the first really well-known early anthropoids, has been discovered there (1, 2). Some of the adaptations and the diversity of these Fayum Eocene primates have been discussed before (3, 4). Unlike most of the previously identified Eocene anthropoids, which are known only from jaw fragments with teeth, in several cases the L-41 primates are documented by complete or nearly complete upper and lower dentitions, as well as mandibles, skulls, and sometimes postcrania. The cranium of one of these species, Proteopithecus sylviae, is here described. To date the bulk of evidence indicates that the Anthropoidea—also referred to as anthropoids, anthropoideans, or simiiform primates—arose in Africa, but for discussion on this subject, as well as other origins see refs. 5–8 and references cited in these papers. Of all the better known African Paleogene anthropoid primates Proteopithecus sylviae is the smallest and most generalized. It exhibits many features that resemble those of platyrrhine monkeys.
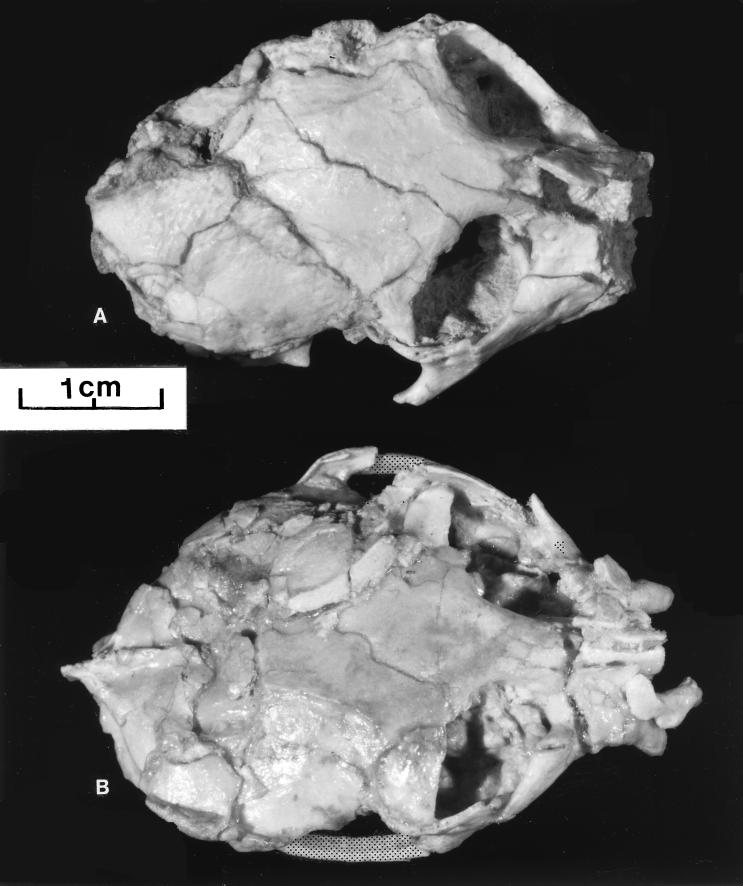
There are two crania of Proteopithecus (Fig. 1). General features detectable on the skulls of Proteopithecus include the relative size and shape of the premaxillae, arrangement and structure of the dentition, position of the lachrymal (or lacrimal) bone and foramen, degree of postorbital closure, shape of the inferior orbital fissure, closure of the metopic suture, disposition of the temporal lines, arrangement of the palate and nares, and structure of the petrosal area and basicranium. Almost all details of these cranial parts are well preserved on one or the other of the skulls of Proteopithecus. Although whole or partial crania of the Oligocene genera Aegyptopithecus and Apidium and of the Eocene Catopithecus and Plesiopithecus have been known for some time, most were damaged. Many of the structural details of Proteopithecus described here are better preserved than on almost any of these earlier found Fayum primates. For instance, basicranial anatomy can be seen more clearly here than in any cranial part of Plesiopithecus, Aegyptopithecus, or Apidium. Only details of the basicrania of Catopithecus rival what can be seen in the two crania of Proteopithecus.
Figure 1.
Dorsal aspects of crania of Proteopithecus sylviae. (A) Cairo Geological Museum (CGM) 42214. (B) Duke University Primate Center (DPC) 14095. Note in B the broadly spaced temporal lines converging to a small sagittal crest posteriorly, moderately long nasals, lachrymal within the orbit, comparatively small premaxilla and broad, rounded braincase.
SYSTEMATICS
Order Primates Linnaeus, 1758; Suborder Anthropoidea Mivart, l864; Superfamily Hominoidea Gray, 1825; Family nov. Proteopithecidae. Emended Diagnosis
Familial diagnosis. As for the genus.
Emended generic diagnosis.
[The generic diagnosis has been extended beyond that proposed in ref. 2 by the discovery of additional specimens.] Proteopithecus differs from both Catopithecus and Oligopithecus in having distinctly larger hypocones on Ml and M2 and also in having a distinct paraconule on M1 and a cusp in the position of hypocone on P4. Proteopithecus sylviae lacks the central upper premolar cusp of parapithecids. Unlike propliopithecids, it retains P2/P2. Upper molars are transversely broader than in Apidium, Catopithecus, and Oligopithecus. Like Serapia in having P2 slightly larger than P3. Differs from Arsinoea in having paraconid crests less extended lingually, in having a higher, more triangular trigonid and Ml and M2 less rounded in basal outline. Resembles Aegyptopithecus and Propliopithecus in general layout of upper molars, but differs from these forms in having P2/P2 and relatively larger postglenoid foramen. Absolute size is smaller than Serapia and larger than Arsinoea. Upper molar series is l5% smaller than the molars of Catopithecus, and the lower P4–M2 of Catopithecus are 20% shorter than in the type of Oligopithecus. Shows reduction in size of jugal foramen compared with parapithecids and relatively smaller premaxillae and ascending premaxillary alae than in Catopithecus or Aegyptopithecus. Possesses full postorbital closure and a medially constricted inferior orbital fissure, metopic sutural fusion, lachrymal within the orbit. Dentition vaguely platyrrhine-like, lacking both specifically catarrhine or derived characters of parapithecids.
Type species. Proteopithecus sylviae.
Distribution. Fayum, Egypt, Quarry L-41.
Species diagnosis. Same as generic diagnosis.
Hypodigm. The full hypodigm as of 1997 is listed in Miller and Simons (9). The two crania discussed here are Cairo Geological Museum (CGM) 42214, first partial skull with right P3–M3 and inner halves of left P3–M3, and second skull Duke University Primate Center (DPC) 14095 with right I1, right P2–M3, and left P2–M3. In addition there are seven fragmentary maxillae.
DESCRIPTIONS AND COMPARISONS
Cranial Size and Braincase.
DPC 14095 (Fig. 1) is relatively uncrushed for an L-41 specimen and is completely preserved from the anterior tip of the right premaxilla (with central incisor) all the way back to the inion or at the position of the external occipital protuberance so that an approximate cranial length of 44.3 mm can be measured (Fig. 1). The orbital margins are somewhat crushed, particularly on the left side, but a reasonably accurate measure across the orbits appears to be about 25.0 mm (Fig. 1). Anteroposterior length of the left nasal appears to be about 11.2 mm and orbital height, 8.5 mm. This is a very small cranium, intermediate in size between that of Callithrix jacchus and Cebuella pygmaea, and approximately the size of crania of Loris tardigradus or Cheirogaleus medius. However, orbits of Proteopithecus sylviae are small in comparison with these latter two nocturnal forms (Table 1). Table 1 shows that, in terms of cranial measures, the skull of Aegyptopithecus is approximately 2 to 2½ times as large as that of Proteopithecus in linear dimensions. Being contemporaries, Proteopithecus crania can be most closely compared with crania of Catopithecus from L-41. Cranial anatomy of the latter has been described in two recent papers (10, 11).
Table 1.
Relative cranial size in Proteopithecus
| Taxonomic name | Skull length, mm | Width across the orbits, mm | Orbital height, mm |
|---|---|---|---|
| Aegyptopithecus zeuxis CGM 40237 | 104.9 | 50.6 | 18.16 |
| Apidium phiomense DPC 9867 and AMNH 14556 | 57.0e | 27.5e | 10.5e |
| Proteopithecus sylviae DPC 14095 | 44.3 | 25.0 | 8.5 |
| Proteopithecus sylviae CGM 42214 | 44.1e | 24.5e | 8.2e |
| Catopithecus browni mean of three best specimens | 50.0 | 28.1 | — |
| Loris tardigradus DPC-O-42 | 43.6 | 28.1 | 14.4 |
| Cheirogaleus medius DPC-O-33 | 42.8 | 26.6 | 11.1 |
AMNH, American Museum of Natural History; e, estimate.
The Brain.
Although impossible to measure with complete accuracy, the brain size of Proteopithecus sylviae appears much smaller relative to tooth size when compared with that of a modern small platyrrhine, such as Callithrix, presumably because Proteopithecus is a very small anthropoidean. In typical skulls of Callithrix—although the upper dentition is distinctly smaller than that of Proteopithecus sylviae—the brain volume must clearly be much larger. A very rough calculation of brain volume in Proteopithecus might be around 2.7 cm3. This was estimated by modeling a clay reconstruction of a brain of a size compatible with the crushed braincases and immersing it in water to measure the volume displaced. Methods for determining the brain size of Catopithecus have been discussed previously (11) and as determined by various methods brain volume estimates range from 2.8 to 3.4 cm3 for this larger primate. These estimates can hardly be accurate, because by taking cranial length × orbital breadth (Table 1) as a rough measure of skull and braincase size, the two-dimensional flattened cranium of Catopithecus proves to be about 10–15% larger than that of Proteopithecus. Because volume increases as the cube of linear measurements the brain size difference between these two primates should be greater. It is also not possible to produce an accurate brain volume estimate for Apidium phiomense (DPC 9867) from the quarry I-M level in the Fayum (12), but judging from a visual comparison between the crushed cranium of DPC 9867 and DPC l4095, the braincase of Proteopithecus, a reasonable guess would be that Apidium phiomense had at least twice the brain volume of Proteopithecus. The brain volume of Aegyptopithecus has been estimated at about 27–34 cm3 (13, 14).
Orbital, Frontal, and Jugal Region.
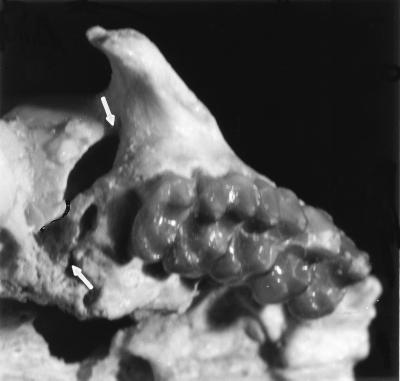
The right orbit of DPC 11434 is well preserved and shows that the lachrymal bone and foramen are within the orbit (Fig. 1). Compared with overall size of the skull and of the zygomatic bone, the zygomaticofacial foramen in Proteopithecus is relatively much smaller than in Parapithecus, Apidium, or some of the small-bodied platyrrhines such as Callithrix (12, 15). Proteopithecus has full postorbital closure and the inferior orbital fissure (Fig. 2) is relatively small, resembling that of Aotus (11). The frontal is a single bone with full closure of the metopic suture (Fig. 1). As in Catopithecus, and quite unlike Tarsius, the large spoon-like, expanded jugal runs back to the braincase and forms the margin of the inferior orbital fissure, where a process dips down from the jugal, as in some platyrrhines, almost dividing the fissure in half (Fig. 2). The broadly rounded posterior jugal resembles that of platyrrhines such as Saguinus, Callithrix, Callimico, or Saimiri because of its smoothly rounded surface, resembling the convex side of a spoon, and the temporal lines do not connect across it to form a sharp crest at the top of the zygomatic arch as in most catarrhines. This feature may be a correlate of absolute size because a continuous ridge is evident here in Lagothrix and even in the smallest of the modern catarrhines, Miopithecus. Interestingly, both the left and right jugal bones of DPC 14095 appear (Fig. 1) to be in the correct position to contact the parietal bones, as in platyrrhines, but breakage and distortion do not allow certainty about this possibility. The comparatively small size of the orbit in Proteopithecus sylviae definitely indicates diurnality. In accord with the general gracility of Proteopithecus there are no supercilliary ridges. Beginning at the dorsal margin of the jugal, the temporal lines can be traced running back toward the midline. These lines meet the sagittal crest much further back than in Apidium, Catopithecus, or Aegyptopithecus, perhaps implying that the temporal musculature was relatively smaller, or alternatively that the braincase was comparatively more expanded (Fig. 1). DPC 14095 shows a slightly elevated sagittal crest running posteriorly from the point where the temporal lines converge.
Figure 2.
Ventral view of CGM 42214, Proteopithecus sylviae, showing the inferior orbital fissure nearly divided into an anterior and a medial part (11). In platyrrhines the anterior part is variably developed.
Simons and Rasmussen (11) calculated angles of orbital convergence in three crania of Catopithecus browni, and these produced an average of 124°. In Proteopithecus DPC 14095 this angle is 148° and in CGM 42214, which is more distorted, the angle is 126°, suggesting that the latter species may have had more frontated orbits like those of Aegyptopithecus zeuxis (angle 142°) and also like Miocene to Recent anthropoids, where the angle is often even higher. However, due to crushing in the L-41 skulls these angles could be somewhat distorted (Fig. 1).
Rostral Region.
The rostrum is almost completely preserved in DPC 14095, and, as in Catopithecus, callitrichids, as well as certain prosimians and catarrhines (11), the interorbital region is broad. The canines have long prominent roots which produce diverging canine pillars distinctly expressed on the lower rostrum. Although both specimens are crushed and hard to interpret it would seem, in CGM 42214, that there was considerable thickness to the interorbital septum posteriorly and that the space between the orbits could not have diminished to a fenestra there as it is in Saimiri. The rostrum of Proteopithecus appears to be proportionately slightly shorter in relation to length of the brain case than is that of Catopithecus, as might be expected for a smaller species. Nearly the entire left nasal bone of DPC 14095 is preserved, although it is badly shattered, so that an accurate length measurement cannot be taken, and the lateral outline of the same bone is also present in CGM 4214. As in Catopithecus these bones are long and widen slightly at the contacts with the frontal where the tips of the nasals are pointed. Contrary to what has been suggested elsewhere (15), this broadening is not a prosimian feature, as it can be found, for instance, in Saguinus, Aotus, and Alouatta as well as in various catarrhines such as Pygathrix and Homo.
Premaxilla and Maxilla.
The right premaxilla is preserved in DPC 14095 and holds the right central incisor (Fig. 1). Compared with incisors and premaxillae in Aegyptopithecus or Catopithecus this bone and tooth appear to be somewhat smaller, relatively, as might be expected for a more diminutive animal with a comparatively smaller rostrum. The ascending wing of the premaxilla is comparatively thin and relatively small, unlike the condition in Catopithecus, Aegyptopithecus, and Afropithecus, where this process is large and expanded; however, its uppermost extension does not taper to a point as in later anthropoids. Proteopithecus also lacks the foramen present in the center of the ascending process of Catopithecus, Aegyptopithecus, and Afropithecus (16). The premaxillary alveolar process, like that of Catopithecus, has a shape and arrangement resembling that of callitrichids.
In both crania the right maxilla is better preserved than the left and in the medial or anterior margin of the orbit appears to be located above the line between P2 and P3. This orbital location is more forward, like its position in small platyrrhines, than it is in Catopithecus. The frontal process of the maxilla, as in Catopithecus, is rather broad and somewhat vertically arranged. This and other features of the face and front of the skull, which resemble Catopithecus in this region, have been interpreted as indicating a callitrichid-like nasal region with heightened olfactory and scent marking capacities (11). Infraorbital foramina are preserved in both specimens and especially in DPC 14095 are relatively small and not multiple. This foramen is perceptibly smaller than the small zygomaticofacial foramen, which is a relationship more typical of platyrrhines than of catarrhines. Size of the infraorbital foramen is comparable to that in callitrichids.
Upper Dentition and Palate.
All upper teeth except for the lateral incisors are preserved in DPC 14095 (Fig. 3). The central incisors are spatulate and, judging from the alveolus of the lateral incisor, the central one is the larger of the two. The upper canines in DPC l4095 are comparatively massive or stout and, unlike the small upper canines of omomyids, are large relative to adjacent teeth. CGM 42214 also retains large, well preserved sockets for left and right canines. On the anterior face of the right canine of DPC 14095 is a vertical groove that ends at the base of the enamel. In this one specimen the canine crowns seem relatively low or blunt but crown height has been diminished by heavy wear. Preservation of three upper pairs of premolars in DPC 14095 settles the question of identification of the teeth preserved in the type specimen (2). In Proteopithecus P2, P3, and P4 increase in size posteriorly and have simple inner and outer cusps, with an accessory cuspule on the posterior base of the inner cusp, much as in Aegyptopithecus. The size increase between P3 and P4 is greatest and P4 is broader than Ml. In Catopithecus the anterior upper premolar (P3) is lengthened somewhat compared with P4 and thus is slightly longer, whereas in Proteopithecus the anterior premolar (P2) is a small, simple tooth. Upper premolars in Catopithecus, Proteopithecus, and Aegyptopithecus do not resemble those of the parapithecids—all of whom have an enlarged and unusual central upper premolar cusp between the inner and outer cusps. Proteopithecus has distinctive upper molars in which M1 is clearly larger than M2 and M3 is much smaller than the other two. All early anthropoideans of the Fayum have very simple upper molars, and yet there are significant proportional and constructional differences between them. Proteopithecus has large and very distinct hypocones on M1 and M2, whereas in Catopithecus the hypocones are poorly developed. In all specimens Proteopithecus has a relatively small M3 where, as is also found in Aegyptopithecus, the metacone is hardly expressed at all. The molars of Aegyptopithecus in their general plan resemble those of both Catopithecus and Proteopithecus except that these teeth in the latter two primates are simpler and more generalized, and their molar cusps are less inflated and are broader labiolingually. Another distinction of Proteopithecus is the small size of the upper third molars. In Catopithecus the surface area of M3 is about 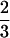 that of M2, whereas in Proteopithecus the M3 surface area is less than ½ that of M2.
that of M2, whereas in Proteopithecus the M3 surface area is less than ½ that of M2.
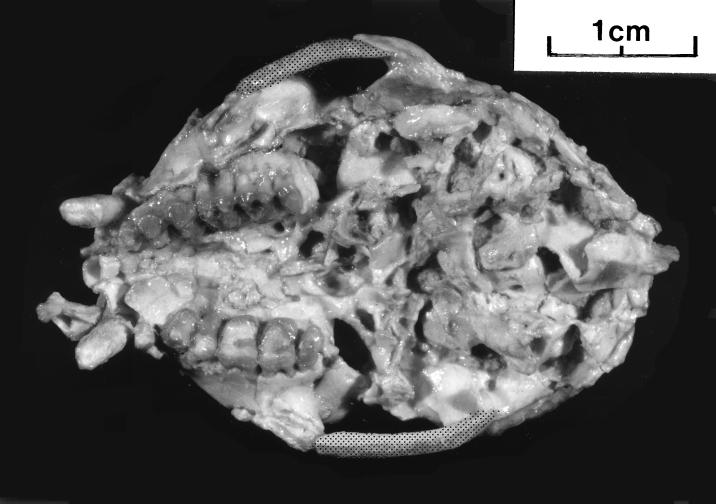
Figure 3.
Basicranial view of the cranium of Proteopithecus sylviae DPC 14095. Note the relative positions of the upper teeth, comparatively narrow palate and small premaxilla, the posterior palatine foramina, and the absence of a posterior palatine torus. On the right side the broad glenoid fossa is well preserved, behind this the postglenoid process and the postglenoid foramen are visible as well as the external auditory opening rimmed by the ectotympanic and the relatively anterior opening of the foramen magnum.
Compared with crania of similar-sized or larger primates such as Cheirogaleus and Callithrix, Proteopithecus has a relatively megadont dentition with combined cheek-tooth breadths about equal to the space across the palate between them. There are large posterior palatine foramina, and as in lemuroids and anthropoids, the openings of the posterior nares begin well forward of a line between the back of the third molars. As in Aegyptopithecus there is no posterior palatine torus. A strong torus in this position is seen in Necrolemur and other omomyids, as well as in Tarsius.
Posterior Braincase.
As mentioned above, the temporal lines in Proteopithecus meet much further back than in Catopithecus or Aegyptopithecus, where they form a slight sagittal crest (Fig. 1). This might mean that the uncrushed braincase posteriorly was relatively more inflated or expanded compared with the whole cranium than in the latter genera, or alternatively that the temporal musculature was less strongly developed. Posteriorly, at inion the sagittal crest joins a sharply developed vertical occipital crest. Less distinct nuchal crests also join the sagittal at inion. As noted elsewhere for Catopithecus (11), this may mean that the posterior aspect of the braincase may have been less “ballooned out” than in small Miocene to Recent anthropoideans, perhaps in correlation with the relatively smaller estimated brain size in the Paleogene genera. Presumably because of the comparatively small brain, there is more distinct cresting posteriorly, and in this respect the posterior cranium of Proteopithecus resembles that of more robust platyrrhines such as Alouatta, where there is also a distinct occipital crest.
Basioccipital.
Although the posterior braincase is crushed dorsoventrally in both specimens, the position of the foramen magnum can be determined. In DPC 14095, the foramen magnum lies nearly as far forward as the foramen of the right posterior carotid artery. This at least suggests that in Proteopithecus the foramen magnum had migrated well forward (Fig. 3) and occupied a position similar to that seen in Saimiri or Callithrix. As has been pointed out (17), the position of the posterior carotid foramen is directly linked to the location of the midline anterior rim of the foramen magnum and usually lies about 1.5 mm anterior to it. Near the shattered occipital condyles and the foramen magnum the posterior lacerate and jugal foramina can be seen; however, much of the rest of the posterior basicranium in CGM 42214 and DPC 14095 are damaged or dislocated to the point that interpretation is unreliable.
Auditory Region.
In the petrosal region there is a relatively uninflated bulla as in small platyrrhines but unlike the expanded bulla of Tarsius. The postglenoid process is small but distinct. There is a postglenoid foramen that is relatively larger than in Catopithecus and positioned much as in Saguinus or Cebus. On the left side the posterior part of the zygomatic process is preserved. On both sides of DPC 14095 the ectotympanic encircles the lateral opening of the auditory meatus much as it does in Catopithecus, Aegyptopithecus, and the platyrrhines. The petrosal regions are not shifted as far laterally as in most platyrrhines but do seem to be more lateral than is typical of many catarrhines. Unlike the condition in Tarsius and Miocene to Recent catarrhines, there is no trace of a tubular extension of the ectotympanic. The position of the posterior carotid foramen is about as in Aegyptopithecus and Catopithecus—well forward on the bullar wall. In general the entire auditory region resembles that of small-bodied platyrrhines in the position of foramina and in relationship of the bulla to the mastoid area, the temperomandibular joint, and the postglenoid process.
Temporomandibular Joint.
The glenoid fossa of DPC 14095 is both mesiodistally and anteroposteriorly broad and flat with a well developed postglenoid process situated just anterolateral to a distinct postglenoid foramen (Fig. 3). The articular surface seems to be slightly concave dorsally. Both this region and the facing mandibular articular process appear to be nearly the same as in Catopithecus.
Mandibular Form and Dentition.
No mandible found to date preserves the antecanine teeth, but since DPC 14095 shows that there were two pairs of upper incisors it is presumed there were two below, and hence a dental formula of I 2/2; C1/C1; P 3/3; M 3/3. Because the anterior tip of the mandibular ramus is unknown, whether or not the symphysis was unfused is also not known. Unlike its structure in many other African Paleogene anthropoideans, the mandibular horizontal ramus is relatively shallow and of even depth throughout most of its length. The articular, coronoid, and angular processes are preserved on DPC 10371. The coronoid process is high and anteroposteriorly narrower than in Catopithecus. The articular process is set somewhat above the tops of the lower teeth, and the angular process is gently rounded or convex posteriorly much as in Catopithecus.
An extensive discussion of the lower dentition of Proteopithecus is presented in ref. 8, so it only need be mentioned here that DPC 13101 and DPC 10371 preserve the ascending ramus and show that Proteopithecus sylviae had a high, narrow coronoid process. DPC 10370 is another right ramus with P3–M2 that are relatively little worn. Finally, DPC 12131, right ramus, holds the lower canine and outer halves of P2 and P3. The most distinctive thing about this lower canine is its massive root and blunted crown as well as its robusticity compared with P2. This corresponds well with the comparatively massive upper canine seen in the crania. In CGM 42209 after the slightly larger P2, lower P3 and P4 increase in size posteriorly but P4 is distinctly larger and there is no evidence that P2 is sectorial. The most marked increase in tooth size posteriorly is between P3 and P4, as is the case in the upper dentition of Proteopithecus. In addition, lower molar paraconid crests are perhaps a little more distinct in Proteopithecus than in Catopithecus and as in the latter there is a twinned hypoconulid/entoconid. There is no cusp in the usual position of the hypoconulid and the twinning of these two cusps is so close that one could speculate that the pairing together or “twinning” of these cusps might rather have arisen from a splitting of the entoconid as from the lateral movement of an hypoconulid. Almost all the Oligocene/Eocene Fayum anthropoideans show this twinned entoconid/hypoconulid.
CONCLUSIONS
Considered overall, the cranial anatomy of Proteopithecus is very distinct from that of strepsirhine prosimians and from that of Tarsius. It seems reasonable to regard Proteopithecus sylviae as the most generalized well known anthropoidean. Marked differences in the dentition (8) and postcranial bones (under study) show that Proteopithecus is certainly not justifiably placed within the parapithecid family, a group that has been ranked as the sister group of all other Anthropoidea (14). Although there are broad dental similarities with Catopithecus, perhaps largely due to shared-primitive characters, there are distinct differences in dental formula, cusp shape and size, relative tooth size, and other details of occlusal morphology. There are many other subtile differences seen throughout the crania and mandibles of the two. Proteopithecus differs from Catopithecus in having the following: (i) a much smaller ascending wing of the premaxilla; (ii) shorter, broader-based canines; (iii) presence of P2/P2 with lower P2 larger than P3; (iv) larger hypocones; (v) greater size of the upper cheek teeth—in proportion to palatal breadth; (vi) seemingly greater frontation of the orbits; (vii) a different inferior orbital fissure; and (viii) a relatively shallower jaw with higher, anteroposteriorly narrower mandibular coronoid process. Several of these features resemble characteristics seen in at least some platyrrhines. Presently what little comparable postcranial evidence there is between the two has been reviewed (18), but the hind limb of Proteopithecus is platyrrhine-like, judging from hind limb bones now under study. Hence, Proteopithecus stands as representing a separate family from the oligopithecine propliopithecids. In turn, some have questioned the ranking of Catopithecus in the Propliopithecidae, but forelimb bones now under study show a close affinity in distal humeral anatomy between Propliopithecus and Catopithecus. Experience has shown that over time, as the earlier anthropoideans have become more clearly known, their diversification has become better demonstrated, as is the case here. This now establishes that there are at least three families of archaic anthropoideans represented in the late Eocene Fayum deposits at L-41: Parapithecidae—Serapia; Propliopithecidae—Catopithecus; and Proteopithecidae—Proteopithecus. A fourth genus and species, Arsinoea callimos, is at present insufficiently known to be ranked with a family group. In view of the long discussion in which the platyrrhines have often been said to have derived from an African origin (19–22) one thing has become clear: if the platyrrhines are to be derived from any known African stock the only remaining serious candidacy for such a stock is with the family Proteopithecidae.
Acknowledgments
I thank F. A. Ankel-Simons, D. T. Rasmussen, and E. R. Miller for comments and criticisms of the manuscript. I thank P. S. Chatrath and F. A. Ankel-Simons for completing the bulk of the preparation of the two specimens described. The first cranium, CGM 42214, was found by Prithijit Chatrath, and the second of the two crania, DPC 14095, was found by the author. Staff of the Egyptian Geological Survey and Mining Authority and the Cairo Geological Museum are thanked for assisting and supporting our field work. Photographs were prepared at Duke by R. Usery. The research reported here was supported by National Science Foundation Grants BNS 91-008445 and SBR 95-07770 and by gifts or grants from Margo Marsh and Verna Cuddeback Simons as well as the Boise Fund of Oxford University and the Ann and Gordon Getty Foundation. This is Duke Primate Center publication no. 655.
ABBREVIATIONS
- CGM
Cairo Geological Museum
- DPC
Duke University Primate Center
References
- 1.Simons E L. Proc Natl Acad Sci USA. 1992;89:10743–10747. doi: 10.1073/pnas.89.22.10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simons E L. Proc Natl Acad Sci USA. 1989;86:9956–9960. doi: 10.1073/pnas.86.24.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons E L, Rasmussen D T, Bown T M, Chatrath P S. In: Anthropoid Origins. Fleagle J G, Kay R F, editors. New York: Plenum; 1994. pp. 179–202. [Google Scholar]
- 4.Simons E L, Rasmussen D T. Evol Anthropol. 1995;3:128–139. [Google Scholar]
- 5.Gingerich P D, Holroyd P A, Ciochon R L. In: Anthropoid Origins. Fleagle J G, Kay R F, editors. New York: Plenum; 1994. pp. 163–178. [Google Scholar]
- 6.Godinot M. In: Anthropoid Origins. Fleagle J G, Kay R F, editors. New York: Plenum; 1994. pp. 235–296. [Google Scholar]
- 7.Beard K C, Qi T, Dawson M R, Wang B, Li C. Nature (London) 1994;368:604–609. doi: 10.1038/368604a0. [DOI] [PubMed] [Google Scholar]
- 8.Chaimanee Y, Suteethorn V, Jaeger J-J, Ducrocq S. Nature (London) 1997;385:429–431. doi: 10.1038/385429a0. [DOI] [PubMed] [Google Scholar]
- 9.Miller E R, Simons E L. Proc Natl Acad Sci USA. 1997;94:13760–13764. doi: 10.1073/pnas.94.25.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons E L. Science. 1990;247:1567–1569. doi: 10.1126/science.2108499. [DOI] [PubMed] [Google Scholar]
- 11.Simons E L, Rasmussen D T. Am J Phys Anthropol. 1996;100:261–292. doi: 10.1002/(SICI)1096-8644(199606)100:2<261::AID-AJPA7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Simons E L. Am Mus Nov. 1995;3124:1–10. [Google Scholar]
- 13.Radinsky L. Am J Phys Anthropol. 1973;39:239–248. doi: 10.1002/ajpa.1330390214. [DOI] [PubMed] [Google Scholar]
- 14.Simons E L. Am J Sci. 1993;293:383–390. [Google Scholar]
- 15.Fleagle J G, Kay R F. J Hum Evol. 1987;16:483–532. [Google Scholar]
- 16.Leakey M G, Leakey R E, Richtsmeier J T, Simons E L, Walker A C. Folia Primatol. 1991;56:65–85. doi: 10.1159/000156531. [DOI] [PubMed] [Google Scholar]
- 17.Simons E L, Rasmussen D T. Am J Phys Anthropol. 1989;79:1–23. doi: 10.1002/ajpa.1330790103. [DOI] [PubMed] [Google Scholar]
- 18.Gebo D L, Simons E L, Rasmussen D T, Dagosto M. In: Anthropoid Origins. Fleagle J G, Kay R F, editors. New York: Plenum; 1994. pp. 203–234. [Google Scholar]
- 19.Hofstetter R. In: Evolutionary Biology of the New World Monkeys and Continental Drift. Ciochon R L, Chiarelli A B, editors. New York: Plenum; 1980. pp. 103–122. [Google Scholar]
- 20.Gingerich P D. In: Evolutionary Biology of the New World Monkeys and Continental Drift. Ciochon R L, Chiarelli A B, editors. New York: Plenum; 1980. pp. 123–138. [Google Scholar]
- 21.Lavocat R. In: Evolutionary Biology of the New World Monkeys and Continental Drift. Ciochon R L, Chiarelli A B, editors. New York: Plenum; 1980. pp. 103–122. [Google Scholar]
- 22.Sarich V M, Cronin J E. In: Evolutionary Biology of the New World Monkeys and Continental Drift. Ciochon R L, Chiarelli A B, editors. New York: Plenum; 1980. pp. 399–422. [Google Scholar]