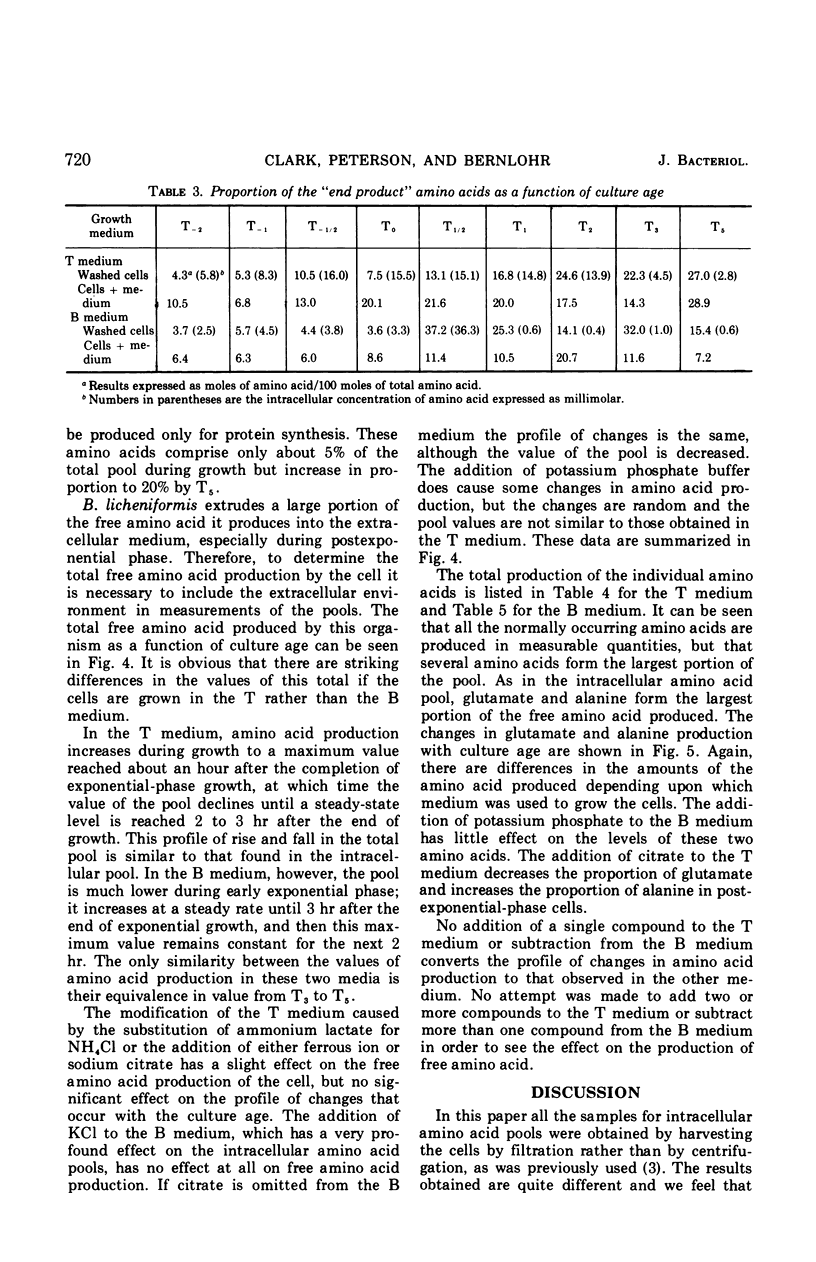
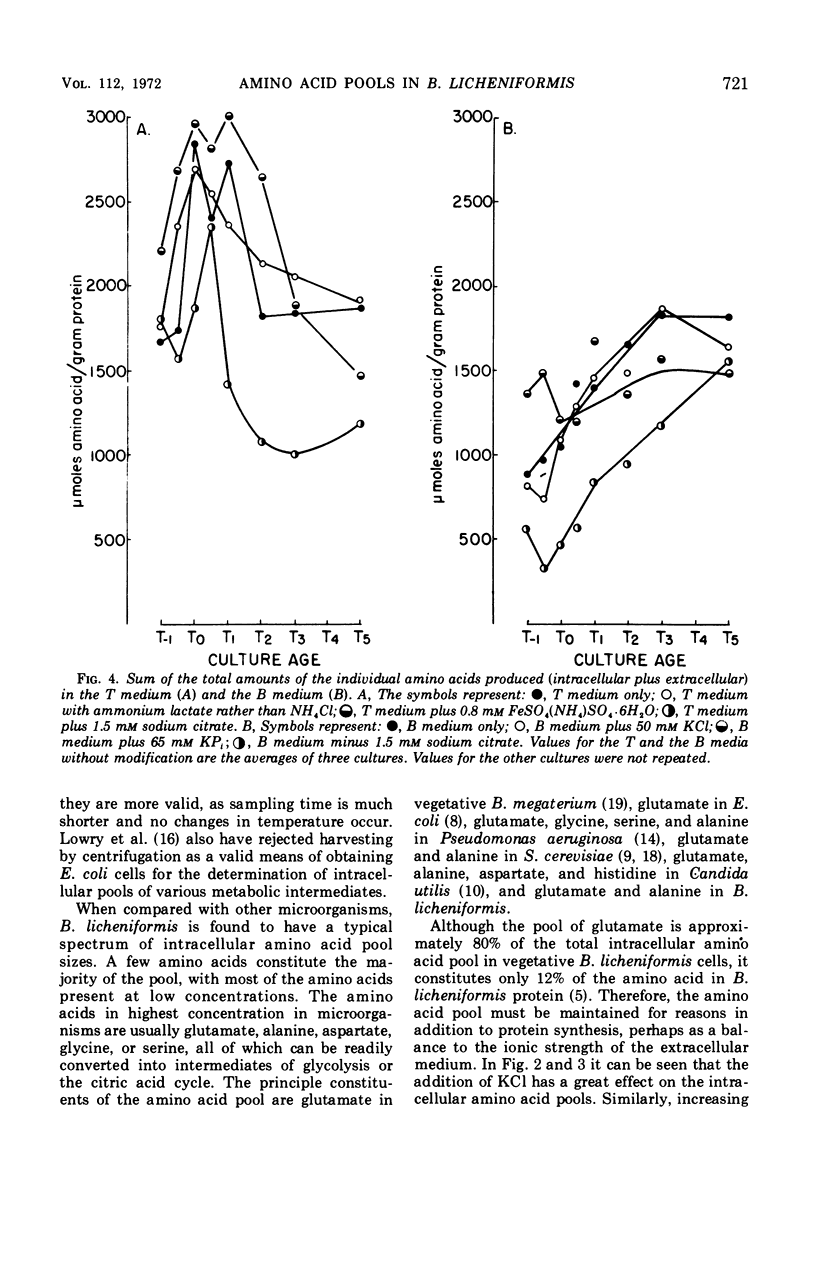
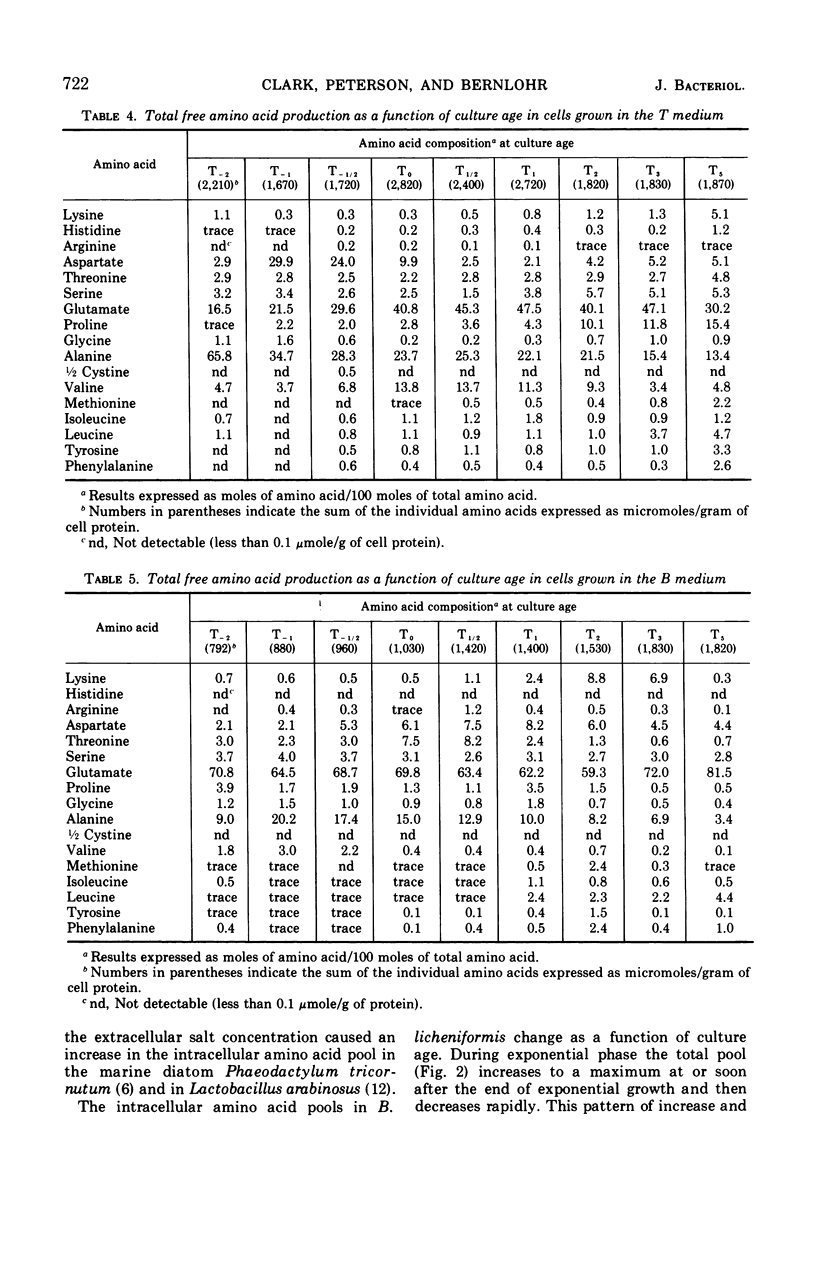
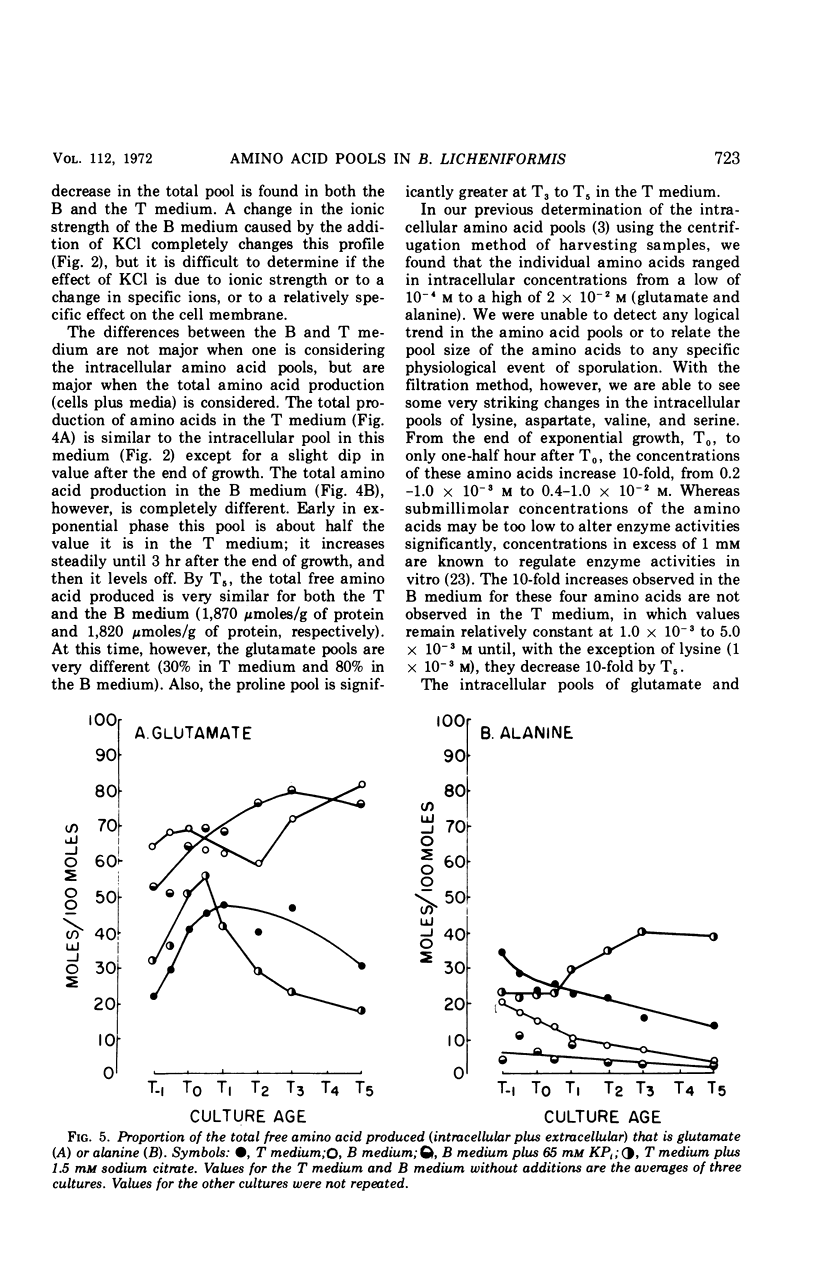
Abstract
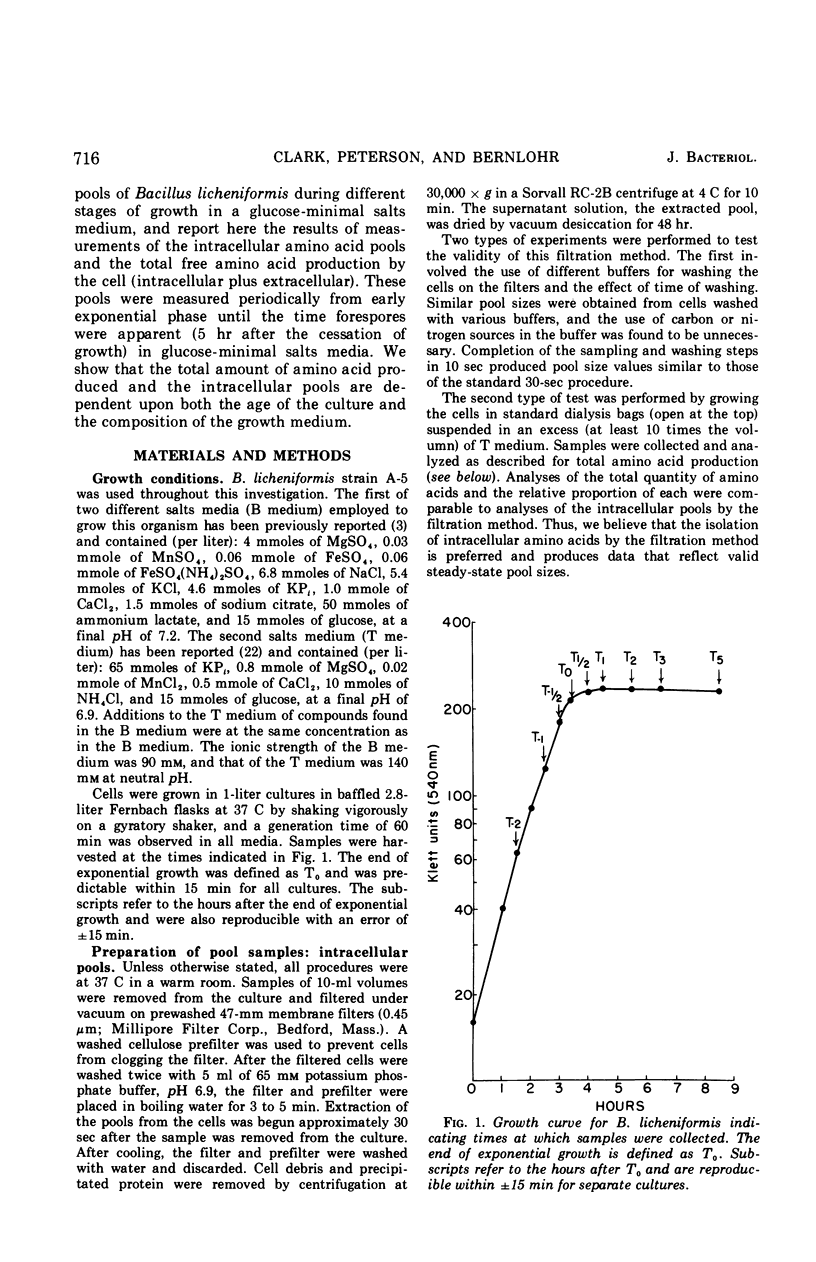
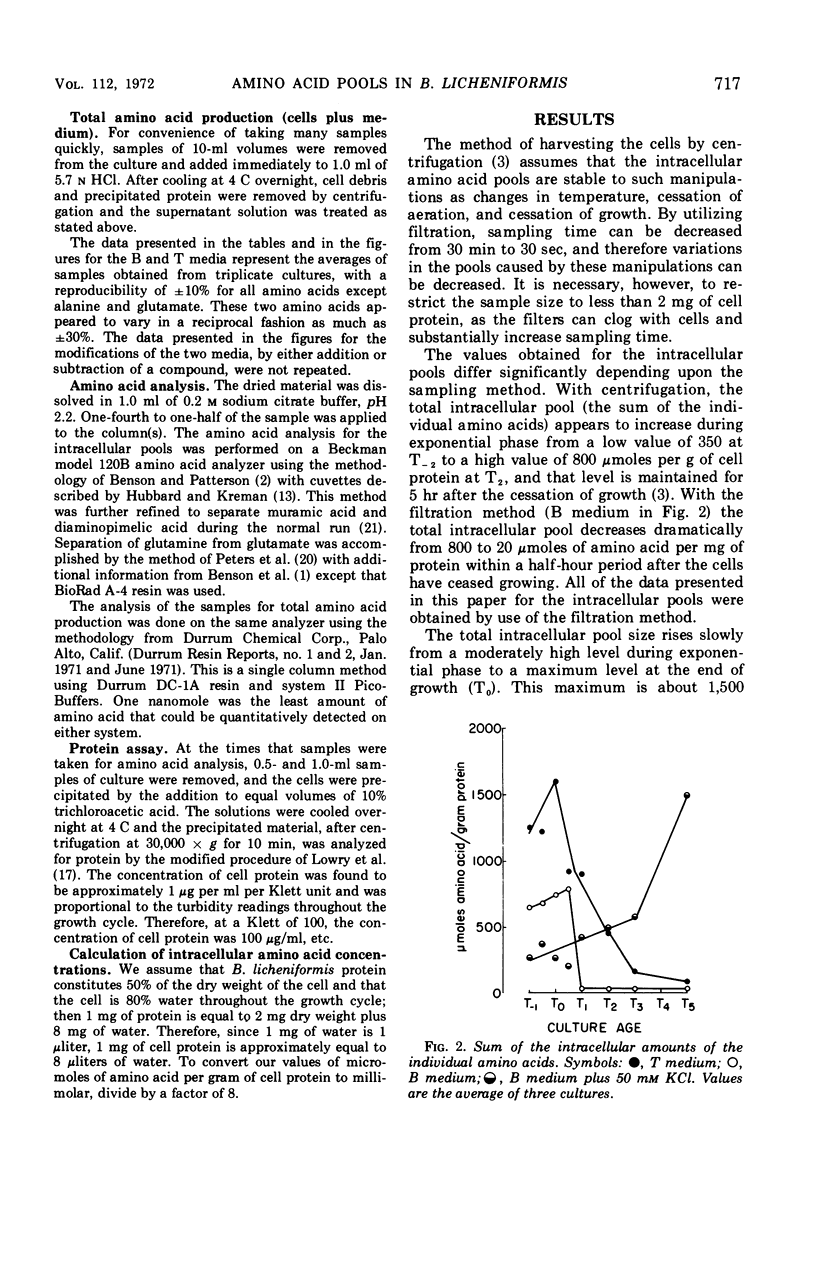
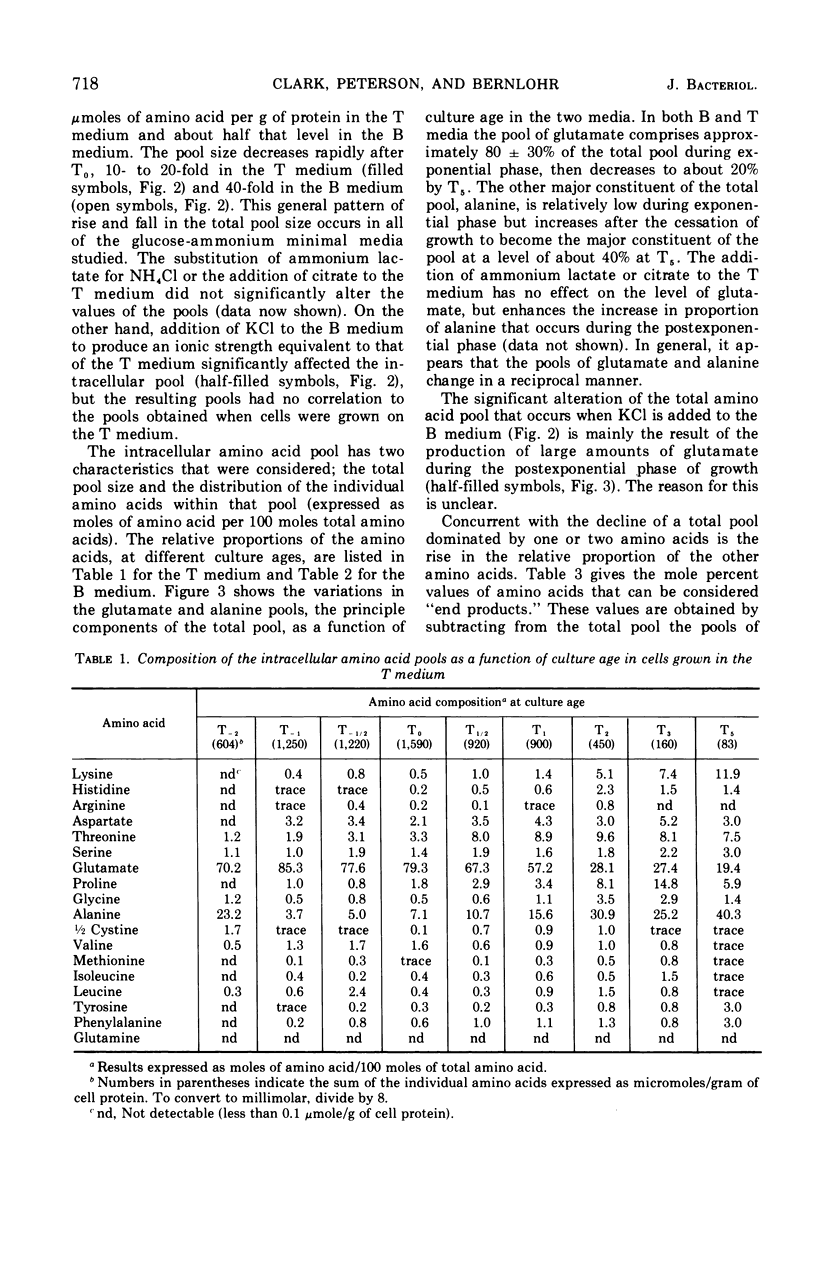
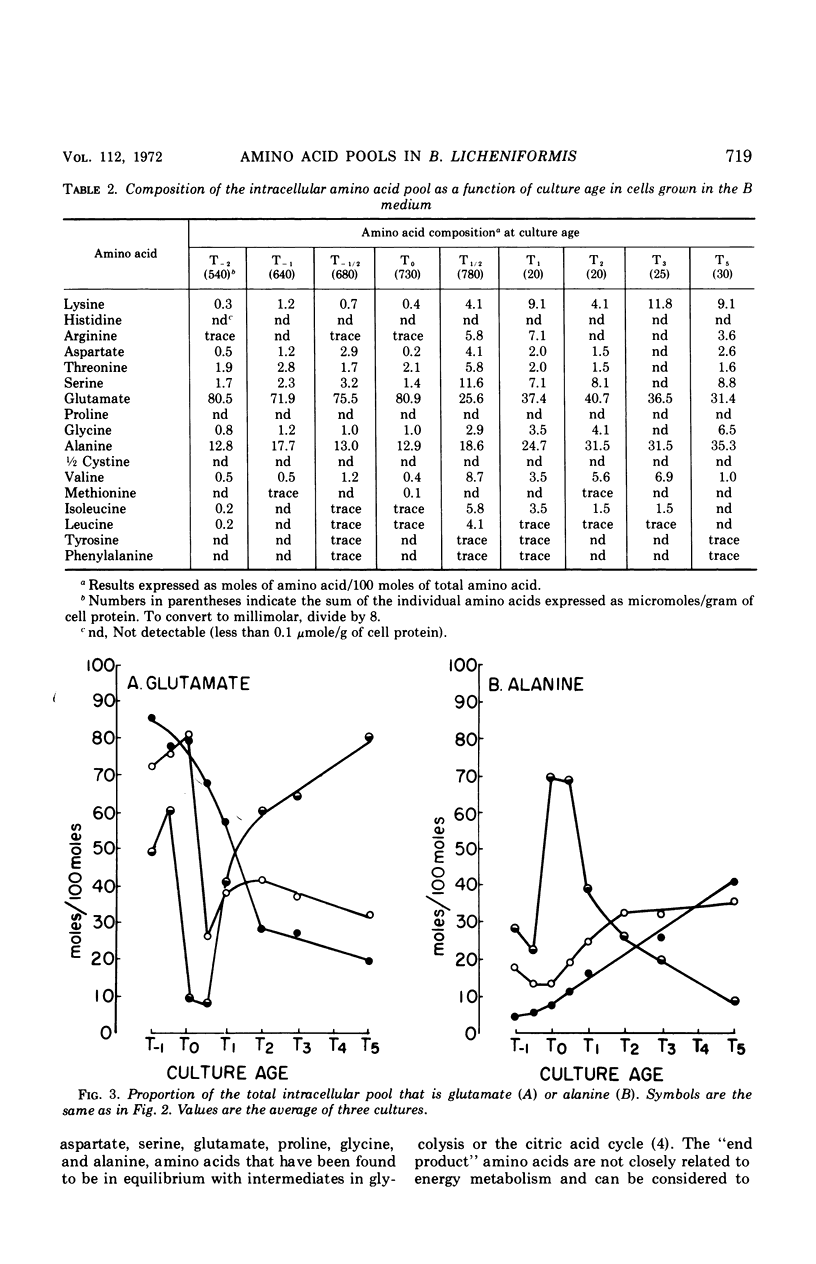
Changes in the endogenous intracellular amino acid pool and total free amino acid production in Bacillus licheniformis grown in minimal media were investigated. The total intracellular pool increased during exponential growth and then decreased rapidly after the end of growth. Most of the amino acids were present at low concentrations, but glutamate and alanine comprised 60 to 90% of the total intracellular free amino acid at most times during the growth cycle. It was concluded that, in addition to providing monomers for protein synthesis, the intracellular amino acid pool may be maintained for the storage of energy-providing metabolic intermediates and possibly as a balance to the ionic strength of the medium. The total free amino acid production by the cell was found to be dependent upon the composition of the salts medium as well as the culture age under conditions in which the carbon and nitrogen sources were the same. A 10-fold increase in extracellular amino acid was observed as the cells changed from vegetative to sporulation metabolism, mostly due to the extrusion of intracellular amino acid. The impact of this increase upon amino acid uptake and pulse-labeling studies using unwashed cells is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. V., Jr, Patterson J. A. Accelerated chromatographic analysis of amino acids commonly found in physiological fluids on a spherical resin of specific design. Anal Biochem. 1965 Nov;13(2):265–280. doi: 10.1016/0003-2697(65)90196-x. [DOI] [PubMed] [Google Scholar]
- Bernlohr R. W. 18 Oxygen probes of protein turnover, amino acid transport, and protein synthesis in Bacillus licheniformis. J Biol Chem. 1972 Aug 10;247(15):4893–4899. [PubMed] [Google Scholar]
- Bernlohr R. W. Changes in amino acid permeation during sporulation. J Bacteriol. 1967 Mar;93(3):1031–1044. doi: 10.1128/jb.93.3.1031-1044.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr R. W., Clark V. Characterization and regulation of protease synthesis and activity in Bacillus licheniformis. J Bacteriol. 1971 Jan;105(1):276–283. doi: 10.1128/jb.105.1.276-283.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnier V., Bazin M., Marchelidon J., Genevet M. Etude de la variation du pool intracellulaire des acides aminés libres d'une diatomée marine en fonction de la salinité. Bull Soc Chim Biol (Paris) 1969 Dec 18;51(9):1255–1262. [PubMed] [Google Scholar]
- COWIE D. B., McCLURE F. T. Metabolic pools and the synthesis of macromolecules. Biochim Biophys Acta. 1959 Jan;31(1):236–245. doi: 10.1016/0006-3002(59)90460-3. [DOI] [PubMed] [Google Scholar]
- Hubbard R. W., Kremen D. M. Increased sensitivity of accelerated amino acid ion-exchange chromatography. Anal Biochem. 1965 Sep;12(3):593–602. doi: 10.1016/0003-2697(65)90227-7. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Amino acid pool formation in Pseudomonas aeruginosa. J Bacteriol. 1969 Jan;97(1):282–291. doi: 10.1128/jb.97.1.282-291.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Amino acid transport in Pseudomonas aeruginosa. J Bacteriol. 1969 Jan;97(1):273–281. doi: 10.1128/jb.97.1.273-281.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- Moat A. G., Ahmad F., Alexander J. K., Barnes I. J. Alteration in the amino acid content of yeast during growth under various nutritional conditions. J Bacteriol. 1969 May;98(2):573–578. doi: 10.1128/jb.98.2.573-578.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. 18. Free amino acids in spores. J Biol Chem. 1970 Mar 10;245(5):1128–1136. [PubMed] [Google Scholar]
- Peters J. H., Berridge B. J., Jr, Cummings J. G., Lin S. C. Column chromatographic analysis of neutral and acidic amino acids using lithium buffers. Anal Biochem. 1968 Jun;23(3):459–465. doi: 10.1016/0003-2697(68)90237-6. [DOI] [PubMed] [Google Scholar]
- Peterson D. E., Bernlohr R. W. Determinaion of muraic acid, ornithine, and diaminopimelic acid during automatic amino aci analysis. Anal Biochem. 1970 Feb;33(2):238–243. doi: 10.1016/0003-2697(70)90292-7. [DOI] [PubMed] [Google Scholar]
- Tuominen F. W., Bernlohr R. W. Pyruvate kinase of the spore-forming bacterium, Bacillus licheniformis. I. Purification, stability, regulation of synthesis, and evidence for multiple molecular states. J Biol Chem. 1971 Mar 25;246(6):1733–1745. [PubMed] [Google Scholar]
- Umbarger H. E. Regulation of amino acid metabolism. Annu Rev Biochem. 1969;38:323–370. doi: 10.1146/annurev.bi.38.070169.001543. [DOI] [PubMed] [Google Scholar]


