Abstract
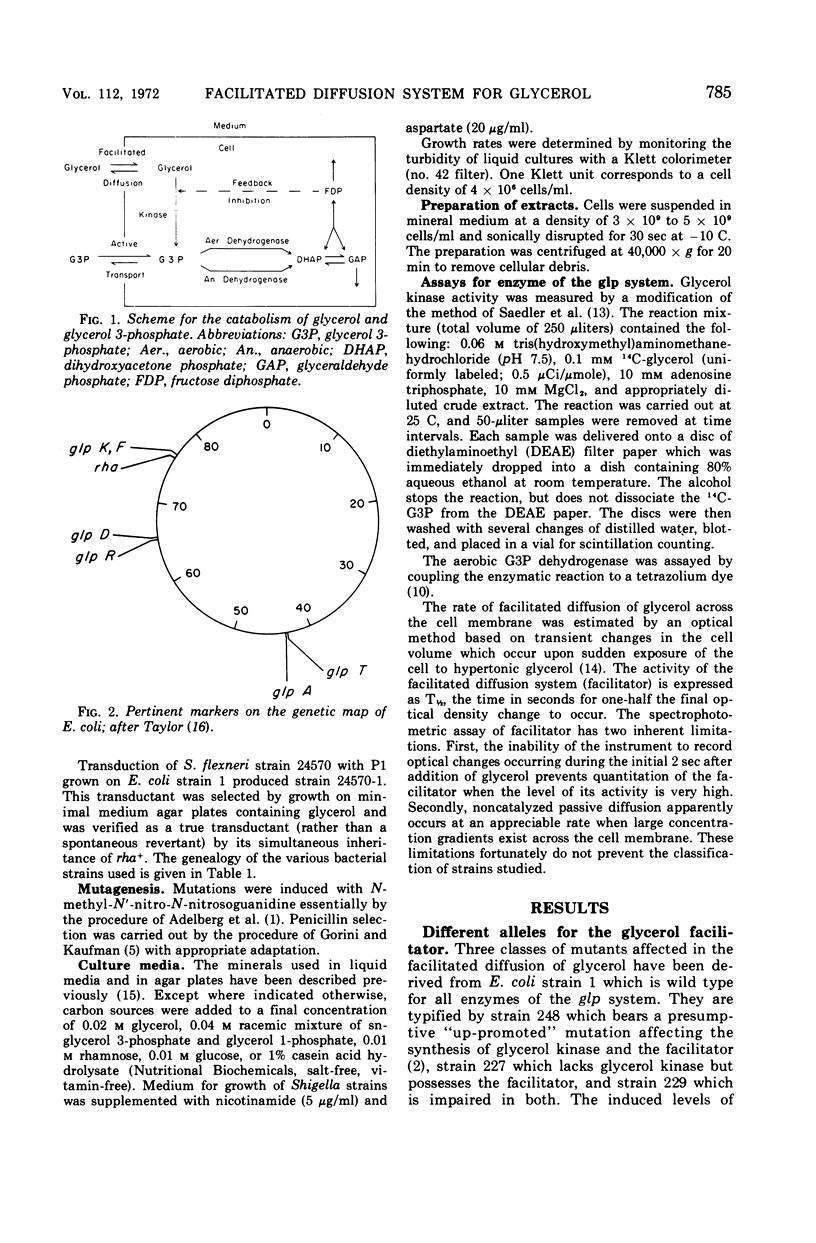
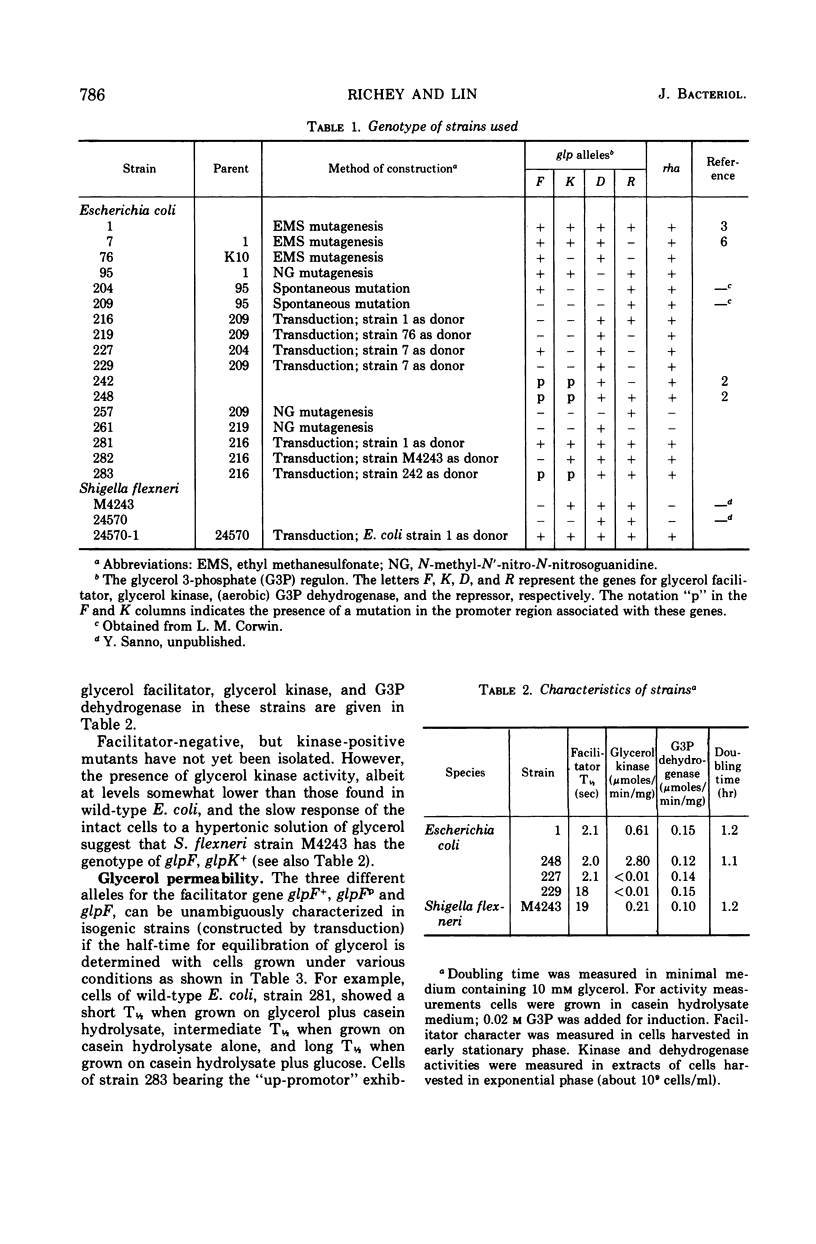
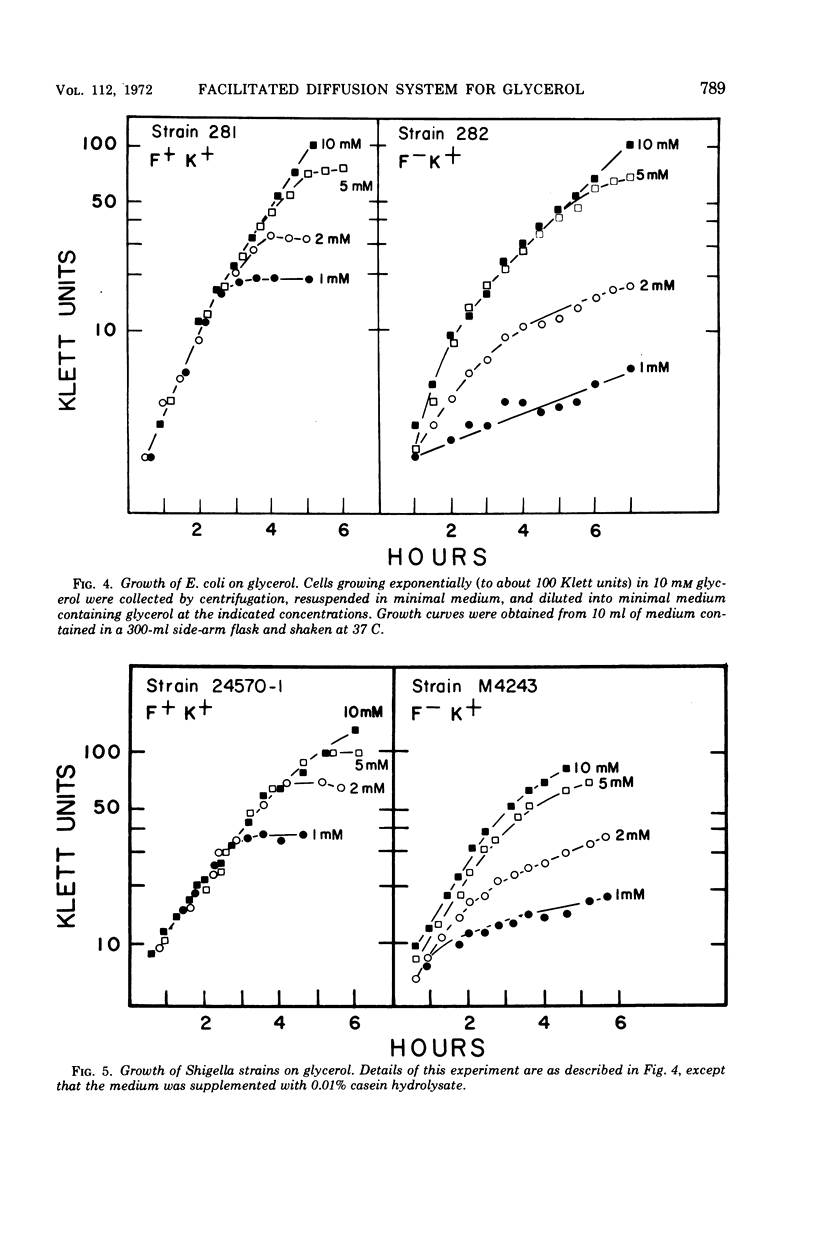
Wild-type Escherichia coli possesses an inducible permeation system which catalyzes facilitated diffusion of glycerol into the cell. A spectrophotometric method can be used to assess the presence of this mechanism. The structural gene for the facilitator (glpF) and the structural gene for glycerol kinase (glpK) apparently belong to a single operon. The glpF+ allele permits effective glycerol utilization by the cells, and, at millimolar concentrations of glycerol, cells carrying the glpF+ allele grow much faster than glpF genotypes. Although the glycerol-scavenging power of the cell depends both on the facilitated entry of the substrate and its subsequent trapping by an adenosine triphosphate-dependent phosphorylation, the two gene products, the facilitator and kinase, function independently. Wild-type Shigella flexneri appears to be glpK+ but glpF. This organism grows slowly in media at low concentrations of glycerol. When the glpF+ and glpK+ alleles of E. coli are inserted into the S. flexneri genome by transduction, the hybrid strain grows rapidly in low glycerol medium. Vice versa, when the glpF and glpK+ alleles of S. flexneri are incorporated into E. coli, the hybrid strain grows slowly in low glycerol medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman-Kurtz M., Lin E. C., Richey D. P. Promoter-like mutant with increased expression of the glycerol kinase operon of Escherichia coli. J Bacteriol. 1971 Jun;106(3):724–731. doi: 10.1128/jb.106.3.724-731.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Lin E. C. Chromosomal location of the structural gene for glycerol kinase in Escherichia coli. J Bacteriol. 1966 May;91(5):1763–1766. doi: 10.1128/jb.91.5.1763-1766.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- HIRSCHMANN H. The nature of substrate asymmetry in stereoselective reactions. J Biol Chem. 1960 Oct;235:2762–2767. [PubMed] [Google Scholar]
- Kistler W. S., Hirsch C. A., Cozzarelli N. R., Lin E. C. Second pyridine nucleotide-independent 1-alpha-glycerophosphate dehydrogenase in Escherichia coli K-12. J Bacteriol. 1969 Nov;100(2):1133–1135. doi: 10.1128/jb.100.2.1133-1135.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Saedler H., Gullon A., Fiethen L., Starlinger P. Negative control of the galactose operon in E. coli. Mol Gen Genet. 1968;102(1):79–88. doi: 10.1007/BF00341872. [DOI] [PubMed] [Google Scholar]
- Sanno Y., Wilson T. H., Lin E. C. Control of permeation to glycerol in cells of Escherichia coli. Biochem Biophys Res Commun. 1968 Jul 26;32(2):344–349. doi: 10.1016/0006-291x(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay S. S., Brown K. K., Gaudy E. T. Transport of glycerol by Pseudomonas aeruginosa. J Bacteriol. 1971 Oct;108(1):82–88. doi: 10.1128/jb.108.1.82-88.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaig N., Kistler W. S., Lin E. C. Glycerol kinase, the pacemaker for the dissimilation of glycerol in Escherichia coli. J Bacteriol. 1970 Jun;102(3):753–759. doi: 10.1128/jb.102.3.753-759.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaig N., Lin E. C. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science. 1966 Aug 12;153(3737):755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]


