Abstract
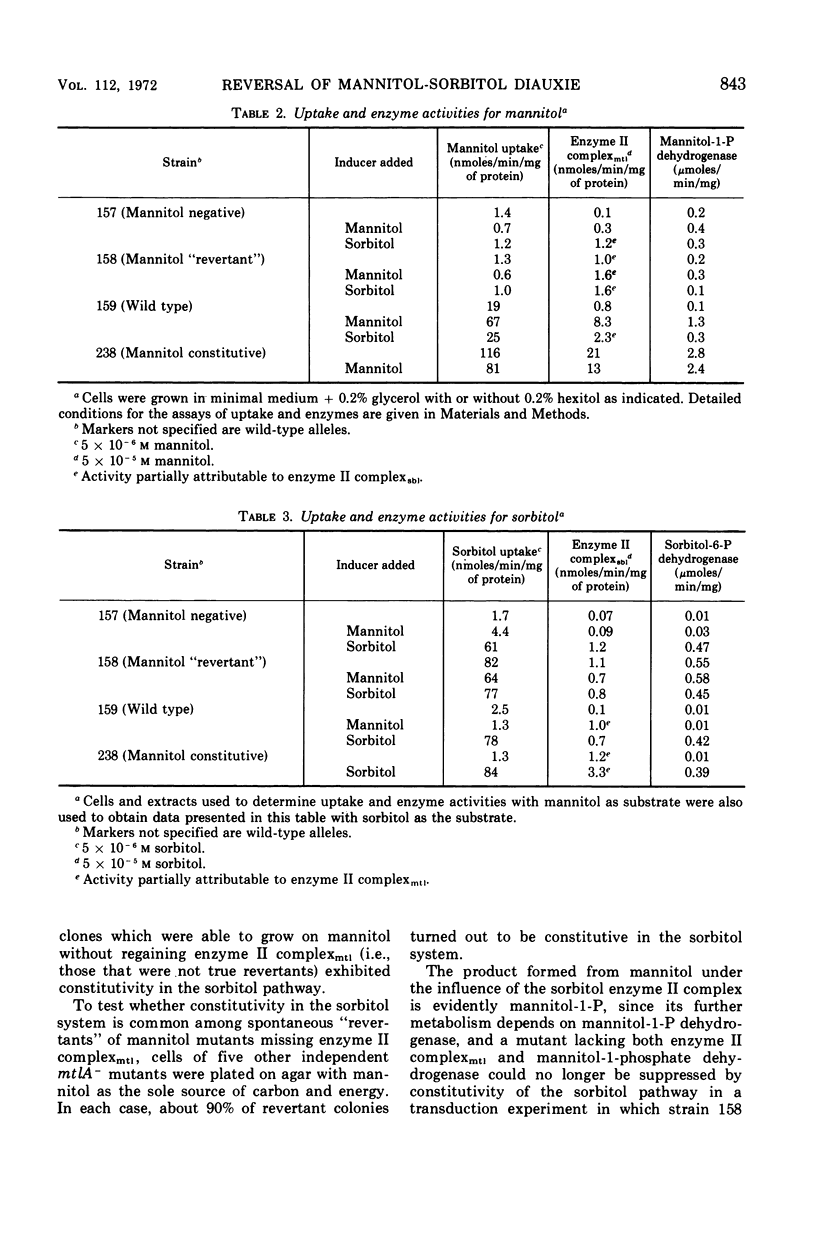
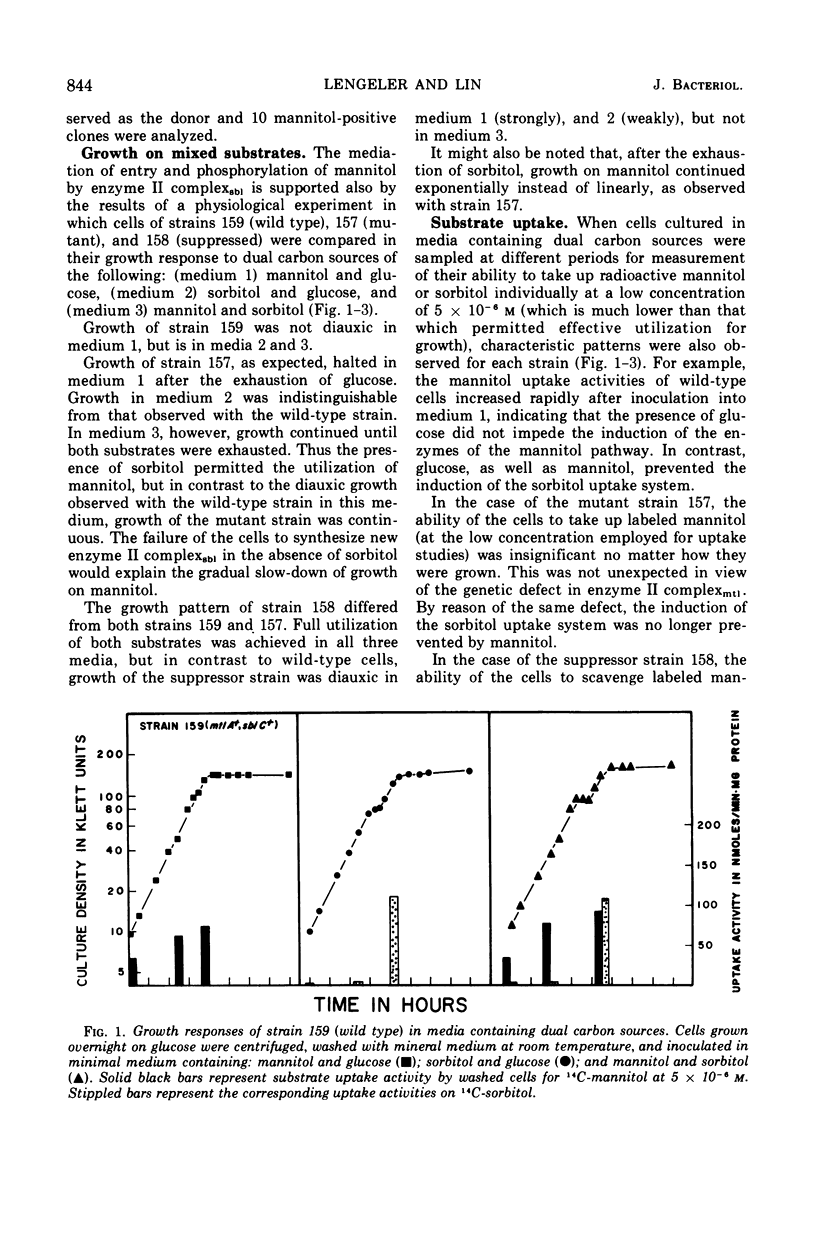
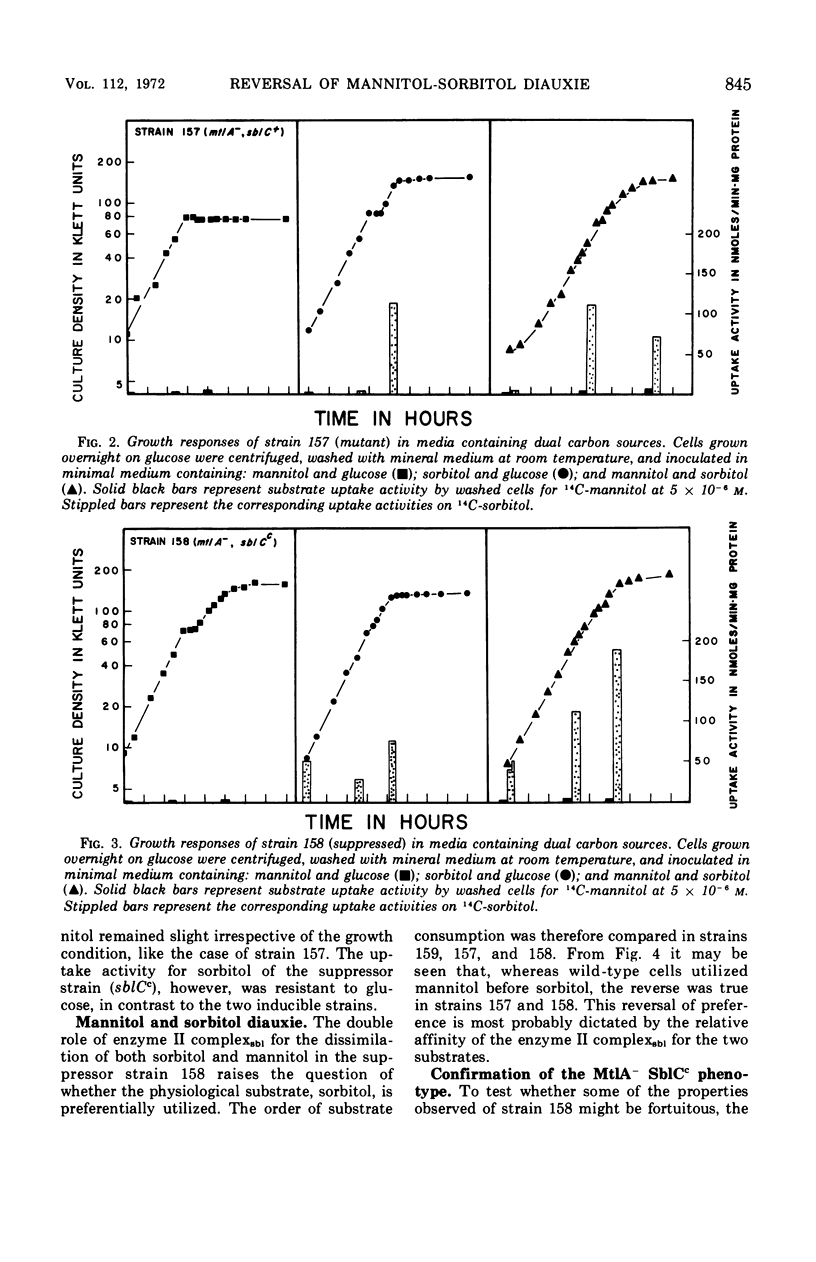
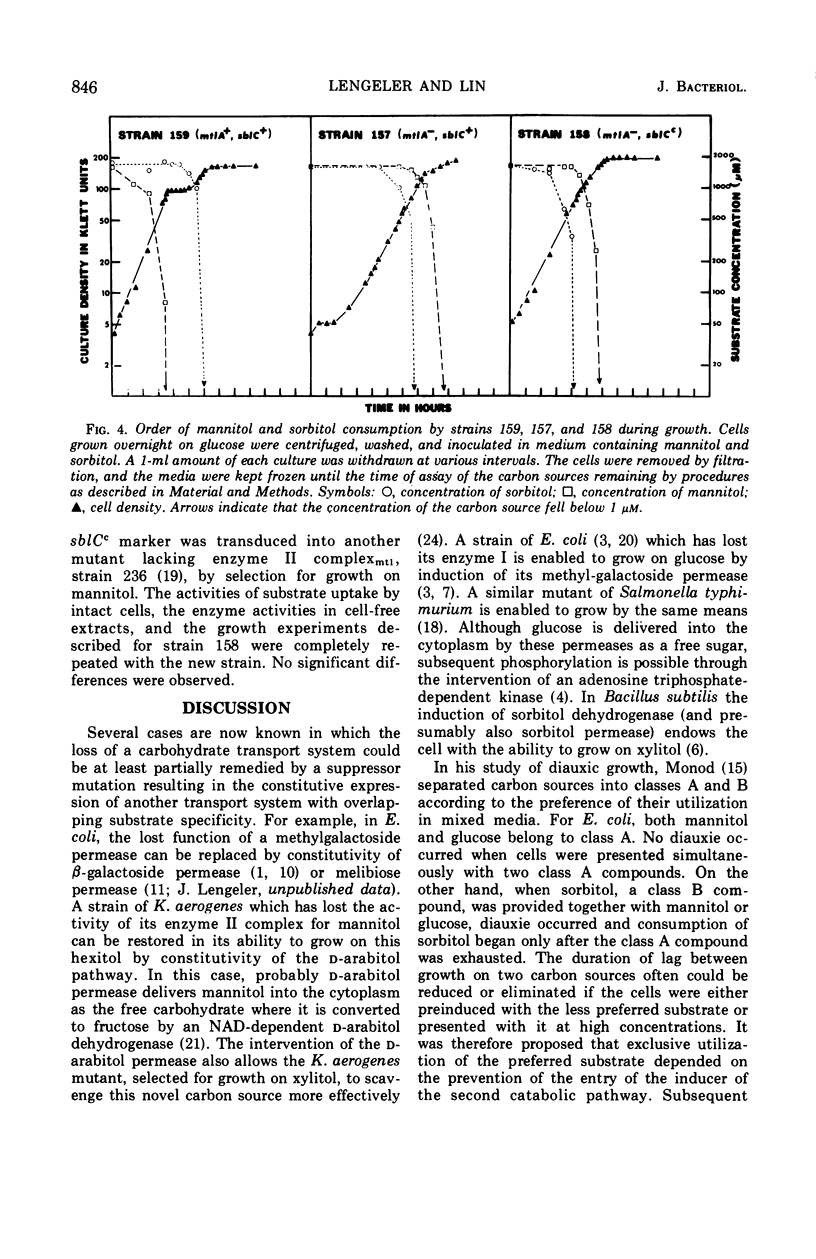
In Escherichia coli K-12 the proteins involved in the dissimilation of mannitol and sorbitol are specified by two separate gene clusters. The mannitol cluster appears to consist of a regulatory gene mtlC, a gene mtlA coding an enzyme II complex of the phosphoenolpyruvate phosphotransferase system, and a gene mtlD coding a mannitol-1-phosphate dehydrogenase. Three corresponding genes, sblC, sblA, and sblD, exist for the sorbitol pathway. In both pathways the hexitol captured from the medium and delivered into the cytoplasm as a phosphorylated compound is dehydrogenated to fructose-6-phosphate. The enzyme II complex for sorbitol is able to catalyze the phosphorylation also of mannitol if this substrate is present at high concentrations. Consequently mtlA− mutants lacking the enzyme II complex for mannitol can grow on mannitol either if the sorbitol phosphorylating system is preinduced by sorbitol or if mtlA is suppressed by a mutation of sblC to constitutivity. In wild-type cells, the induction of the enzymes in the mannitol pathway and dissimilation of the substrate are not prevented by glucose. The sorbitol system, however, is sensitive to glucose and to mannitol as well. In the suppressed strains (mtlA−, sblCc) in which mannitol is utilized through the sorbitol enzyme, glucose becomes effective in restraining the consumption of mannitol, causing a definite diauxie. Moreover, in a mixture of mannitol and sorbitol, the latter is utilized preferentially. This reversal of normal diauxic pattern is consequent to the fact that the enzyme II complex for sorbitol has relatively poor affinity for mannitol.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- ASENSIO C., AVIGAD G., HORECKER B. L. PREFERENTIAL GALACTOSE UTILIZATION IN A MUTANT STRAIN OF E. COLI. Arch Biochem Biophys. 1963 Dec;103:299–309. doi: 10.1016/0003-9861(63)90419-3. [DOI] [PubMed] [Google Scholar]
- Adhya S., Echols H. Glucose effect and the galactose enzymes of Escherichia coli: correlation between glucose inhibition of induction and inducer transport. J Bacteriol. 1966 Sep;92(3):601–608. doi: 10.1128/jb.92.3.601-608.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W., Lengeler J., Hermann K. O., Unsöld H. J. The regulation of the beta-methylgalactoside transport system and of the galactose binding protein of Escherichia coli K12. Eur J Biochem. 1971 Apr 30;19(4):457–470. doi: 10.1111/j.1432-1033.1971.tb01336.x. [DOI] [PubMed] [Google Scholar]
- FRAENKEL D. G., FALCOZ-KELLY F., HORECKER B. L. THE UTILIZATION OF GLUCOSE 6-PHOSPHATE BY GLUCOKINASELESS AND WILD-TYPE STRAINS OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Nov;52:1207–1213. doi: 10.1073/pnas.52.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORWITZ S. B., KAPLAN N. O. HEXITOL DEHYDROGENASES OF BACILLUS SUBTILIS. J Biol Chem. 1964 Mar;239:830–838. [PubMed] [Google Scholar]
- KORN E. D. Clearing factor, a heparin-activated lipoprotein lipase. I. Isolation and characterization of the enzyme from normal rat heart. J Biol Chem. 1955 Jul;215(1):1–14. [PubMed] [Google Scholar]
- Kamogawa A., Kurahashi K. Inhibitory effect of glucose on the growth of a mutant strain of Escherichia coli defective in glucose transport system. J Biochem. 1967 Feb;61(2):220–230. doi: 10.1093/oxfordjournals.jbchem.a128534. [DOI] [PubMed] [Google Scholar]
- Kelker N. E., Anderson R. L. Sorbitol metabolism in Aerobacter aerogenes. J Bacteriol. 1971 Jan;105(1):160–164. doi: 10.1128/jb.105.1.160-164.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C. An inducible D-arabitol dehydrogenase from Aerobacter aerogenes. J Biol Chem. 1961 Jan;236:31–36. [PubMed] [Google Scholar]
- Lengeler J. Untersuchungen zum Glukose-Effekt bei der Synthese der Galaktose-Enzyme von Escherichia coli. Z Vererbungsl. 1966;98(3):203–229. [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- Pascal M. C., Puig J., Lepelletier M. Etude génétique d'une mutation affectant l'activité L-actate-déshydrogénase chez escherichia coli K 12. C R Acad Sci Hebd Seances Acad Sci D. 1969 Jan 27;268(4):737–739. [PubMed] [Google Scholar]
- Solomon E., Lin E. C. Mutations affecting the dissimilation of mannitol by Escherichia coli K-12. J Bacteriol. 1972 Aug;111(2):566–574. doi: 10.1128/jb.111.2.566-574.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFF J. B., KAPLAN N. O. Hexitol metabolism in Escherichia coli. J Bacteriol. 1956 May;71(5):557–564. doi: 10.1128/jb.71.5.557-564.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T., Lin E. C., Tanaka S. Mutants of Aerobacter aerogenes capable of utilizing xylitol as a novel carbon. J Bacteriol. 1968 Aug;96(2):447–456. doi: 10.1128/jb.96.2.447-456.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaig N., Kistler W. S., Lin E. C. Glycerol kinase, the pacemaker for the dissimilation of glycerol in Escherichia coli. J Bacteriol. 1970 Jun;102(3):753–759. doi: 10.1128/jb.102.3.753-759.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


