Abstract
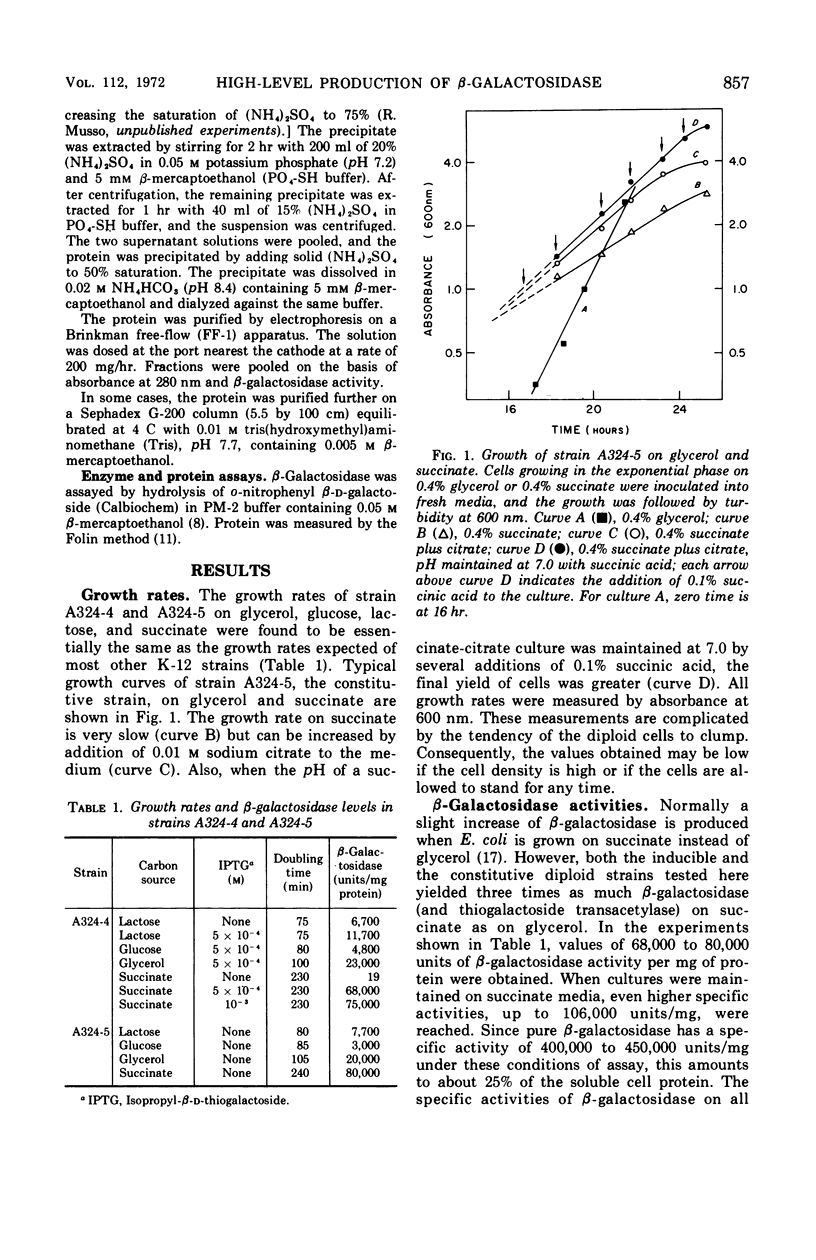
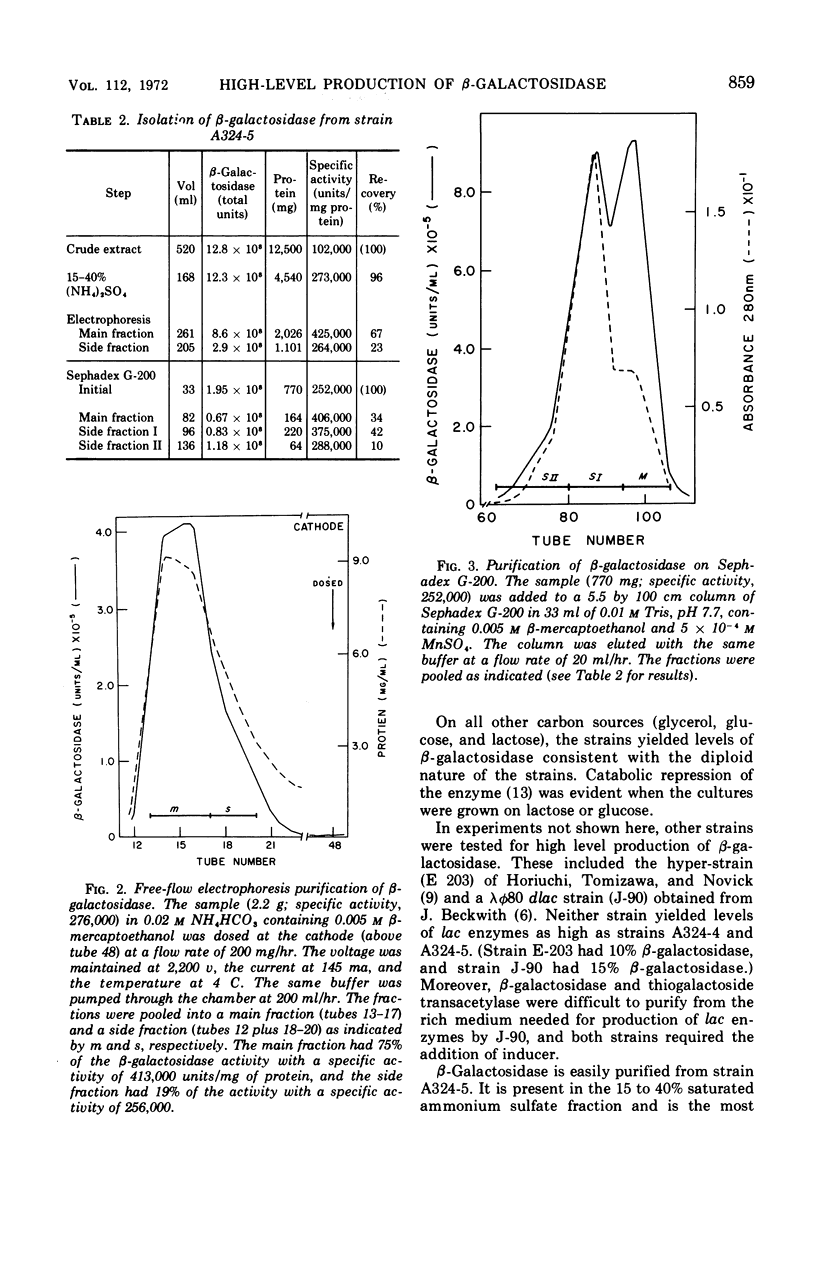
Two merodiploids of Escherichia coli that contain genes for the lac operon on both chromosome and episome were tested for production of lac enzymes after growth on various carbon sources. The specific activity of β-galactosidase (and of thiogalactoside transacetylase) was about twice that from haploid cells when grown on glycerol. With succinate as carbon source, the specific activity increased by an additional factor of 3. Up to 25% of the soluble cell protein is β-galactosidase in these strains, one of which is inducible and the other constitutive. The enzyme is purified easily in high yield by ammonium sulfate fractionation and electrophoresis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPEL S. H., ALPERS D. H., TOMKINS G. M. MULTIPLE MOLECULAR FORMS OF BETA-GALACTOSIDASE. J Mol Biol. 1965 Jan;11:12–22. doi: 10.1016/s0022-2836(65)80167-x. [DOI] [PubMed] [Google Scholar]
- CRAVEN G. R., STEERS E., Jr, ANFINSEN C. B. PURIFICATION, COMPOSITION, AND MOLECULAR WEIGHT OF THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2468–2477. [PubMed] [Google Scholar]
- Fowler A. V. The amino acid sequence of -galactosidase. II. Tryptic peptides of the maleylated protein and sequences of some tryptic peptides. J Biol Chem. 1972 Sep 10;247(17):5425–5431. [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Co-linearity of beta-galactosidase with its gene by immunological detection of incomplete polypeptide chains. Science. 1966 Nov 25;154(3752):1027–1029. doi: 10.1126/science.154.3752.1027. [DOI] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. The amino acid sequence of beta galactosidase. I. Isolation and composition of tryptic peptides. J Biol Chem. 1970 Oct 10;245(19):5032–5041. [PubMed] [Google Scholar]
- HERZENBERG L. A. Studies on the induction of beta-galactosidase in a cryptic strain of Escherichia coli. Biochim Biophys Acta. 1959 Feb;31(2):525–538. doi: 10.1016/0006-3002(59)90029-0. [DOI] [PubMed] [Google Scholar]
- HORIUCHI T., TOMIZAWA J. I., NOVICK A. Isolation and properties of bacteria capable of high rates of beta-galactosidase synthesis. Biochim Biophys Acta. 1962 Jan 22;55:152–163. doi: 10.1016/0006-3002(62)90941-1. [DOI] [PubMed] [Google Scholar]
- Hamlin J., Zabin I. -Galactosidase: immunological activity of ribosome-bound, growing polypeptide chains. Proc Natl Acad Sci U S A. 1972 Feb;69(2):412–416. doi: 10.1073/pnas.69.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARLSSON U., KOORAJIAN S., ZABIN I., SJOESTRAND F. S., MILLER A. HIGH RESOLUTION ELECTRON MICROSCOPY ON HIGHLY PURIFIED BETA-GALACTOSIDASE FROM ESCHERICHIA COLI. J Ultrastruct Res. 1964 Jun;10:457–469. doi: 10.1016/s0022-5320(64)80022-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin S., Zabin I. Beta-galactosidase. Rates of synthesis and degradation of incomplete chains. J Biol Chem. 1972 Apr 10;247(7):2205–2211. [PubMed] [Google Scholar]
- Marchesi S. L., Steers E., Jr, Shifrin S. Purification and characterization of the multiple forms of beta-galactosidase of Escherichia coli. Biochim Biophys Acta. 1969 May;181(1):20–34. doi: 10.1016/0005-2795(69)90223-2. [DOI] [PubMed] [Google Scholar]
- Newton W. A., Beckwith J. R., Zipser D., Brenner S. Nonsense mutants and polarity in the lac operon of Escherichia coli. J Mol Biol. 1965 Nov;14(1):290–296. doi: 10.1016/s0022-2836(65)80250-9. [DOI] [PubMed] [Google Scholar]
- Villarejo M., Zamenhof P. J., Zabin I. Beta-galactosidase. In vivo -complementation. J Biol Chem. 1972 Apr 10;247(7):2212–2216. [PubMed] [Google Scholar]
- Zabin I., Fowler A. V. The amino acid sequence of -galactosidase. 3. The sequences of NH 2 - and COOH-terminal tryptic peptides. J Biol Chem. 1972 Sep 10;247(17):5432–5435. [PubMed] [Google Scholar]


