Abstract
Minicells produced by abnormal cell division in a strain of Escherichia coli (K-12) have been employed here to investigate the phenomenon of “entry exclusion.” When purified minicells from strains containing F′ or R factors, or both, are mated with radioactive thymidine-labeled Hfr or R+ donors, the recipient minicells can be conveniently separated from normal-sized donors following mating, and the products of conjugation can be analyzed in the absence of donors and of further growth of the recipients. Transmissible plasmids or episomes are transferred less efficiently to purified minicells derived from strains carrying similar or related elements than to strains without them. Measurement of deoxyribonucleic acid (DNA) degradation and determination of weight-average molecular weights following transfer indicate that degradation of transferred DNA or transfer of smaller pieces cannot account for the comparative reduction in transfer to entry-excluding recipients. Therefore, we conclude that entry exclusion operates to prevent the physical entry of DNA into recipients expressing the exclusion phenotype. The R-produced repressor (product of the drd+ gene), which represses fertility (i.e., ability to act as donor), reduces exclusion mediated by R or F factor, or both, in matings between strains carrying homologous elements. Furthermore, the data suggest that the presence of the F pilus or F-like R pilus on recipient cells ensures maximum expression of the exclusion phenotype but is not essential for its expression. In contrast to previous suggestions, we found no evidence for a reduction of entry exclusion attributable to the DNA temperature-sensitive chromosomal mutation dnaB(TS).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Paper strip method for assaying gradient fractions containing radioactive macromolecules. Anal Biochem. 1971 Oct;43(2):427–432. doi: 10.1016/0003-2697(71)90272-7. [DOI] [PubMed] [Google Scholar]
- Cohen A., Fisher W. D., Curtiss R., 3rd, Adler H. I. DNA isolated from Escherichia coli minicells mated with F+ cells. Proc Natl Acad Sci U S A. 1968 Sep;61(1):61–68. doi: 10.1073/pnas.61.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd Bacterial conjugation. Annu Rev Microbiol. 1969;23:69–136. doi: 10.1146/annurev.mi.23.100169.000441. [DOI] [PubMed] [Google Scholar]
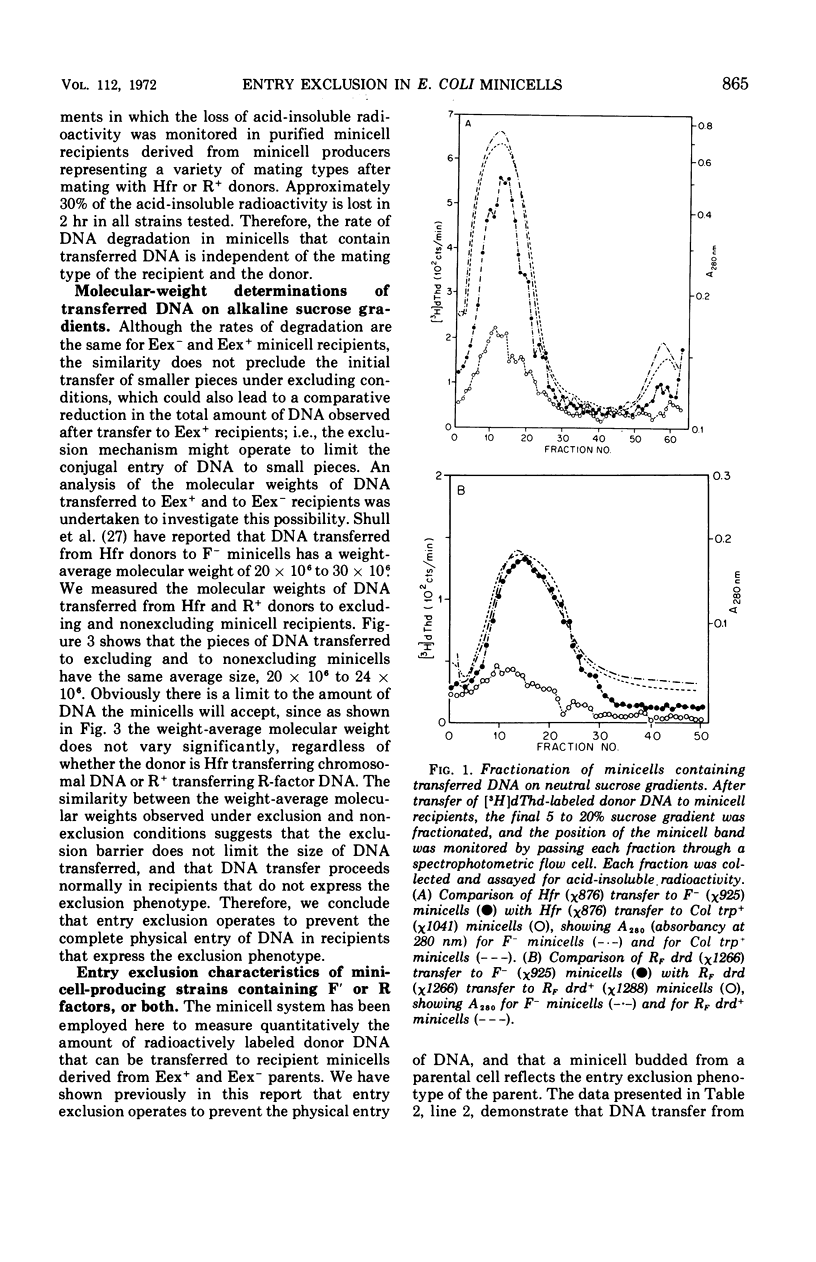
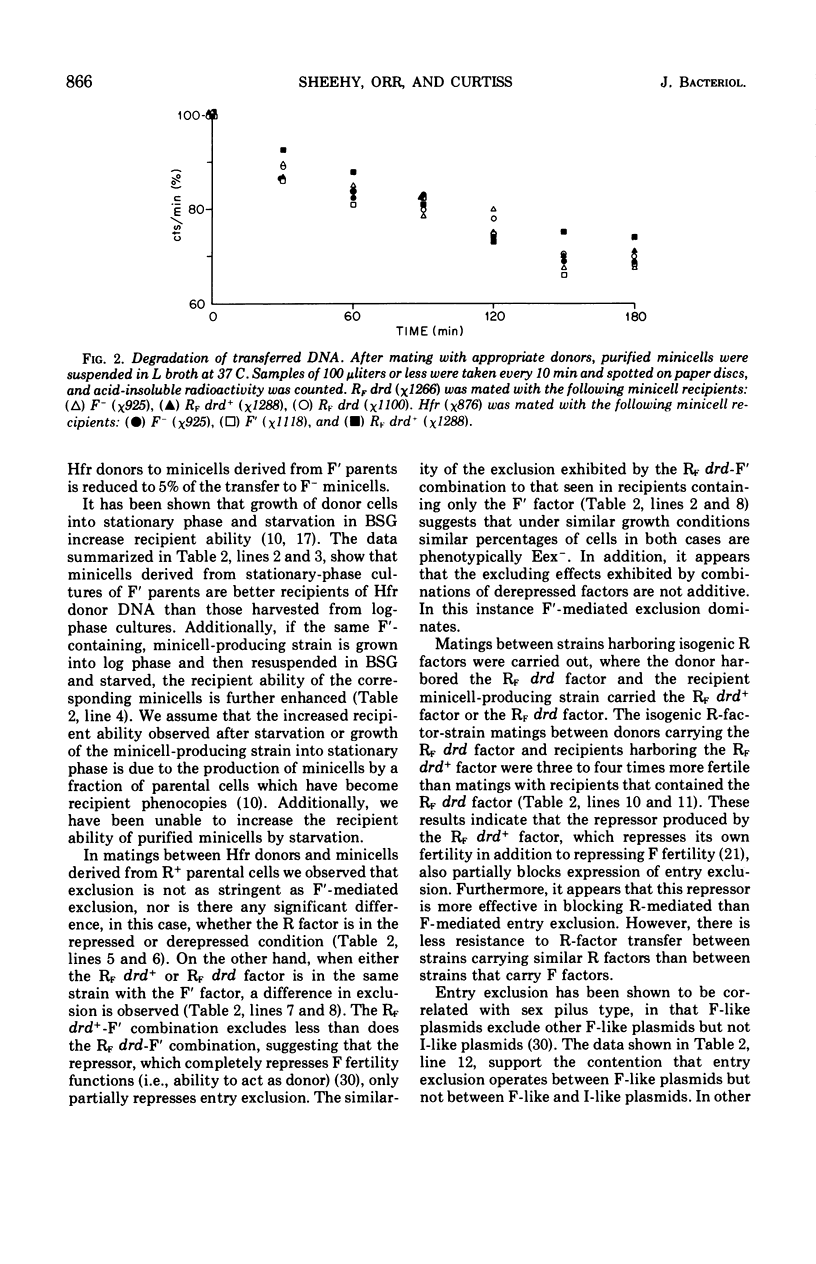
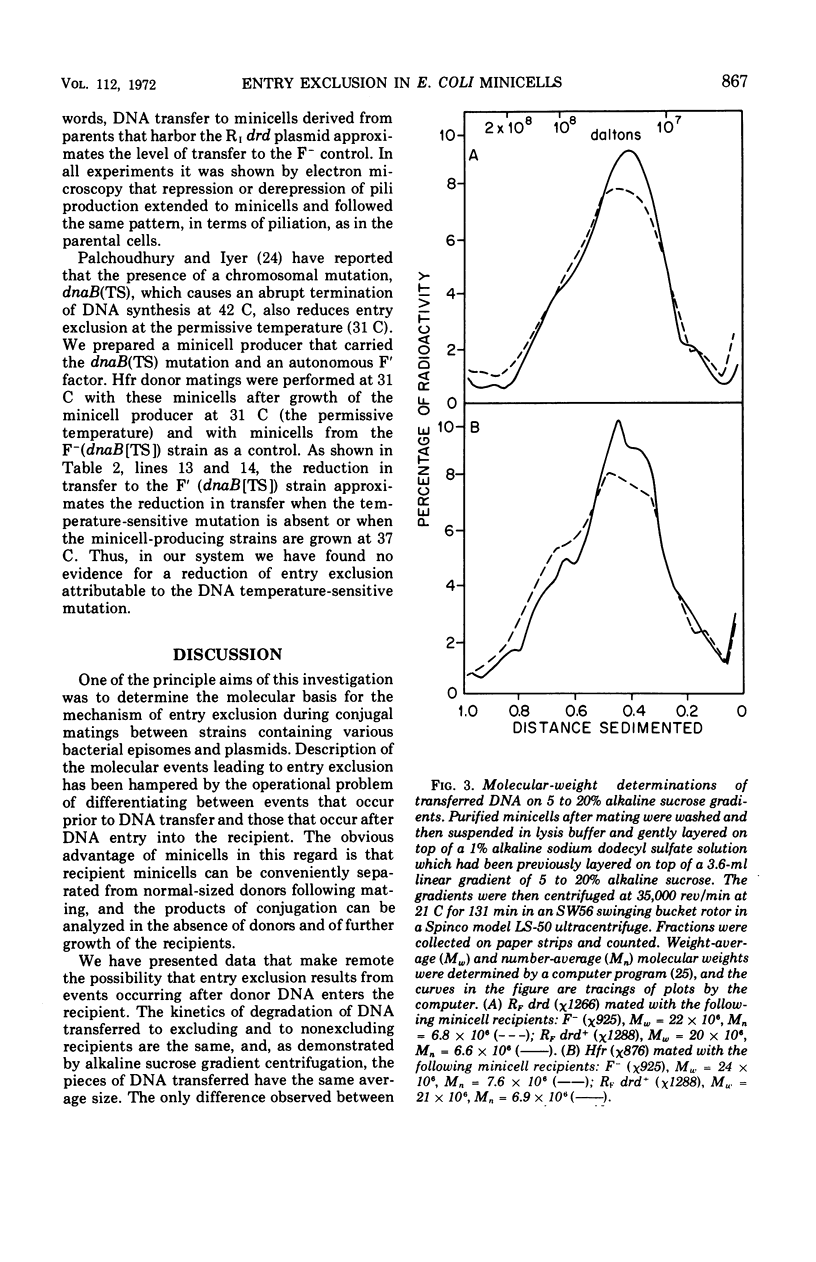
- Curtiss R., 3rd, Caro L. G., Allison D. P., Stallions D. R. Early stages of conjugation in Escherichia coli. J Bacteriol. 1969 Nov;100(2):1091–1104. doi: 10.1128/jb.100.2.1091-1104.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Stallions D. R., Mays J. A. Parental functions during conjugation in Escherichia coli K-12. Bacteriol Rev. 1968 Dec;32(4 Pt 1):320–348. [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd Ultraviolet-induced genetic recombination in a partially diploid strain of Escherichia coli. Genetics. 1968 Jan;58(1):9–54. doi: 10.1093/genetics/58.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Tompkins L. S., Silver R. P., Guerry P., Le Blanc D. J. The problems of drug-resistant pathogenic bacteria. The replication of R-factor DNA in Escherichia coli K-12 following conjugation. Ann N Y Acad Sci. 1971 Jun 11;182:153–171. doi: 10.1111/j.1749-6632.1971.tb30654.x. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J., Willetts N. S. Two classes of Flac mutants insensitive to transfer inhibition by an F-like R factor. Mol Gen Genet. 1971;111(3):256–264. doi: 10.1007/BF00433110. [DOI] [PubMed] [Google Scholar]
- Freifelder D. R., Freifelder D. Studies on Escherichia coli sex factors. I. Specific labeling of F'Lac DNA. J Mol Biol. 1968 Feb 28;32(1):15–23. doi: 10.1016/0022-2836(68)90141-1. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lederberg J, Iino T. Phase Variation in Salmonella. Genetics. 1956 Sep;41(5):743–757. doi: 10.1093/genetics/41.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATNEY T. S., ACHENBACH N. E. New uses for membrane filters III. Bacterial mating procedure. J Bacteriol. 1962 Oct;84:874–875. doi: 10.1128/jb.84.4.874-875.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic D. Genetic loads affecting fecundity in natural populations of Drosophila pseudoobscura. Genetics. 1967 May;56(1):61–71. doi: 10.1093/genetics/56.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Malamy M. H. Comparisons of F factors and R factors: existence of independent regulation groups in F factors. J Bacteriol. 1970 Jul;103(1):81–88. doi: 10.1128/jb.103.1.81-88.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchoudhury S. R., Iyer V. N. Compatibility between two F' factors in an Escherichia coli strain bearing a chromosomal mutation affecting DNA synthesis. J Mol Biol. 1971 Apr 28;57(2):319–333. doi: 10.1016/0022-2836(71)90349-4. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B., Ley R. D. Normal and defective repair of damaged DNA in human cells: a sensitive assay utilizing the photolysis of bromodeoxyuridine. Proc Natl Acad Sci U S A. 1971 Apr;68(4):708–712. doi: 10.1073/pnas.68.4.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull F. W., Jr, Fralick J. A., Stratton L. P., Fisher W. D. Membrane association of conjugally transferred deoxyribonucleic acid in Escherichia coli minicells. J Bacteriol. 1971 May;106(2):626–633. doi: 10.1128/jb.106.2.626-633.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneath P. H., Lederberg J. INHIBITION BY PERIODATE OF MATING IN ESCHERICHIA COLI K-12. Proc Natl Acad Sci U S A. 1961 Jan;47(1):86–90. doi: 10.1073/pnas.47.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallions D. R., Curtiss R., 3rd Chromosome transfer and recombinant formation with deoxyribonucleic acid temperature-sensitive strains of Escherichia coli. J Bacteriol. 1971 Mar;105(3):886–895. doi: 10.1128/jb.105.3.886-895.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., NISHIDA H., OGATA C., ARAI T., SATO S. EPISOME-MEDIATED TRANSFER OF DRUG RESISTANCE IN ENTEROBACTERIACEAE. VII. TWO TYPES OF NATURALLY OCCURRING R FACTORS. J Bacteriol. 1964 Sep;88:716–726. doi: 10.1128/jb.88.3.716-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Finnegan D. J. Characteristics of E. coli K12 strains carrying both an F prime and an R factor. Genet Res. 1970 Aug;16(1):113–122. doi: 10.1017/s0016672300002329. [DOI] [PubMed] [Google Scholar]
- Willetts N. S. Plasmid specificity of two proteins required for conjugation in E. coli K12. Nat New Biol. 1971 Apr 7;230(14):183–185. doi: 10.1038/newbio230183a0. [DOI] [PubMed] [Google Scholar]



