Abstract
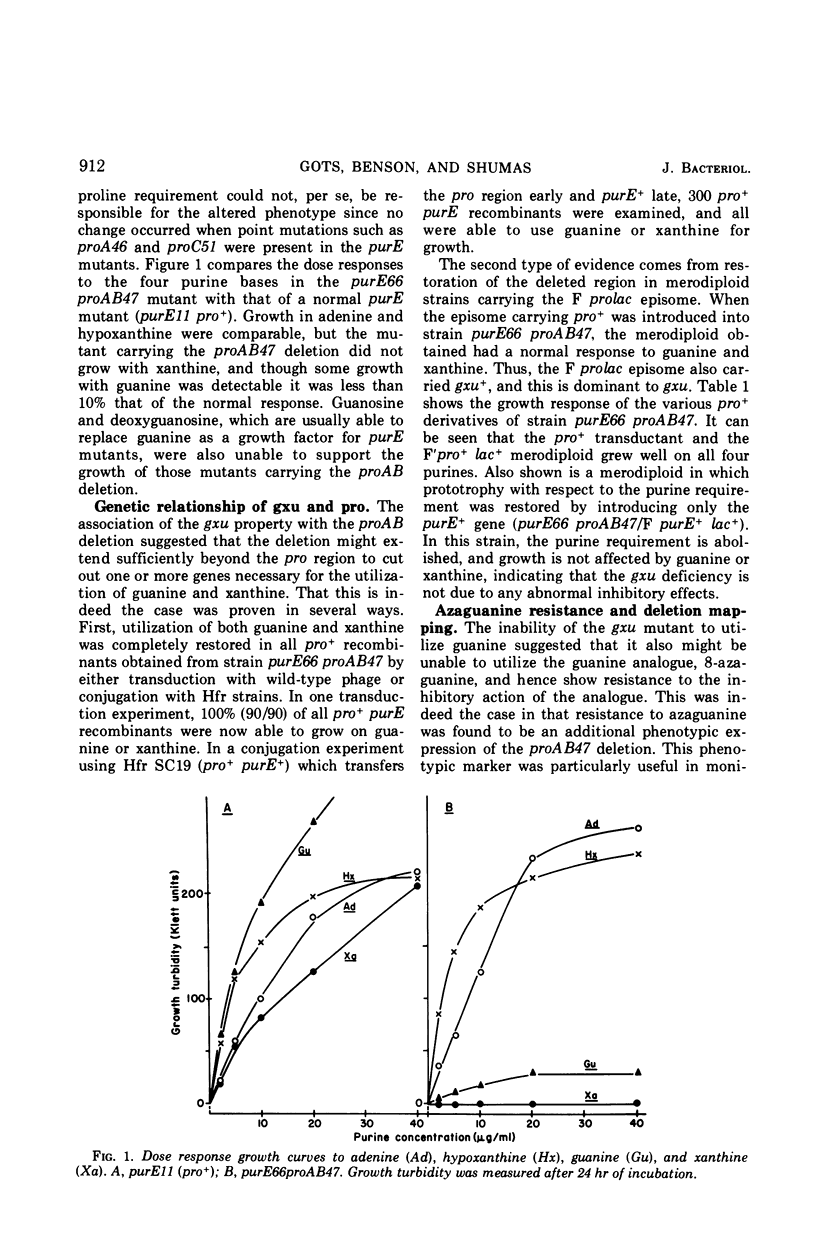
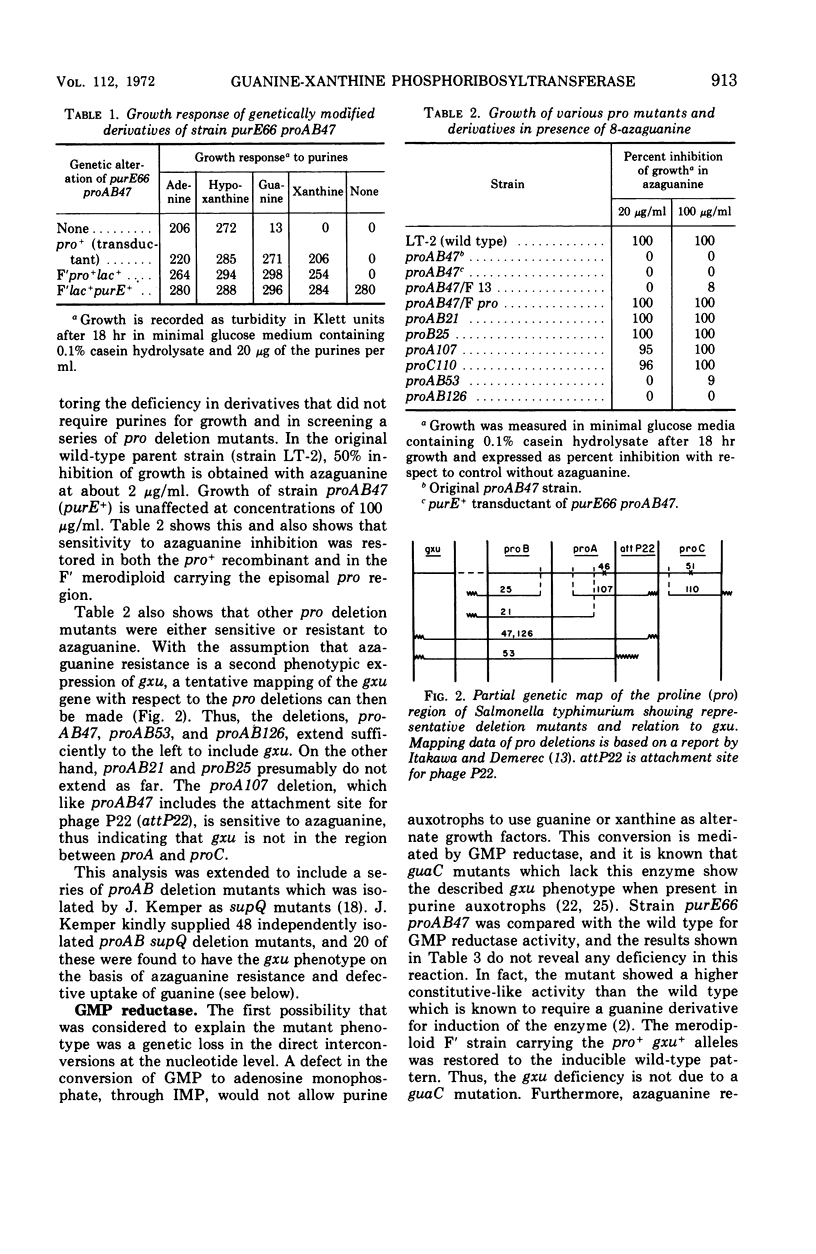
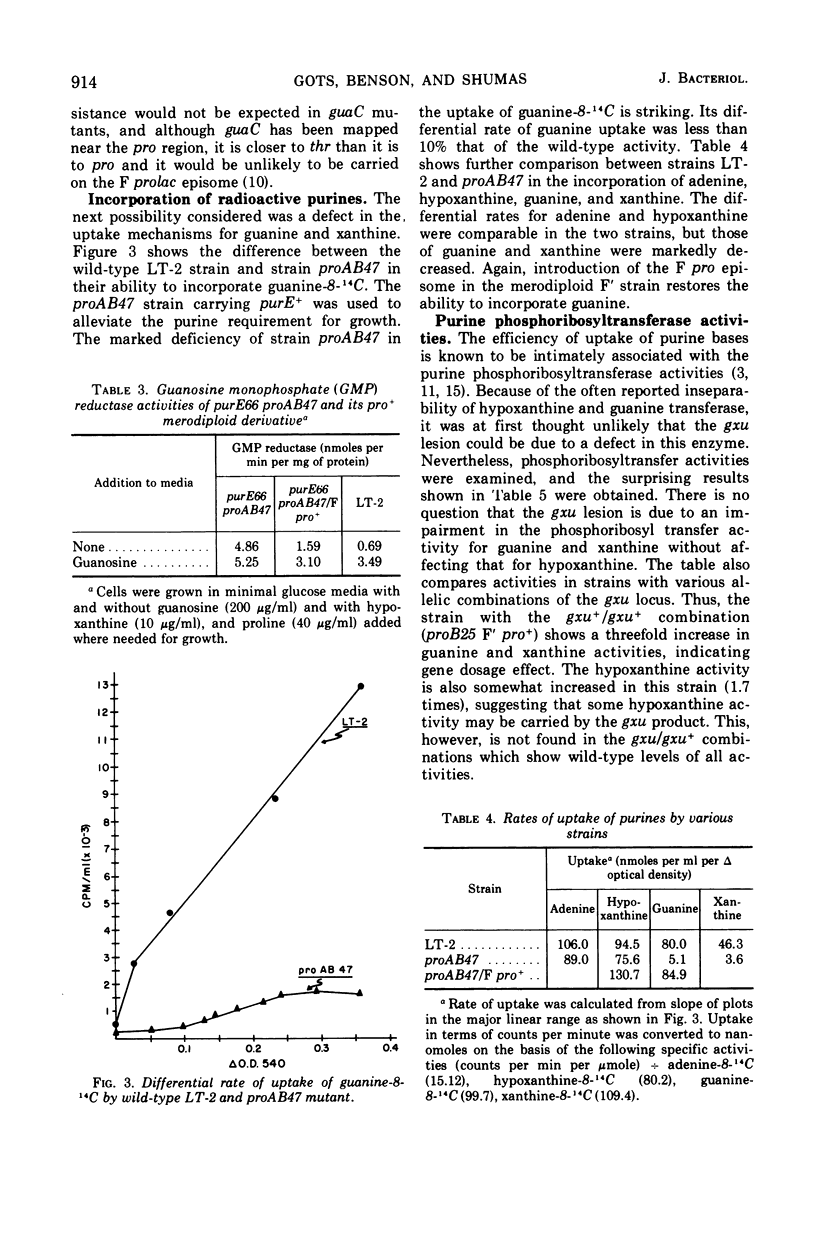
Certain proAB deletion mutants of Salmonella typhimurium were found to be simultaneously deleted in a gene required for the utilization of guanine and xanthine (designated gxu). These mutants were resistant to 8-azaguanine and when carrying an additional pur mutation were unable to use guanine or xanthine as a purine source. The defect was correlated with deficiencies in the uptake and phosphoribosyltransferase activities for guanine and xanthine. Hypoxanthine and adenine activities were unaltered. The deficiency was restored to normal by transduction to pro+ and in F′ merodiploids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adye J. C., Gots J. S. Further studies on genetically altered purine nucleotide pyrophosphorylases of Salmonella. Biochim Biophys Acta. 1966 May 5;118(2):344–350. doi: 10.1016/s0926-6593(66)80043-7. [DOI] [PubMed] [Google Scholar]
- Benson C. E., Brehmeyer B. A., Gots J. S. Requirement of cyclic AMP for induction of GMP reductase in Escherichia coli. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1089–1094. doi: 10.1016/0006-291x(71)90573-0. [DOI] [PubMed] [Google Scholar]
- Berlin R. D., Stadtman E. R. A possible role of purine nucleotide pyrophosphorylases in the regulation of purine uptake by Bacillus subtilis. J Biol Chem. 1966 Jun 10;241(11):2679–2686. [PubMed] [Google Scholar]
- Brockman R. W. Resistance to purine antagonists in experimental leukemia systems. Cancer Res. 1965 Oct;25(9):1596–1605. [PubMed] [Google Scholar]
- CARTER C. E. Reaction of 6-mercaptopurine with inosine- and guanosine-5'-phosphate pyrophosphorylase purified from E. coli. Biochem Pharmacol. 1959 Aug;2:105–111. doi: 10.1016/0006-2952(59)90077-2. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Purine phosphoribosyltransferases of Salmonella typhimurium. J Bacteriol. 1972 Nov;112(2):1010–1013. doi: 10.1128/jb.112.2.1010-1013.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggin J. H., Loosemore M., Martin W. R. Metabolism of 6-Mercaptopurine by Resistant Escherichia coli Cells. J Bacteriol. 1966 Aug;92(2):446–454. doi: 10.1128/jb.92.2.446-454.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan P. G., Hoekstra W. P., Verhoef C., Felix H. S. Recombination in Escherichia coli. 3. Mapping by the gradient of transmission. Mutat Res. 1969 Nov-Dec;8(3):505–512. doi: 10.1016/0027-5107(69)90067-0. [DOI] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. III. The involvement of purine phosphoribosyltransferases in the uptake of adenine and other nucleic acid precursors by intact resting cells. J Biol Chem. 1971 Sep 10;246(17):5312–5320. [PubMed] [Google Scholar]
- Hoffmeyer J., Neuhard J. Metabolism of exogenous purine bases and nucleosides by Salmonella typhimurium. J Bacteriol. 1971 Apr;106(1):14–24. doi: 10.1128/jb.106.1.14-24.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itikawa H., Demerec M. Salmonella typhimurium proline mutants. J Bacteriol. 1968 Mar;95(3):1189–1190. doi: 10.1128/jb.95.3.1189-1190.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLE G. P., GOTS J. S. Alterations in purine nucleotide pyrophosphorylases and resistance to purine analogues. Biochim Biophys Acta. 1961 Oct 14;53:166–173. doi: 10.1016/0006-3002(61)90803-4. [DOI] [PubMed] [Google Scholar]
- KALLE G. P., GOTS J. S. GENETIC ALTERATION OF ADENYLIC PYROPHOSPHORYLASE IN SALMONELLA. Science. 1963 Nov 8;142(3593):680–681. doi: 10.1126/science.142.3593.680. [DOI] [PubMed] [Google Scholar]
- KALLE G. P., GOTS J. S. Mechanism of resistance to 2,6-diaminopurine in Salmonella typhimurium. Biochim Biophys Acta. 1961 Jul 22;51:130–137. doi: 10.1016/0006-3002(61)91023-x. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. Xanthine phosphoribosyltransferase in man: relationship to hypoxanthine-guanine phosphoribosyltransferase. Biochem Biophys Res Commun. 1967 Aug 7;28(3):340–345. doi: 10.1016/0006-291x(67)90315-4. [DOI] [PubMed] [Google Scholar]
- Kemper J., Margolin P. Suppression by gene substitution for the leuD gene of Salmonella typhimurium. Genetics. 1969 Oct;63(2):263–279. doi: 10.1093/genetics/63.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenitsky T. A., Neil S. M., Miller R. L. Guanine and xanthine phosphoribosyltransfer activities of Lactobacillus casei and Escherichia coli. Their relationship to hypoxanthine and adenine phosphoribosyltransfer activities. J Biol Chem. 1970 May 25;245(10):2605–2611. [PubMed] [Google Scholar]
- Krenitsky T. A., Papaioannou R., Elion G. B. Human hypoxanthine phosphoribosyltransferase. I. Purification, properties, and specificity. J Biol Chem. 1969 Mar 10;244(5):1263–1270. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGASANIK B., KARIBIAN D. Purine nucleotide cycles and their metabolic role. J Biol Chem. 1960 Sep;235:2672–2681. [PubMed] [Google Scholar]
- Miller R. L., Bieber A. L. Substrate binding specificity and properties of inosine monophosphate: pyrophosphate phosphoribosyltransferase (EC 2.4.2.8) from Brewers yeast. Biochemistry. 1969 Feb;8(2):603–608. doi: 10.1021/bi00830a021. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Elliott D. C., Atkinson M. R. Nucleotide biosynthesis from preformed purines in mammalian cells: regulatory mechanisms and biological significance. Prog Nucleic Acid Res Mol Biol. 1970;10:87–119. doi: 10.1016/s0079-6603(08)60562-0. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J., De Haan P. G. Genetic and biochemical studies of the guanosine 5'-monophosphate pathway in Escherichia coli. Biochim Biophys Acta. 1967 Aug 22;145(1):31–40. doi: 10.1016/0005-2787(67)90651-x. [DOI] [PubMed] [Google Scholar]
- Thakar J. H., Kalle G. P. Defective guanine uptake in an 8-azaguanine-resistant mutant of Salmonella typhimurium. J Bacteriol. 1968 Feb;95(2):458–464. doi: 10.1128/jb.95.2.458-464.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]



