Abstract
Subcutaneous atrophy and central fat accumulation are common among HIV-infected patients receiving highly active antiretroviral therapy, and may be accompanied by dyslipidemia and insulin resistance. These fat changes, although commonly referred to together as lipodystrophy, are best considered as separate disorders, with distinct pathogeneses and treatment approaches. These morphological and metabolic abnormalities first appeared after introduction of protease inhibitors more than 10 yr ago, but research has demonstrated that their pathogenesis is multifactorial, with contributions from other antiretroviral medications, patient-related factors, and HIV itself. Switching to a less toxic highly active antiretroviral therapy regimen has shown partial effectiveness for the management of fat atrophy and lipid abnormalities. Lifestyle modification or surgical approaches are the treatment of choice for lipohypertrophy, although novel therapies targeting the GH axis show promise. HIV-related dyslipidemia may be difficult to treat, and can be complicated by drug-drug interactions between some lipid-lowering medications and antiretroviral medications. Treatment of diabetes in HIV-infected patients should generally follow established guidelines, but thiazolidinediones, rather than metformin, may be considered first-line treatment in a patient with lipoatrophy, given their potential to increase sc fat. The contribution of body fat changes and metabolic abnormalities to cardiovascular risk and the changing risk profiles of newer antiretroviral regimens are under intense investigation.
Subcutaneous fat atrophy and central fat accumulation are common among HIV-infected patients receiving highly active antiretroviral therapy (HAART) and may be accompanied by dyslipidemia and insulin resistance. This article suggests guidelines for treating HIV-infected patients with lipodystrophy.
A 59-yr-old white male is referred by his HIV provider for body composition changes, diabetes, and dyslipidemia in the setting of antiretroviral therapy. He was diagnosed with HIV 10 yr earlier with a nadir CD4 cell count of 166 cells per mm3. He was immediately started on highly active antiretroviral therapy (HAART), including stavudine, didanosine, and indinavir, with an excellent immunological and virological response. Within 12 months of HAART initiation, he began to notice reduced fat in his buttocks and thighs, with a prominence of the veins in his legs, and changes to his facial appearance. He also noticed increased fat in his abdomen. Triglycerides (TGs) increased to more than 2000 mg/dl, and a random glucose was found to be more than 600 mg/dl. Gemfibrozil and insulin were started, and he stopped HAART. His diabetes and hyperlipidemia did not resolve after HAART discontinuation. One year later, his CD4 cell count decreased less than 200 cells per mm3, and antiretroviral therapy was restarted.
Currently, his HIV disease is well controlled on tenofovir/emtricitabine/efavirenz. He reports a 20-lb weight gain in the past 3 yr. He admits that his diet is poor, and his physical activity level is low. In addition to his antiretroviral medications, he is receiving glyburide 20 mg daily, pioglitazone 45 mg daily, and pravastatin 80 mg daily. He has hypertension, and is on a regimen of diltiazem 360 mg daily and olmesartan 40 mg daily. He quit smoking in 2005. He has no family history of coronary artery disease.
On physical examination, blood pressure is 126/74, weight 184 lb, and body mass index (BMI) 28 kg/m2. Waist circumference (at the level of the iliac crest) is 107 cm, and hip circumference (at the level of the greater trochanter) is 87 cm. He has marked facial wasting in the malar region with prominence of the nasolabial folds and moderate lipoatrophy of the buttocks and thighs. A small dorsocervical fat pad is noted. Glycosylated hemoglobin is 6.6%, total cholesterol 182 mg/dl, TGs 254 mg/dl, high-density lipoprotein (HDL) 37 mg/dl, and low-density lipoprotein (LDL) 95 mg/dl. Serum creatinine is 1.7 mg/dl.
Background
With the introduction of HAART in the mid-1990s, HIV-related morbidity and mortality have been dramatically reduced (1,2). Coinciding with the clinical success of HAART, changes in body composition, including sc fat wasting and central fat accumulation, have been observed among HIV-infected patients, often in conjunction with dyslipidemia and insulin resistance, commonly called the HIV lipodystrophy syndrome or the fat redistribution syndrome (3). Because the HIV protease inhibitors (PIs) were important components of many early HAART regimens, this class of medications was implicated in playing the primary causative role in the pathogenesis of these disorders. However, over the past decade, intensive basic and clinical investigation has demonstrated that, whereas some PIs contribute to the development of adipose tissue alterations in HIV-infected patients (4), the pathogenesis of these problems is multifactorial, with contributions from other HIV medications, patient-related factors, and HIV infection itself. In addition, most current evidence suggests that sc lipoatrophy and central lipohypertrophy are distinct processes (5,6), and should be considered separately rather than collectively as a single syndrome of lipodystrophy or fat redistribution.
Clinical Features of Lipoatrophy
The generalized depletion of sc fat, which is commonly observed in the face, buttocks, legs, and arms, is the most frequent morphological change among HIV-infected patients receiving HAART, with prevalence estimates ranging from 13–34% (7,8,9,10), depending on the population studied and the measurement technique used. The proportion of patients with subclinical lipoatrophy is even larger (11) because at least 30% limb fat loss is necessary for lipoatrophy to be noticed by either the clinician or patient (12). This process is distinct from HIV-related wasting that is associated with advanced AIDS and loss of muscle mass.
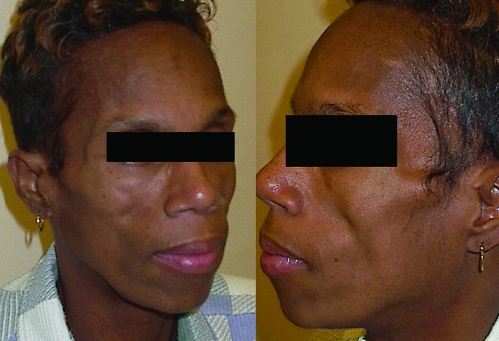
Facial lipoatrophy generally begins as a symmetrical loss of fat in the malar regions, giving prominence to the nasolabial folds. In more severe cases, there is extension to the cheeks, with prominence of the bony landmarks and visibility of the underlying musculature (13) (Fig. 1). These changes may be particularly stigmatizing for patients and may affect antiretroviral adherence (14).
Figure 1.
A 45-yr-old HIV-infected woman with moderate/severe facial lipoatrophy with marked fat wasting in the malar areas giving prominence to the nasolabial folds, appearance of the facial musculature (zygomaticus major) and bony landmarks through the skin, and extension of fat wasting to buccal and temporal areas.
There are multiple risk factors for the development of lipoatrophy. Fat wasting is more common in males, older patients, and those who started HAART with advanced HIV (7,8,10). Although lipoatrophy was initially recognized after the introduction of PIs (3), most evidence suggests that the medications most closely tied to its development are the nucleoside reverse transcriptase inhibitors (NRTIs), stavudine (Zerit, d4T; Bristol-Myers Squibb Co., Princeton, NJ) and, to a lesser extent, zidovudine (Retrovir, AZT; GlaxoSmithKline, Research Triangle Park, NC) (15). These medications inhibit the synthesis of mitochondrial DNA preferentially in adipocytes, leading to mitochondrial dysfunction and adipocyte apoptosis (16). Some members of the PI class, such as nelfinavir, can act synergistically with stavudine to worsen lipoatrophy (4). A recent large clinical trial showed unexpected worsening of lipoatrophy in HIV-infected patients randomized to the non-NRTI, efavirenz, but the mechanism underlying this observation is not clear (17).
This patient developed fat wasting in the first 12–24 months after initiation of the stavudine-containing HAART regimen. Although he has since changed to other antiretroviral medications, he has not experienced significant recovery of his sc fat. In the assessment of HIV-infected patients with body composition changes, it is useful to have objective measures of body composition to be able to gauge longitudinal changes. I typically measure the waist at the iliac crest, the hip around the greater trochanter, and thigh at a fixed distance from the top of the patella. In select patients who continue to receive stavudine or zidovudine and who have minimal or no lipoatrophy on physical examination, whole body dual x-ray absorptiometry can be a useful tool to document subclinical worsening of lipoatrophy, which may be an important factor in the consideration of modifying the antiretroviral regimen. Previous photographs can be useful for the assessment of facial lipoatrophy, and grading scales have been developed based on abnormalities in facial contour, prominence of bony structures, and visibility of the underlying musculature (13,18,19).
Clinical Features of Lipohypertrophy
In addition to lipoatrophy, this patient also presented with abdominal and dorsocervical fat accumulation. In other patients, circumscribed lipoma in other areas of the body (e.g. suprapubic) and lipomastia in both men and women can also be observed. Unlike lipoatrophy, the pathogenesis of lipohypertrophy among HIV-infected patients has been elusive and cannot be linked to a specific antiretroviral medication or class of medications. With the initiation of antiretroviral therapy, most studies have shown an increase in central fat over the first 6 months, which then levels off (4,20). Some of this increase may be due to a return to premorbid body composition with effective control of the HIV virus and “catch-up” to HIV-negative peers. However, visceral fat in HIV-infected patients with lipodystrophy exceeds that observed in HIV-negative controls when matched on BMI (21).
The assessment of HIV-lipohypertrophy can be difficult in practice, and there is no commonly accepted definition. Because central fat accumulation is common in the general population, it can be challenging to ascertain the contribution of the fat gain that is specific to HIV infection or its therapy. In addition, because some patients have a combination of sc lipoatrophy in the abdominal region and visceral lipohypertrophy, clinical measurements of central adiposity that do not distinguish between sc and visceral fat, such as waist circumference, may be misleading. For example, a waist circumference that is considered normal in the general population may indicate a significant amount of visceral fat in a HIV-infected patient with concomitant sc lipoatrophy.
Previously, it was believed that lipoatrophy and lipohypertrophy were reciprocal processes, whereby the loss of sc fat was associated with gains in the visceral compartment. This notion was challenged when it was found that those HIV-infected patients with clinical lipoatrophy were found to have either equal or less visceral fat compared with those HIV-infected patients without lipoatrophy, arguing against a strict “fat redistribution syndrome” (5,6).
Associated Metabolic Abnormalities: Dyslipidemia
Dyslipidemia, characterized by increased LDL cholesterol and TGs, and decreased HDL cholesterol, is common among HIV-infected patients receiving HAART (22). Before the HAART era, decreased HDL, LDL, and total cholesterol, accompanied by decrease TG clearance were recognized among HIV-infected patients (23). As was seen in this patient, initiation of antiretroviral therapy can be associated with dramatic changes in lipid concentrations, particularly TGs, although HDL and LDL also increase but to a lesser extent (24). In vitro studies have shown that certain PIs increase TG synthesis (25), and there is variability among the clinical effect of individual agents within the PI class (24). However, it should be noted that individual PIs are often given in combination low doses of ritonavir, a PI that inhibits cytochrome P450 3A4, which is the primary metabolic pathway of PIs. Coadministration with ritonavir increases PI serum concentrations and improves the pharmacokinetic profile. Although not active against HIV at these “boosting” doses, ritonavir may also worsen dyslipidemia (26). Other antiretroviral medications may contribute to dyslipidemia, including efavirenz, stavudine, and zidovudine (27,28,29). In addition, recent evidence suggests that both increases in visceral fat and reductions in lower body sc fat are independently associated with dyslipidemia in both HIV-infected men and women (30,31).
Associated Metabolic Abnormalities: Insulin Resistance/Diabetes Mellitus
The risk of diabetes mellitus and insulin resistance is higher among HAART-treated HIV-infected patients, compared with HIV-uninfected controls (32,33). Similar to the pathogenesis of dyslipidemia in this population, the etiology is multifactorial with contributions from the effects of HIV itself, patient-related characteristics, and antiretroviral therapy. Although some PIs may directly induce peripheral and hepatic insulin resistance (34,35,36,37,38), other medications such as stavudine and zidovudine also have a direct effect on glucose metabolism (39,40), and also may induce insulin resistance indirectly, through effects on body composition. Visceral fat accumulation and sc lipoatrophy have both been associated with insulin resistance (41,42).
Treatment Considerations
Stopping or switching HAART
After this patient developed severe hypertriglyceridemia and hyperglycemia in the setting of antiretroviral therapy, his HIV medications were discontinued. This type of structured treatment interruption showed initial promise as a strategy to minimize antiretroviral toxicity (43). However, a recent large randomized, controlled trial comparing structured treatment interruption with continued therapy not only showed a 2-fold increase in death and opportunistic disease in the structured treatment interruption arm but also, unexpectedly, showed an increase in non-AIDS-related complications, including myocardial infarction (44). These results, along with those of a similar trial (45), provide compelling evidence against structured treatment interruption as a safe and effective strategy for managing the metabolic toxicities of antiretroviral therapy.
Switching strategies have been extensively evaluated in their effect on reversing body composition and metabolic abnormalities (46). Substituting less toxic NRTIs, such as abacavir or tenofovir, for stavudine or zidovudine, has clearly shown benefit in reversing lipoatrophy, although the extent of fat recovery after switching has not yet been determined (47,48,49). Switching off of these medications has also been associated with improvements in lipid profiles and insulin resistance markers (50,51,52). Substitutions for certain PIs have also been an important strategy to improve lipid concentrations (53). Of the most frequently used PIs, atazanavir, darunavir, and saquinavir are associated with the most favorable lipid profiles (24). However, because PIs generally require low-dose ritonavir for its boosting effect, the impact from switching from one ritonavir-boosted PI to another may be modest (54). Antiretroviral switch studies examining the changes in central fat have generally shown no benefit (55,56,57). Because alterations in the antiretroviral regimen may be an important strategy to improve the metabolic abnormalities in HIV-infected patients, close collaboration between endocrine and HIV specialists is essential.
Lipoatrophy
Pharmacological approaches aimed at increasing sc fat have shown only limited success. Thiazolidinediones have shown efficacy in in vitro models of antiretroviral-associated adipocyte loss (58), and have been effective in increasing sc fat and improving metabolic abnormalities among HIV-uninfected persons with lipodystrophy (59). However, results from clinical trials of rosiglitazone in HIV-infected patients with lipoatrophy have been mixed (60,61,62,63). A recent randomized 48-wk trial of pioglitazone in 131 HIV-infected patients with lipoatrophy showed significant improvements in limb fat (0.38 kg pioglitazone group vs. 0.05 kg placebo group; P = 0.05), thigh circumference, and skin-fold thickness, most pronounced in patients not taking d4T (64). Although statistically significant using these objective measures, the change in fat was not perceptible to the patient. It is unclear if continued improvement would be seen with longer exposure, and further studies are needed before thiazolidinediones can be routinely recommended for the treatment of lipoatrophy.
Statin therapy may also improve lipoatrophy. In one, small, randomized controlled trial designed to assess the effect of pravastatin (40 mg at bedtime) on lipid parameters over 16 wk, limb fat measured by dual x-ray absorptiometry was shown unexpectedly to increase in those receiving pravastatin compared with placebo (0.72 vs. 0.19 kg increase with placebo) (65). Further research is needed to confirm these findings, elucidate the underlying mechanism, and determine whether other statins have similar effects.
Another potential approach is supplementation with uridine. In in vitro models, uridine has prevented and reversed mitochondrial toxicity induced by stavudine (66). Clinical trial data are limited. One randomized trial of 20 HIV-infected patients with lipoatrophy showed that limb fat increased to a greater extent over 3 months in those receiving uridine compared with placebo (0.88 ± 0.14 vs. 0.23 ± 0.27 kg; P < 0.05) (67). A uridine-rich dietary supplement is currently available (www.nucleomaxx.com) and is being evaluated in a multicenter, randomized trial in the United States. The efficacy, safety, and optimal dosing are not known, and it is unclear if this approach will be successful in those who have already discontinued stavudine or zidovudine.
Given the negative psychological effects and stigmatization of facial lipoatrophy, facial fillers, generally administered by a plastic surgeon or dermatologist, have gained popularity. Both permanent and absorbable compounds have been successful in improving lipoatrophy grading, improving quality of life, and decreasing anxiety and depression symptoms (68,69,70).
Lipohypertrophy
Central fat accumulation is difficult to treat in HIV-infected patients. Lifestyle modification is an important treatment approach and should be recommended. However, although lipohypertrophy has been associated with lower activity levels (71), studies evaluating the effect of exercise regimens involving resistance training, aerobic exercise, or stretching and relaxation techniques have all showed only modest benefit in improving body composition and metabolic abnormalities in HIV-infected patients (72,73,74,75). Metformin decreases visceral fat in HIV-infected patients in some studies, as well as blood pressure and markers of impaired fibrinolysis (76,77,78,79), and its potential benefits are enhanced with concomitant aerobic and resistance training (80). However, some studies have not shown a similar benefit (81,82), and metformin may worsen lipoatrophy in HIV-infected patients with mixed lipodystrophy (83). Nevertheless, metformin, particularly in combination with exercise training, may be useful in HIV-infected patients with significant lipohypertrophy with minimal lipoatrophy.
The GH axis has also been the target of pharmacological strategies to improve visceral fat accumulation. HIV-infected patients with body composition changes have demonstrated reduced GH mean pulse amplitude on overnight testing, which has been correlated with central fat accumulation (84) and failure rates of 18–38% on standard GHRH-arginine stimulation testing depending on the cutpoint used (3.3 vs. 7.5 ng/ml peak GH response) (85), providing a rationale for this approach. GHRH (86), a GHRH analog (tesamorelin) (87), and human GH (hGH) at both physiological and supraphysiological doses (88,89,90,91,92) have been evaluated in clinical trials. Although Phase III trials are ongoing, none of these therapies are currently approved for use in HIV-infected patients with central fat accumulation. However, the studies to date are promising. For example, tesamorelin decreases visceral adipose tissue by 15% at 6 months, and is associated with improved lipid parameters and body image compared with placebo in a recent large, randomized trial (87).
Supraphysiological hGH may be associated with worsening of lipoatrophy and impaired glucose tolerance (90,91,93,94), which is dose dependent. These adverse effects were not observed with GHRH or tesamorelin (86,87). Similar to other interventions, such as lifestyle modifications or metformin, the treatment effect of hGH, GHRH, or tesamorelin reverses upon discontinuation. Although visceral fat accumulation has a strong association with metabolic abnormalities and cardiovascular disease in the general population, it is unclear whether visceral fat reduction with GH axis-targeted therapies is associated with reduction in clinical endpoints.
Surgical approaches, including liposuction, have been used with success in patients with dorsocervical fat pad accumulation or sc fat accumulation in other sites (95), but recurrence is a potential problem.
Dyslipidemia
The treatment of dyslipidemia should follow the same guidelines as in the general population (96), although guidelines specific to HIV-infected patients are available (97,98), and the risk of myocardial infarction may be underestimated using risk equations derived from the general population (99). LDL cholesterol should be the first target of therapy for those with fasting TGs less than 500 mg/dl. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors are the preferred therapy, but simvastatin and lovastatin should be avoided in HIV-infected patients receiving ritonavir to avoid potential drug-drug interactions (100). Atorvastatin also interacts with ritonavir, but to a lesser extent, such that lower doses are safe in a patient receiving ritonavir (101). Although a drug-drug interaction with ritonavir was not predicted based on in vitro models, rosuvastatin concentrations increased by more than 2-fold in a recent pharmacokinetic study, whereas its LDL-lowering efficacy was attenuated (102) (Table 1). Conversely, coadministration of statins with efavirenz reduces atorvastatin, simvastatin, and pravastatin serum concentrations, through induction of statin metabolism, thereby diminishing the effect (103). Modest LDL lowering with ezetimibe has also been observed in HIV-infected patients, although its effect on clinical endpoints is unclear (104,105).
Table 1.
The Risk of Pharmacologic Interactions between HMG coA-reductase inhibitors and ritonavir (Norvir), a common component of protease inhibitor containing HAART regimens
| Safe to coadminister | Use with caution | Contraindicated |
|---|---|---|
| Pravastatin | Atorvastatin | Simvastatin |
| Fluvastatin | Rosuvastatin | Lovastatin |
For patients whose LDL goal has been reached or who have TG levels more than 500 mg/dl, fibric acid derivatives (106,107), omega-3 fatty acids (108,109), and niacin (110) have each shown efficacy in the reduction of TGs and non-HDL cholesterol. HIV-associated dyslipidemia can be difficult to treat, and often multiple agents are necessary to reach target lipid concentrations, in addition to dietary modifications (111).
Diabetes mellitus/insulin resistance
In general, treatment guidelines outlined by the American Diabetes Association and European Association for the Study of Diabetes should be followed in HIV-infected patients with diabetes mellitus (112). However, in patients with lipoatrophy, metformin should be used with caution because further reductions in sc fat may be seen (82). Thiazolidinediones can be considered the preferred approach in those with lipoatrophy, given the possibility of increasing sc fat, albeit modest. Among the members of this class, pioglitazone is associated with a more favorable cardiovascular risk profile in the general population (113,114,115). Because of the severity of insulin resistance in many HIV-infected patients with diabetes, it is reasonable to favor insulin sensitizers over insulin secretagogues. Incretin mimetics have not been systematically evaluated in HIV-infected patients, but similar effects to the general population would be expected. There are no known pharmacological interactions between any hypoglycemic agents and antiretroviral agents.
Similar to the general population, pharmacological treatment of insulin resistance, impaired fasting glucose, or impaired glucose tolerance is not currently indicated, except for perhaps younger patients with a BMI more than or equal to 35 kg/m2, with both impaired glucose tolerance and impaired fasting glucose, in whom the use of metformin may be considered along with lifestyle modifications to reduce the risk of progression to diabetes (116).
Controversial and Unanswered Questions
Risk of cardiovascular disease
There is widespread concern that the metabolic and morphological abnormalities in HIV-infected patients lead to an increased risk of cardiovascular disease. Some observational studies suggest an increased risk of cardiovascular disease among HIV-infected patients compared with the general population (117) and an increased risk of myocardial infarction in HIV-infected persons with each year of HAART exposure, particularly to the PI class (118). Other observational data sets have shown that the risk of cardiovascular mortality has not changed in the HAART era (119), and that cardiovascular events in HIV-infected patients are most closely associated with traditional cardiovascular risk factors (120), such as dyslipidemia and impaired glucose tolerance, which may be more common among HIV-infected patients. The extent of the increased risk of cardiovascular disease in this population and its association with factors related to HIV infection, antiretroviral therapy, and its metabolic consequences are topics of intense investigation.
Role of new antiretroviral agents
Antiretroviral therapy is rapidly evolving. In the last 2 yr, two new classes of antiretrovirals have been introduced, as well as several new additions to existing classes. In general, newer antiretroviral agents have been associated with fewer toxicities (121,122). However, the optimal combination of these medications has not yet been determined, and the recent finding of unexpected cardiovascular toxicity with short-term exposure to a preferred nucleoside analog reminds us that careful scrutiny of the metabolic and cardiovascular effects of antiretroviral medications is warranted (123).
Back to the Patient
Although the patient had significant lipoatrophy, his central fat accumulation had the largest negative impact on his quality of life. Strategies to increase his physical activity were discussed in detail with the patient, including 20–30 min walking during his weekday lunch break and adopting a regular walking regimen on the weekends. He was referred to a dietician for recommendations regarding medical nutrition therapy for diabetes mellitus and dyslipidemia, including mild caloric restriction with the goal of a 10% weight loss in the next 6 months.
With a glycosylated hemoglobin of 6.6%, no intensification of his diabetes regimen was recommended. However, it was discussed that, with adoption of lifestyle changes, discontinuation of his sulfonylurea may be possible and may enhance further weight loss. Metformin was not considered because of his elevated serum creatinine. His LDL cholesterol was at a goal with 80 mg pravastatin given his cardiovascular risk factors (<100 mg/dl). We discussed the fact that his non-HDL cholesterol (145 mg/dl) was above the goal (<130 mg/dl) but elected to evaluate the effects of weight loss before further pharmacological intervention was considered. Low-dose aspirin was recommended for primary prevention of cardiovascular disease.
Conclusions
Lipoatrophy, lipohypertrophy, dyslipidemia, and abnormalities in glucose metabolism are common among HIV-infected patients receiving HAART. Although these problems are interrelated, they can be seen independently, and their pathophysiologies are distinct. Switching to less-toxic antiretroviral regimens is an important treatment strategy for lipoatrophy and dyslipidemia, and close communication between endocrine and HIV-specialists is essential. Specific pharmacological therapies for fat changes are limited, but new agents are currently being evaluated. Dyslipidemia and diabetes mellitus should be treated using established guidelines in the general population. However, the choice of pharmacological agent may be complicated by pharmacokinetic interactions with certain antiretroviral medication and additional considerations regarding toxicity profiles of individual agents (e.g. metformin in a patient with lipoatrophy). Further research is underway to clarify the risk of cardiovascular disease in HIV-infected populations, evaluate newer antiretroviral agents for metabolic and cardiovascular toxicity, and identify other treatment strategies.
Footnotes
This work was supported by National Institutes of Health Grant K23 AT002862-01.
Disclosure Summary: T.T.B. has served as a consultant for EMD-Serono and Abbott Laboratories and has received research support from Reliant Pharmaceuticals, Theratechnologies, Abbott Laboratories, GlaxoSmithKline, and Merck.
Abbreviations: BMI, Body mass index; HAART, highly active antiretroviral therapy; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TG, triglyceride.
References
- Palella Jr FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD 1998 Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338:853–860 [DOI] [PubMed] [Google Scholar]
- Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, Sax PE, Weinstein MC, Freedberg KA 2006 The survival benefits of AIDS treatment in the United States. J Infect Dis 194:11–19 [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Carr A 2005 Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 352:48–62 [DOI] [PubMed] [Google Scholar]
- Dube MP, Parker RA, Tebas P, Grinspoon SK, Zackin RA, Robbins GK, Roubenoff R, Shafer RW, Wininger DA, Meyer III WA, Snyder SW, Mulligan K 2005 Glucose metabolism, lipid, and body fat changes in antiretroviral-naive subjects randomized to nelfinavir or efavirenz plus dual nucleosides. AIDS 19:1807–1818 [DOI] [PubMed] [Google Scholar]
- Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, Osmond D, Saag M, Scherzer R, Shlipak M, Tien P 2005 Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr 40:121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fat distribution in women with HIV infection 2006. J Acquir Immune Defic Syndr 42:562–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson DL, Knox T, Spiegelman D, Skinner S, Gorbach S, Wanke C 2005 Prevalence of, evolution of, and risk factors for fat atrophy and fat deposition in a cohort of HIV-infected men and women. Clin Infect Dis 40:1837–1845 [DOI] [PubMed] [Google Scholar]
- Lichtenstein KA, Delaney KM, Armon C, Ward DJ, Moorman AC, Wood KC, Holmberg SD 2003 Incidence of and risk factors for lipoatrophy (abnormal fat loss) in ambulatory HIV-1-infected patients. J Acquir Immune Defic Syndr 32:48–56 [DOI] [PubMed] [Google Scholar]
- Tien PC, Grunfeld C 2004 What is HIV-associated lipodystrophy? Defining fat distribution changes in HIV infection. Curr Opin Infect Dis 17:27–32 [DOI] [PubMed] [Google Scholar]
- Miller J, Carr A, Emery S, Law M, Mallal S, Baker D, Smith D, Kaldor J, Cooper DA 2003 HIV lipodystrophy: prevalence, severity and correlates of risk in Australia. HIV Med 4:293–301 [DOI] [PubMed] [Google Scholar]
- Kosmiski L, Kuritzkes D, Hamilton J, Sharp T, Lichtenstien K, Hill J, Eckel R 2003 Fat distribution is altered in HIV-infected men without clinical evidence of the HIV lipodystrophy syndrome. HIV Med 4:235–240 [DOI] [PubMed] [Google Scholar]
- Podzamczer D, Ferrer E, Martinez E, Del Rio L, Ruiz I, Rosales J, Ribera E, Barrufet P, Llibre JAM How much fat loss is needed for lipoatrophy to become clinically evident? Program and Abstracts of the 15th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 2008, Abstract 941 [Google Scholar]
- Ascher B, Coleman S, Alster T, Bauer U, Burgess C, Butterwick K, Donofrio L, Engelhard P, Goldman MP, Katz P, Vleggaar D 2006 Full scope of effect of facial lipoatrophy: a framework of disease understanding. Dermatol Surg 32:1058–1069 [DOI] [PubMed] [Google Scholar]
- Santos CP, Felipe YX, Braga PE, Ramos D, Lima RO, Segurado AC 2005 Self-perception of body changes in persons living with HIV/AIDS: prevalence and associated factors. AIDS 19(Suppl 4):S14–S21 [DOI] [PubMed] [Google Scholar]
- Bogner JR, Vielhauer V, Beckmann RA, Michl G, Wille L, Salzberger B, Goebel FD 2001 Stavudine versus zidovudine and the development of lipodystrophy. J Acquir Immune Defic Syndr 27:237–244 [DOI] [PubMed] [Google Scholar]
- Cherry CL, Nolan D, James IR, McKinnon EJ, Mallal SA, Gahan ME, Lal L, McArthur JC, Wesselingh SL 2006 Tissue-specific associations between mitochondrial DNA levels and current treatment status in HIV-infected individuals. J Acquir Immune Defic Syndr 42:435–440 [DOI] [PubMed] [Google Scholar]
- Haubrich R, Riddler SA, DiRenzo G, Komarow L, Powderly WG, Garren K, George T, Rooney J, Mellors J, Havlir D Metabolic outcomes of ACTG 5142: a prospective, randomized, phase III trial of NRTI-, PI-, and NNRTI-sparing regimens for initial treatment of HIV-1 infection. Program and Abstracts of the 14th Conference of Retroviruses and Opportunistic Infections, Los Angeles, CA, 2007, Abstract 38 [Google Scholar]
- Funk E, Brissett AE, Friedman CD, Bressler FJ 2007 HIV-associated facial lipoatrophy: establishment of a validated grading scale. Laryngoscope 117:1349–1353 [DOI] [PubMed] [Google Scholar]
- James J, Carruthers A, Carruthers J 2002 HIV-associated facial lipoatrophy. Dermatol Surg 28:979–986 [DOI] [PubMed] [Google Scholar]
- Mallon PW, Miller J, Cooper DA, Carr A 2003 Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS 17:971–979 [DOI] [PubMed] [Google Scholar]
- Joy T, Keogh HM, Hadigan C, Dolan SE, Fitch K, Liebau J, Johnsen S, Lo J, Grinspoon S 2008 Relation of body composition to body mass index in HIV-infected patients with metabolic abnormalities. J Acquir Immune Defic Syndr 47:174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JH, Klein MA, Bellehumeur JL, McBride PE, Wiebe DA, Otvos JD, Sosman JM 2001 Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation 104:257–262 [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR 1992 Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab 74:1045–1052 [DOI] [PubMed] [Google Scholar]
- Hill A, Sawyer W, Gazzard B Effects of nucleoside analogues versus ritonavir-boosted protease inhibitors on lipid levels-analysis of12 clinical trials in 4231 antiretroviral naive patients. Program and Abstracts of the 14th Conference of the British HIV Association, Belfast, Northern Ireland, 2008, Abstract P79 [Google Scholar]
- Lenhard JM, Croom DK, Weiel JE, Winegar DA 2000 HIV protease inhibitors stimulate hepatic triglyceride synthesis. Arterioscler Thromb Vasc Biol 20:2625–2629 [DOI] [PubMed] [Google Scholar]
- Shafran SD, Mashinter LD, Roberts SE 2005 The effect of low-dose ritonavir monotherapy on fasting serum lipid concentrations. HIV Med 6:421–425 [DOI] [PubMed] [Google Scholar]
- Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, Coakley DF, Lu B, Toole JJ, Cheng AK, 903 Study Group 2004 Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 292:191–201 [DOI] [PubMed] [Google Scholar]
- Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, Lu B, McColl D, Chuck S, Enejosa J, Toole JJ, Cheng AK 2006 Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med 19:354:251–260 [DOI] [PubMed] [Google Scholar]
- van Leth F, Phanuphak P, Stroes E, Gazzard B, Cahn P, Raffi F, Wood R, Bloch M, Katlama C, Kastelein JJ, Schechter M, Murphy RL, Horban A, Hall DB, Lange JM, Reiss P 2004 Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy-naive patients infected with HIV-1. PLoS Med [Erratum (2004) 1:e73] 1:e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier J, Scherzer R, Bacchetti P, Heymsfield S, Lee D, Sidney S, Tien PC, Fat Redistribution and Metabolic Changes in HIV Infection Study Investigators 2008 Regional adipose tissue and lipid and lipoprotein levels in HIV-infected women. J Acquir Immune Defic Syndr 48:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohl D, Scherzer R, Heymsfield S, Simberkoff M, Sidney S, Bacchetti P, Grunfeld C, FRAM Study Investigators 2008 The associations of regional adipose tissue with lipid and lipoprotein levels in HIV-infected men. J Acquir Immune Defic Syndr 48:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, Visscher BR, Margolick JB, Dobs AS 2005 Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the Multicenter AIDS Cohort Study. Arch Int Med [Erratum (2005) 165:2541] 165:1179–1184 [DOI] [PubMed] [Google Scholar]
- Hadigan C, Meigs JB, Corcoran C, Rietschel P, Piecuch S, Basgoz N, Davis B, Sax P, Stanley T, Wilson PW, D'Agostino RB, Grinspoon S 2001 Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis 32:130–139 [DOI] [PubMed] [Google Scholar]
- Lee GA, Seneviratne T, Noor MA, Lo JC, Schwarz JM, Aweeka FT, Mulligan K, Schambelan M, Grunfeld C 2004 The metabolic effects of lopinavir/ritonavir in HIV-negative men. AIDS 18:641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor MA, Seneviratne T, Aweeka FT, Lo JC, Schwarz JM, Mulligan K, Schambelan M, Grunfeld C 2002 Indinavir acutely inhibits insulin-stimulated glucose disposal in humans: a randomized, placebo-controlled study. AIDS 16:F1–F8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JM, Lee GA, Park S, Noor MA, Lee J, Wen M, Lo JC, Mulligan K, Schambelan M, Grunfeld C 2004 Indinavir increases glucose production in healthy HIV-negative men. AIDS 18:1852–1854 [DOI] [PubMed] [Google Scholar]
- Lee GA, Rao MN, Schwarz J, Aweeka FT, Grunfeld C A single dose of amprenavir does not induce insulin resistance in healthy normal volunteers. Program and Abstracts of the 7th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV, Dublin, Ireland, 2005, Abstract 15 [Google Scholar]
- Lee GA, Mafong DD, Lo JC, Schwarz J, Aweeka FT, Mulligan K, Schambelan M, Grunfeld C Ritonavir acutely induces insulin resistance in healthy normal volunteers. Program and Abstracts of the 6th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV, Washington, DC, 2004, Abstract 7 [Google Scholar]
- Fleischman A, Johnsen S, Systrom DM, Hrovat M, Farrar CT, Frontera W, Fitch K, Thomas BJ, Torriani M, Cote HCF, Grinspoon SK 2007 Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle in healthy adults. Am J Physiol Endocrinol Metab 292:E1666–E1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer RM, van Vonderen MG, Sutinen J, Hassink E, Ackermans M, van Agtmael MA, Yki-Jarvinen H, Danner SA, Reiss P, Sauerwein HP 2008 Zidovudine/lamivudine contributes to insulin resistance within 3 months of starting combination antiretroviral therapy. AIDS 22:227–236 [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Rimland D, Gibert CL, Powderly WG, Sidney S, Shlipak MG, Bacchetti P, Scherzer R, Haffner SM, Heymsfield SB 2007 Association of upper trunk and visceral adipose tissue volume with insulin resistance in control and HIV-infected subjects in the FRAM study. J Acquir Immune Defic Syndr 46:283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mynarcik DC, McNurlan MA, Steigbigel RT, Fuhrer J, Gelato MC 2000 Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J Acquir Immune Defic Syndr 25:312–321 [DOI] [PubMed] [Google Scholar]
- Hatano H, Miller KD, Yoder CP, Yanovski JA, Sebring NG, Jones EC, Davey Jr RT 2000 Metabolic and anthropometric consequences of interruption of highly active antiretroviral therapy. AIDS 14:1935–1942 [DOI] [PubMed] [Google Scholar]
- El Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, Cohn D, Cooper D, Darbyshire J, Emery S, Fatkenheuer G, Gazzard B, Grund B, Hoy J, Klingman K, Losso M, Markowitz N, Neuhaus J, Phillips A, Rappoport C 2006 CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 355:2283–2296 [DOI] [PubMed] [Google Scholar]
- Ananworanich J, Gayet-Ageron A, Le Braz M, Prasithsirikul W, Chetchotisakd P, Kiertiburanakul S, Munsakul W, Raksakulkarn P, Tansuphasawasdikul S, Sirivichayakul S, Cavassini M, Karrer U, Genne D, Nuesch R, Vernazza P, Bernasconi E, Leduc D, Satchell C, Yerly S, Perrin L, Hill A, Perneger T, Phanuphak P, Furrer H, Cooper D, Ruxrungtham K, Hirschel B, Staccato Study Group, Swiss HIV Cohort Study 2006 CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: results of the Staccato randomised trial. Lancet 368:459–465 [DOI] [PubMed] [Google Scholar]
- Drechsler H, Powderly WG 2002 Switching effective antiretroviral therapy: a review. Clin Infect Dis 35:1219–1230 [DOI] [PubMed] [Google Scholar]
- McComsey GA, Ward DJ, Hessenthaler SM, Sension MG, Shalit P, Lonergan JT, Fisher RL, Williams VC, Hernandez JE, Trial to Assess the Regression of Hyperlactatemia and to Evaluate the Regression of Established Lipodystrophy in HIV-1-Positive Subjects (TARHEEL; ESS40010) Study Team 2004 Improvement in lipoatrophy associated with highly active antiretroviral therapy in human immunodeficiency virus-infected patients switched from stavudine to abacavir or zidovudine: the results of the TARHEEL study. Clin Infect Dis 38:263–270 [DOI] [PubMed] [Google Scholar]
- Carr A, Workman C, Smith DE, Hoy J, Hudson J, Doong N, Martin A, Amin J, Freund J, Law M, Cooper DA 2002 Abacavir substitution for nucleoside analogs in patients with HIV lipoatrophy: a randomized trial. JAMA 288:207–215 [DOI] [PubMed] [Google Scholar]
- Moyle G, Sabin C, Cartledge J, Johnson M, Wilkins E, Churchill D, Hay P, Fakoya A, Murphy M, Scullard G, Leen C, Reilly G A 48-week, randomized open-label comparative study of tenofovir DF vs abacavir as substitutes for a thymidine analog in persons with lipoatrophy and sustained virological suppression on HAART. Program and Abstracts of the 12th Conference of Retroviruses and Opportunistic Infections, Boston, MA, 2005, Abstract 45LB [Google Scholar]
- da Silva B, Albrecht M, Benson C, Eron J, Gulick R, Glesby M, Hicks C, Kessler H, Murphy R, Thompson M, White A, Wolfe P, McMillan F, Niemi K, King M, Hanna G Improved metabolic profile with replacement of stavudine by tenofovir DF after 6 years of a lopinavir/ritonavir-based regimen. Program and Abstracts of the 7th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV, Dublin, Ireland, 2006, Abstract L38 [Google Scholar]
- Podzamczer D, Ferrer E, Sanchez P, Gatell JM, Crespo M, Fisac C, Lonca M, Sanz J, Niubo J, Veloso S, Llibre JM, Barrufet P, Ribas MA, Merino E, Ribera E, Martinez-Lacasa J, Alonso C, Aranda M, Pulido F, Berenguer J, Delegido A, Pedreira JD, Lerida A, Rubio R, del Rio L 2007 Less lipoatrophy and better lipid profile with abacavir as compared to stavudine: 96-week results of a randomized study. J Acquir Immune Defic Syndr 44:139–147 [DOI] [PubMed] [Google Scholar]
- Valdez JR, Cassetti I, Suleiman JM, Etzel A, Zhong L, Holmes CB, Cheng AK, Enejosa J 2007 The safety and efficacy of switching stavudine to tenofovir df in combination with lamivudine and efavirenz in hiv-1-infected patients: three-year follow-up after switching therapy. HIV Clin Trials 8:381–390 [DOI] [PubMed] [Google Scholar]
- Martinez E, Arnaiz JA, Podzamczer D, Dalmau D, Ribera E, Domingo P, Knobel H, Riera M, Pedrol E, Force L, Llibre JM, Segura F, Richart C, Cortes C, Javaloyas M, Aranda M, Cruceta A, de Lazzari E, Gatell JM 2003 Substitution of nevirapine, efavirenz, or abacavir for protease inhibitors in patients with human immunodeficiency virus infection. N Engl J Med 349:1036–1046 [DOI] [PubMed] [Google Scholar]
- Busti AJ, Bedimo R, Margolis DM, Hardin DS 2008 Improvement in insulin sensitivity and dyslipidemia in protease inhibitor-treated adult male patients after switch to atazanavir/ritonavir. J Investig Med 56:539–544 [DOI] [PubMed] [Google Scholar]
- Martinez E, Garcia-Viejo MA, Blanco JL, Bianchi L, Buira E, Conget I, Casamitjana R, Mallolas J, Gatell JM 2000 Impact of switching from human immunodeficiency virus type 1 protease inhibitors to efavirenz in successfully treated adults with lipodystrophy. Clin Infect Dis 31:1266–1273 [DOI] [PubMed] [Google Scholar]
- Ruiz L, Negredo E, Domingo P, Paredes R, Francia E, Balague M, Gel S, Bonjoch A, Fumaz CR, Johnston S, Romeu J, Lange J, Clotet B 2001 Antiretroviral treatment simplification with nevirapine in protease inhibitor-experienced patients with HIV-associated lipodystrophy: 1-year prospective follow-up of a multicenter, randomized, controlled study. J Acquir Immune Defic Syndr 27:229–236 [DOI] [PubMed] [Google Scholar]
- Carr A, Hudson J, Chuah J, Mallal S, Law M, Hoy J, Doong N, French M, Smith D, Cooper DA 2001 HIV protease inhibitor substitution in patients with lipodystrophy: a randomized, controlled, open-label, multicentre study. AIDS 15:1811–1822 [DOI] [PubMed] [Google Scholar]
- Caron M, Auclair M, Vigouroux C, Glorian M, Forest C, Capeau J 2001 The HIV protease inhibitor indinavir impairs sterol regulatory element-binding protein-1 intranuclear localization, inhibits preadipocyte differentiation, and induces insulin resistance. Diabetes 50:1378–1388 [DOI] [PubMed] [Google Scholar]
- Arioglu E, Duncan-Morin J, Sebring N, Rother KI, Gottlieb N, Lieberman J, Herion D, Kleiner DE, Reynolds J, Premkumar A, Sumner AE, Hoofnagle J, Reitman ML, Taylor SI 2000 Efficacy and safety of troglitazone in the treatment of lipodystrophy syndromes. Ann Intern Med 133:263–274 [DOI] [PubMed] [Google Scholar]
- Carr A, Workman C, Carey D, Rogers G, Martin A, Baker D, Wand H, Law M, Samaras K, Emery S, Cooper DA 2004 No effect of rosiglitazone for treatment of HIV-1 lipoatrophy: randomised, double-blind, placebo-controlled trial. Lancet 363:429–438 [DOI] [PubMed] [Google Scholar]
- Sutinen J, Hakkinen AM, Westerbacka J, Seppala-Lindroos A, Vehkavaara S, Halavaara J, Jarvinen A, Ristola M, Yki-Jarvinen H 2003 Rosiglitazone in the treatment of HAART-associated lipodystrophy–a randomized double-blind placebo-controlled study. Antivir Ther 8:199–207 [PubMed] [Google Scholar]
- Mulligan K, Yang Y, Koletar S, Wininger DA, Parker R, Alston-Smith B, Basar M, Grinspoon S 2006 Effects of metformin and rosiglitazone on body composition in HIV-infected patients with hyperinsulinemia and elevated waist/hip ratio: a randomized, placebo-controlled trial. Program and Abstracts of the 13th Conference of Retroviruses and Opportunistic Infections, Denver, CO, Abstract 147 [Google Scholar]
- Hadigan C, Yawetz S, Thomas A, Havers F, Sax PE, Grinspoon S 2004 Metabolic effects of rosiglitazone in HIV lipodystrophy: a randomized, controlled trial. Ann Intern Med 140:786–794 [DOI] [PubMed] [Google Scholar]
- Slama L, Lanoy E, Valantin MA, Bastard JP, Chermak A, Boutekatjirt A, William-Faltaos D, Billaud E, Molina JM, Capeau J, Costagliola D, Rozenbaum W 2008 Effect of pioglitazone on HIV-1-related lipodystrophy: a randomized double-blind placebo-controlled trial (ANRS 113). Antivir Ther 13:67–76 [PubMed] [Google Scholar]
- Mallon PW, Miller J, Kovacic JC, Kent-Hughes J, Norris R, Samaras K, Feneley MP, Cooper DA, Carr A 2006 Effect of pravastatin on body composition and markers of cardiovascular disease in HIV-infected men–a randomized, placebo-controlled study. AIDS 20:1003–1010 [DOI] [PubMed] [Google Scholar]
- Walker UA, Venhoff N, Koch EC, Olschewski M, Schneider J, Setzer B 2003 Uridine abrogates mitochondrial toxicity related to nucleoside analogue reverse transcriptase inhibitors in HepG2 cells. Antivir Ther 8:463–470 [PubMed] [Google Scholar]
- Sutinen J, Walker UA, Sevastianova K, Klinker H, Hakkinen AM, Ristola M, Yki-Jarvinen H 2007 Uridine supplementation for the treatment of antiretroviral therapy-associated lipoatrophy: a randomized, double-blind, placebo-controlled trial. Antivir Ther 12:97–105 [PubMed] [Google Scholar]
- Moyle GJ, Brown S, Lysakova L, Barton SE 2006 Long-term safety and efficacy of poly-L-lactic acid in the treatment of HIV-related facial lipoatrophy. HIV Med 7:181–185 [DOI] [PubMed] [Google Scholar]
- Carey DL, Baker D, Rogers GD, Petoumenos K, Chuah J, Easey N, Machon K, Cooper DA, Emery S, Carr A 2007 A randomized, multicenter, open-label study of poly-L-lactic acid for HIV-1 facial lipoatrophy. J Acquir Immune Defic Syndr 46:581–589 [DOI] [PubMed] [Google Scholar]
- Loutfy MR, Raboud JM, Antoniou T, Kovacs C, Shen S, Halpenny R, Ellenor D, Ezekiel D, Zhao A, Beninger F 2007 Immediate versus delayed polyalkylimide gel injections to correct facial lipoatrophy in HIV-positive patients. AIDS 21:1147–1155 [DOI] [PubMed] [Google Scholar]
- Florindo AA, Oliveira Latorre MR, Jaime PC, Segurado AA 2007 Leisure time physical activity prevents accumulation of central fat in HIV/AIDS subjects on highly active antiretroviral therapy. Int J STD AIDS 18:692–696 [DOI] [PubMed] [Google Scholar]
- Engelson ES, Agin D, Kenya S, Werber-Zion G, Luty B, Albu JB, Kotler DP 2006 Body composition and metabolic effects of a diet and exercise weight loss regimen on obese, HIV-infected women. Metabolism 55:1327–1336 [DOI] [PubMed] [Google Scholar]
- Dolan SE, Frontera W, Librizzi J, Ljungquist K, Juan S, Dorman R, Cole ME, Kanter JR, Grinspoon S 2006 Effects of a supervised home-based aerobic and progressive resistance training regimen in women infected with human immunodeficiency virus: a randomized trial. Arch Intern Med 166:1225–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry L, Sprinz E, Stein R, Medeiros NB, Oliveira J, Ribeiro JP 2006 Exercise training in HIV-1-infected individuals with dyslipidemia and lipodystrophy. Med Sci Sports Exerc 38:411–417 [DOI] [PubMed] [Google Scholar]
- Nixon S, O'Brien K, Glazier RH, Tynan AM 2005 Aerobic exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev CD001796 [DOI] [PubMed] [Google Scholar]
- Hadigan C, Rabe J, Grinspoon S 2002 Sustained benefits of metformin therapy on markers of cardiovascular risk in human immunodeficiency virus-infected patients with fat redistribution and insulin resistance. J Clin Endocrinol Metab 87:4611–4615 [DOI] [PubMed] [Google Scholar]
- Saint-Marc T, Touraine JL 1999 Effects of metformin on insulin resistance and central adiposity in patients receiving effective protease inhibitor therapy. AIDS 13:1000–1002 [DOI] [PubMed] [Google Scholar]
- van Wijk JP, de Koning EJ, Cabezas MC, op't Roodt J, Joven J, Rabelink TJ, Hoepelman AI 2005 Comparison of rosiglitazone and metformin for treating HIV lipodystrophy: a randomized trial. Ann Intern Med 143:337–346 [DOI] [PubMed] [Google Scholar]
- Hadigan C, Meigs JB, Rabe J, D'Agostino RB, Wilson PW, Lipinska I, Tofler GH, Grinspoon SS, Framingham Heart Study 2001 Increased PAI-1 and tPA antigen levels are reduced with metformin therapy in HIV-infected patients with fat redistribution and insulin resistance. J Clin Endocrinol Metab 86:939–943 [DOI] [PubMed] [Google Scholar]
- Driscoll SD, Meininger GE, Ljungquist K, Hadigan C, Torriani M, Klibanski A, Frontera WR, Grinspoon S 2004 Differential effects of metformin and exercise on muscle adiposity and metabolic indices in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab 89:2171–2178 [DOI] [PubMed] [Google Scholar]
- Mulligan K, Yang Y, Wininger DA, Koletar SL, Parker RA, Alston-Smith BL, Schouten JT, Fielding RA, Basar MT, Grinspoon S 2007 Effects of metformin and rosiglitazone in HIV-infected patients with hyperinsulinemia and elevated waist/hip ratio. AIDS 21:47–57 [DOI] [PubMed] [Google Scholar]
- Kohli R, Shevitz A, Gorbach S, Wanke C 2007 A randomized placebo-controlled trial of metformin for the treatment of HIV lipodystrophy. HIV Med 8:420–426 [DOI] [PubMed] [Google Scholar]
- Kohli R, Wanke C, Gorbach S, Shevitz A A randomized placebo-controlled trial of metformin for the treatment of HIV lipodystrophy. Program and Abstracts of the 13th Conference of Retroviruses and Opportunistic Infections, Denver, CO, 2006, Abstract 148 [Google Scholar]
- Rietschel P, Hadigan C, Corcoran C, Stanley T, Neubauer G, Gertner J, Grinspoon S 2001 Assessment of growth hormone dynamics in human immunodeficiency virus-related lipodystrophy. J Clin Endocrinol Metab 86:504–510 [DOI] [PubMed] [Google Scholar]
- Koutkia P, Canavan B, Breu J, Grinspoon S 2005 Growth hormone (GH) responses to GH-releasing hormone-arginine testing in human immunodeficiency virus lipodystrophy. J Clin Endocrinol Metab 90:32–38 [DOI] [PubMed] [Google Scholar]
- Koutkia P, Canavan B, Breu J, Torriani M, Kissko J, Grinspoon S 2004 Growth hormone-releasing hormone in HIV-infected men with lipodystrophy: a randomized controlled trial. JAMA 292:210–218 [DOI] [PubMed] [Google Scholar]
- Falutz J, Allas S, Blot K, Potvin D, Kotler D, Somero M, Berger D, Brown S, Richmond G, Fessel J, Turner R, Grinspoon S 2007 Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med 357:2359–2370 [DOI] [PubMed] [Google Scholar]
- Bickel M, Zangos S, Jacobi V, Lutz T, Knecht G, Goebel F, Staszewski S, Klauke S 2006 A randomized, open-label study to compare the effects of two different doses of recombinant human growth hormone on fat reduction and fasting metabolic parameters in HIV-1-infected patients with lipodystrophy. HIV Med 7:397–403 [DOI] [PubMed] [Google Scholar]
- D'Amico S, Shi J, Sekhar RV, Jahoor F, Ellis KJ, Rehman K, Willis J, Maldonado M, Balasubramanyam A 2006 Physiologic growth hormone replacement improves fasting lipid kinetics in patients with HIV lipodystrophy syndrome. Am J Clin Nutr 84:204–211 [DOI] [PubMed] [Google Scholar]
- Engelson ES, Glesby MJ, Mendez D, Albu JB, Wang J, Heymsfield SB, Kotler DP 2002 Effect of recombinant human growth hormone in the treatment of visceral fat accumulation in HIV infection. J Acquir Immune Defic Syndr 30:379–391 [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Thompson M, Brown SJ, Richmond G, Lee D, Muurahainen N, Kotler DP 2007 Recombinant human growth hormone to treat HIV-associated adipose redistribution syndrome: 12 week induction and 24-week maintenance therapy. J Acquir Immune Defic Syndr 45:286–297 [DOI] [PubMed] [Google Scholar]
- Lo J, You S, Canavan B, Beltrani G, Koutkia P, Lee H, Grinspoon S Effects of 18-month physiological GH replacement in relatively GH-deficient patients with HIV lipodystrophy. Program and Abstracts of the 15th Conference of Retroviruses and Opportunistic Infections, Boston, MA, 2008, Abstract 146LB [Google Scholar]
- Lo JC, Mulligan K, Noor MA, Schwarz JM, Halvorsen RA, Grunfeld C, Schambelan M 2001 The effects of recombinant human growth hormone on body composition and glucose metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab 86:3480–3487 [DOI] [PubMed] [Google Scholar]
- Wanke C, Gerrior J, Kantaros J, Coakley E, Albrecht M 1999 Recombinant human growth hormone improves the fat redistribution syndrome (lipodystrophy) in patients with HIV. AIDS 13:2099–2103 [DOI] [PubMed] [Google Scholar]
- Hultman CS, McPhail LE, Donaldson JH, Wohl DA 2007 Surgical management of HIV-associated lipodystrophy: role of ultrasonic-assisted liposuction and suction-assisted lipectomy in the treatment of lipohypertrophy. Ann Plast Surg 58:255–263 [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 2002 Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106:3143–3421 [PubMed] [Google Scholar]
- Lundgren JD, Battegay M, Behrens G, De Wit S, Guaraldi G, Katlama C, Martinez E, Nair D, Powderly WG, Reiss P, Sutinen J, Vigano A, EACS Executive Committee 2008 European AIDS Clinical Society (EACS) guidelines on the prevention and management of metabolic diseases in HIV. HIV Med 9:72–81 [DOI] [PubMed] [Google Scholar]
- Dubé MP, Stein JH, Aberg JA, Fichtenbaum CJ, Gerber JG, Tashima KT, Henry WK, Currier JS, Sprecher D, Glesby MJ, Adult AIDS Clinical Trials Group Cardiovascular Subcommittee, HIV Medical Association of the Infectious Disease Society of America 2003 Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis 37:613–627 [DOI] [PubMed] [Google Scholar]
- Law MG, Friis-Moller N, El Sadr WM, Weber R, Reiss P, D'Arminio MA, Thiebaut R, Morfeldt L, De Wit S, Pradier C, Calvo G, Kirk O, Sabin CA, Phillips AN, Lundgren JD, D:A:D Study Group 2006 The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D Study. HIV Med 7:218–230 [DOI] [PubMed] [Google Scholar]
- Fichtenbaum CJ, Gerber JG 2002 Interactions between antiretroviral drugs and drugs used for the therapy of the metabolic complications encountered during HIV infection. Clin Pharmacokinet 41:1195–1211 [DOI] [PubMed] [Google Scholar]
- Fichtenbaum CJ, Gerber JG, Rosenkranz SL, Segal Y, Aberg JA, Blaschke T, Alston B, Fang F, Kosel B, Aweeka F 2002 Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS 16:569–577 [DOI] [PubMed] [Google Scholar]
- Kiser JJ, Gerber JG, Predhomme JA, Wolfe P, Flynn DM, Hoody DW 2008 Drug/drug interaction between lopinavir/ritonavir and rosuvastatin in healthy volunteers. J Acquir Immune Defic Syndr 47:570–578 [DOI] [PubMed] [Google Scholar]
- Gerber JG, Rosenkranz SL, Fichtenbaum CJ, Vega JM, Yang A, Alston BL, Brobst SW, Segal Y, Aberg JA, AIDS Clinical Trials Group 5108 Team 2005 Effect of efavirenz on the pharmacokinetics of simvastatin, atorvastatin, and pravastatin: results of AIDS Clinical Trials Group 5108 Study. J Acquir Immune Defic Syndr 39:307–312 [DOI] [PubMed] [Google Scholar]
- Berg-Wolf MV, Klibanov OM, Gaughan JP, Tedaldi EM 2008 Ezetimibe combined with low-dose statin effectively lowers LDL in protease inhibitor treated patients. AIDS Patient Care STDS 22:483–488 [DOI] [PubMed] [Google Scholar]
- Coll B, Aragones G, Parra S, Alonso-Villaverde C, Masana L 2006 Ezetimibe effectively decreases LDL-cholesterol in HIV-infected patients. AIDS 20:1675–1677 [DOI] [PubMed] [Google Scholar]
- Calza L, Manfredi R, Chiodo F 2002 Use of fibrates in the management of hyperlipidemia in HIV-infected patients receiving HAART. Infection 30:26–31 [DOI] [PubMed] [Google Scholar]
- Rao A, D'Amico S, Balasubramanyam A, Maldonado M 2004 Fenofibrate is effective in treating hypertriglyceridemia associated with HIV lipodystrophy. Am J Med Sci 327:315–318 [DOI] [PubMed] [Google Scholar]
- De Truchis P, Kirsteller M, Perier A, Meunier C, Zucman D, Force G, Doll J, Katlama C, Rozenbaum W, Masson H, Gardette J, Melchoir JC 2007 Reduction in triglyceride level with N-3 polyunsaturated fatty acids in HIV-infected patients taking potent antiretroviral therapy: a randomized prospective study. J Acquir Immune Defic Syndr 44:278–285 [DOI] [PubMed] [Google Scholar]
- Wohl DA, Tien HC, Busby M, Cunningham C, Macintosh B, Napravnik S, Danan E, Donovan K, Hossenipour M, Simpson Jr RJ 2005 Randomized study of the safety and efficacy of fish oil (omega-3 fatty acid) supplementation with dietary and exercise counseling for the treatment of antiretroviral therapy-associated hypertriglyceridemia. Clin Infect Dis 41:1498–1504 [DOI] [PubMed] [Google Scholar]
- Dube MP, Wu JW, Aberg JA, Deeg MA, McGovern ME, Alston BL, Shriver SL, Greenwald ML, Lee D, Stein JH Safety and efficacy of extended-release niacin for the treatment of dyslipidemia in patients with HIV-infection: a prospective, multicenter study (ACTG 5148). Program and Abstracts of the 7th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV, Dublin, Ireland, 2005, Abstract 12 [Google Scholar]
- Aberg JA, Zackin RA, Brobst SW, Evans SR, Alston BL, Henry WK, Glesby MJ, Torriani FJ, Yang Y, Owens SI, Fichtenbaum CJ, ACTG 5087 Study Team 2005 A randomized trial of the efficacy and safety of fenofibrate versus pravastatin in HIV-infected subjects with lipid abnormalities: AIDS Clinical Trials Group Study 5087. AIDS Res Hum Retroviruses 21:757–767 [DOI] [PubMed] [Google Scholar]
- Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B 2006 Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care [Erratum (2006) 49:2816–2818] 29:1963–1972 [DOI] [PubMed] [Google Scholar]
- Goldberg RB, Kendall DM, Deeg MA, Buse JB, Zagar AJ, Pinaire JA, Tan MH, Khan MA, Perez AT, Jacober SJ 2005 A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 28:1547–1554 [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Wolski K, Nicholls SJ, Nissen SE 2007 Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 298:1180–1188 [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K 2007 Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356:2457–2471 [DOI] [PubMed] [Google Scholar]
- Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B 2007 Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30:753–759 [DOI] [PubMed] [Google Scholar]
- Triant VA, Lee H, Hadigan C, Grinspoon SK 2007 Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 92:2506–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis-Moller N, Sabin CA, Weber R, d'Arminio MA, El Sadr WM, Reiss P, Thiebaut R, Morfeldt L, De Wit S, Pradier C, Calvo G, Law MG, Kirk O, Phillips AN, Lundgren JD 2003 Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 349:1993–2003 [DOI] [PubMed] [Google Scholar]
- Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA 2003 Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med 348:702–710 [DOI] [PubMed] [Google Scholar]
- Lichtenstein K, Armon C, Buchacz K, Moorman AC, Wood KC, Brooks J Analysis of cardiovascular risk factors in the HIV Outpatient Study Cohort. Program and Abstracts of the 13th Conference of Retroviruses and Opportunistic Infections, Denver, CO, 2006, Abstract 735 [Google Scholar]
- Markowitz M, Nguyen BY, Gotuzzo E, Mendo F, Ratanasuwan W, Kovacs C, Prada G, Morales-Ramirez JO, Crumpacker CS, Isaacs RD, Gilde LR, Wan H, Miller MD, Wenning LA, Teppler H 2007 Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr 46:125–133 [DOI] [PubMed] [Google Scholar]
- DeJesus E, Walmsley S, Cohen C, Cooper D, Hirschel B, Goodrich J, Valdez J, Rajicic N, Mayer H Fasted lipid changes after administration of maraviroc or efavirenz in combination with zidovudine and lamivudine for 48 weeks to treatment-naïve HIV-infected patients. Program and Abstracts of the 15th Conference of Retroviruses and Opportunistic Infections, Boston, MA, 2008, Abstract 929 [Google Scholar]
- D:A:D Study Group, Sabin CA, Worm SW, Weber R, Reiss P, El Sadr W, Dabis F, De Wit S, Law M, D'Arminio MA, Friis-Moller N, Kirk O, Pradier C, Weller I, Phillips AN, Lundgren JD 2008 Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet 371:1417–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]