Abstract
Context: Alterations of protein turnover may contribute to the progressive decline of muscle mass with aging.
Objective: Our objective was to examine the effects of near-physiological recombinant human GH and/or testosterone (T) administration to older men on whole body protein kinetics and muscle gene expression.
Design, Settings, and Participants: A 6-month randomized, double-blind, placebo-controlled trial in 21 healthy elderly men aged 65–75 yr, was performed. Participants were randomized to receive placebo GH and placebo T, rhGH and placebo T (GH), T and placebo GH (T), or rhGH and T (GHT).
Interventions: The leucine rate of appearance (index of proteolysis), nonoxidative leucine disposal rate (an index of protein synthesis), and leucine oxidation rate were measured with an infusion of l-[1-13C] leucine. Muscle biopsies for the measurement of gene expression were performed. Body composition and aerobic capacity (maximal oxygen capacity) were measured.
Results: Serum IGF-I levels increased significantly with GH and GHT (P < 0.001) compared with placebo. T increased significantly only in the T group (P = 0.028). Leucine rate of appearance and nonoxidative leucine disposal rate increased with GH (P = 0.015, P = 0.019) and GHT (P = 0.017, P = 0.02), but leucine oxidation did not change significantly in any treatment group. Midthigh muscle mass and maximal oxygen capacity increased (P < 0.04) with GHT only. Expression of muscle function genes did not change significantly, but the within-group comparisons revealed a significant increase of androgen receptor expression in the GHT group (P = 0.001).
Conclusion: This study showed that 6-month treatment with low-dose GH alone or with T in healthy elderly men produces comparable increments in whole body protein turnover and protein synthesis.
Treatment with low-dose GH with or without testosterone in elderly men produces similar increments in whole body protein turnover and synthesis.
Sarcopenia, an involuntary loss of muscle mass and strength, is a well-recognized feature of normal aging. The mechanism for this is probably multifactorial but may be due in part to age-related hormonal changes (1). Normal aging in men is marked by decreases in both GH and testosterone (T) secretion. The 24-h GH production rate decreases by approximately 14% per decade of adult life after puberty (2). Serum T levels begin to decline in the late third decade and then decrease progressively throughout adult life (3). Both GH and T are strong anabolic agents, which promote nitrogen retention, and increase lean body mass (LBM) and protein metabolism in GH deficient (GHD) and hypogonadal men, respectively (4,5). It has been suggested that the changes in body composition in older subjects may be related to the age-related decrease in GH and T.
In healthy elderly men, the rate of muscle protein synthesis and myosin heavy chain (MHC) synthesis has correlated positively with IGF-I and T levels (6,7). In elderly women, GH administration of 0.025 mg recombinant human GH (rhGH)/kg·d−1 for 1 month increased whole body protein synthesis (WBPS) and breakdown, and increased muscle protein synthesis (8). However, in healthy elderly men, GH administration for 3 months increased muscle mass and strength but failed to affect whole body protein metabolism or muscle protein synthesis (9). Near-physiological T treatment for 6 months increased LBM and strength, and reduced muscle protein breakdown in older men (10), whereas low-dose T treatment for 2 yr in elderly men with low T has shown no beneficial effect on muscle mass and strength, suggesting a dose-dependent effect (11). Both GH and T elicit their effect on protein synthesis by binding to their receptors and increasing muscle gene transcription, including that of IGF-I (10,12). There is some evidence that im IGF-I rather that of the systemic circulation is of major importance for the anabolic action of GH (13). Therefore, there may be a synergistic effect between the anabolic action of GH and T because the administration of T in elderly men increased skeletal muscle IGF-I mRNA and up-regulated the androgen receptor (AR) (10).
We have investigated the effect of near-physiological GH and/or T on whole body protein turnover in healthy elderly men with relatively low-circulating IGF-I and T levels. Low-dose GH replacement was tailored individually with the aim being to reverse some of the aging-associated changes without significant adverse effects. To investigate a possible effect of treatment on skeletal muscle gene expression, biopsies were taken from the vastus lateralis.
Subjects and Methods
Participants
Twenty-four community dwelling elderly men (65–75 yr old) who were healthy and independent, were randomized to receive either: 1) placebo GH and placebo T (PL), 2) rhGH and placebo T (GH), 3) T and placebo rhGH (T), or 4) rhGH and T (GHT) for 6 months in a double-blind trial. Hospital pharmacists not otherwise involved in the study randomized participants into treatments using computer-generated pre-allocated study numbers. These subjects were a subgroup of a larger study of 80 subjects. The effect of the different hormone treatments on LBM, fat body mass, midthigh muscle cross-sectional area (CSA), muscle strength, aerobic capacity [maximal oxygen capacity (VO2max)], and quality of life has been published previously (14). Exclusion criteria were: any clinically significant disease, body mass index more than 30 kg/m2, evidence of active malignancy, any history of pituitary disease or prostate cancer and elevated age-specific levels of prostate-specific antigen, or a palpable prostate nodule. From volunteers meeting these criteria whose circulating IGF-I levels were lower than the 50th percentile for the local age-specific reference range (145 ng/ml), we selected 80 subjects with the lowest T levels but excluded those with frank T deficiency (<173 ng/dl: 6.0 nmol/liter). The mean screening T level of our study participants was 397.6 ± 10 ng/dl. A total of 24 volunteered to participate in the isotope study. The Research Ethics Committee of Guy’s and St. Thomas’ Hospitals approved the protocol, and subjects gave informed written consent.
Study protocol
The study was a double-blind, randomized, and placebo-controlled trial. At baseline participants gave fasting morning blood samples for IGF-I, IGF binding proteins (IGFBPs) 1 and 3, T, SHBG, prostate-specific antigen, glucose, insulin, and C peptide. We encountered a problem measuring the insulin levels at the end of the study in two of the subjects in the GH group, and they were excluded from the analysis. The same day, subjects underwent body composition analysis using dual-emission x-ray absorptiometry (Hologic QDR-4500W scanner; Waltham, MA) and midthigh muscle CSA measurements by computed tomography (Philips Medical Systems, Eindhoven, The Netherlands). VO2max was measured using a step-wise maximal exercise test on an electromagnetically braked bicycle ergometer (Lode Excalibur Sport; Lode, Groningen, The Netherlands), as described (14). Strength was measured in the afternoon of the same day isometrically on handgrip and on the right knee. Right knee concentric flexion/extension at angular velocities of 60°/sec, 90°/sec, and 180°/sec were also performed using a Kin-Com model 125E (Chattecx, Chattanooga, TN). Peak torque was calculated using Kin-Com computer software.
A second appointment was made less than 1 wk from the initial assessment, and participants were asked to present in the metabolic research area of the Diabetes and Endocrinology Day Center at St. Thomas’ Hospital, at 0800 h after an overnight fast. Subjects were instructed not to exercise for 3 d before this appointment. A priming dose of sodium [13C]bicarbonate (0.2 mg/kg) and [1-13C]leucine (15 mg/ml) (both Tracer Technologies, Somerville, MA; 13C enrichment 99%) was administered, followed by a constant infusion of [1-13C]leucine (1 mg/kg·h−1) for 9 h.
Blood samples were obtained 5 min before, at baseline, and at 180, 240, 360, 480, and 540 min to measure the isotopic enrichment and concentration of α-ketoisocaproic acid (KIC). Expired air was collected at the same time points using a GaSampler Breath Collection System (Quintron Instrument Co., Inc., Milwaukee, WI) for the measurement of 13CO2 enrichment. At baseline, and at 2, 5, and 8 h after the start of the infusion, the subject’s CO2 production and oxygen consumption were measured by indirect calorimetry (CPX-D; Medical Graphics, Minneapolis, MN).
At the end of the study, a percutaneous muscle biopsy of the vastus lateralis was performed under local anesthesia for the measurement of skeletal muscle gene expression. The study was repeated at 6 months. At visit 1 (wk 0 of the treatment period), within 2-wk of baseline assessment, participants were trained to self-administer their treatment, which commenced that day and continued daily for 6 months.
Dosage and hormone administration
T or placebo was given by transdermal patches in a standard dose of 5 mg/d. Dosage and administration protocol of rhGH or placebo started at a low-fixed dose (0.1 mg/d), and the dose increased at each study visit until a prearranged target IGF-I level was reached. The rhGH dose was reduced if the IGF-I level was above the 100th percentile of the age-specific reference range (250 ng/ml), and there were likely side effects or if it was above the 75th percentile of the young adult range (380 ng/ml). Compliance was monitored by counting the empty vials and used patches, which had to be returned to our nurse specialist.
Analytical methods
Using gas chromatography-mass spectrometry (5971A MSD; Hewlett-Packard, Palo Alto, CA) and selected ion monitoring, αKIC enrichment was measured as the quinoxalinol-trimethylsilyl derivative at m/z 232 and 233 (15) [within-assay coefficient of variation (CV) < 2%]. 13C enrichment of breath CO2 was measured on a VG SIRA series II isotope ratio mass spectrometer (VG Isotech, Cheshire, UK; within-assay CV, < 1%).
Total T was measured on the Bayer ADVIA Centaur analyzer (Bayer Diagnostics, Tarrytown, NY) by a competitive immunoassay using direct chemiluminescent technology. The intraassay CV was 6.2%, and the interassay CV was 4.4% at 95.5 ng/dl, 4.7% and 4.7% at 365.4 ng/dl, 2.6% and 4.3% at 798.3 ng/dl, and 2.3% and 4.3% at 1009.5 ng/dl. The sensitivity and assay range was 10–1500 ng/dl (0.35–52.1 nmol/liter). Free testosterone (FT) was calculated from the formula: FT (pmol/liter) = 6.11 − [2.38 × log (SHBG nmol/liter)] × T result × 10. IGFBP-1 and IGFBP-3 were measured using coated tube immunoradiometric assay kits, DSL 7800 and DSL 6600 respectively (Diagnostics Systems Laboratories UK Ltd., Oxon, UK). The intraassay CV for IGFBP-3 was 1.8% at 82.7 ng/ml and 3.9% at 7.3 ng/ml. The interassay CV was 1.9% at 76.9 ng/ml and 0.6% at 8.03 ng/ml. The intraassay CV for IGFBP-1 was 5.2% at 5.2 ng/ml and 2.7% at 144.6 ng/ml. The interassay CV was 3.5% at 5.16 ng/ml and 3.6% at 142.03 ng/ml. Insulin and C peptide were measured by double-antibody RIA (within-assay CV, 6 and 9%, respectively). Glucose was determined by an oxygen rate method using a Beckman oxygen electrode (Beckman Instruments, Inc., Fullerton, CA) with a within-assay CV of 2%.
Calculations
The kinetics of leucine metabolism was calculated using standard isotope dilution equations for steady-state conditions. KIC enrichment was used as a measure of intracellular leucine enrichment (16). The leucine oxidation rate was calculated from the enrichment of CO2 and the CO2 production rate assuming 80% recovery of 13CO2 (17). Rate of disappearance (Rd) was assumed to equal rate of appearance (Ra) because subjects were studied in steady state. The nonoxidative leucine disposal rate (NOLD) (a measure of protein synthesis) was calculated as the difference between leucine Rd and leucine oxidation rate. Leucine kinetics was expressed as μmol/kg body weight·min−1.
Muscle biopsy
Muscle biopsies were performed at the end of the study. The vastus lateralis was anesthetized locally using lignocaine. A 150-mg tissue sample was removed using a Bergström needle and was immediately frozen in liquid nitrogen.
Muscle mRNA
A real-time quantitative PCR system (PE Biosystems Prism 7700; Foster City, CA) was used to measure the abundance of selected mRNAs in muscle tissue. Transcripts quantified were MHC type 1 (MHC I), MHC IIa, MHC IIx, IGF-I, IGF-II, IGFBP-5, peroxisome proliferator-activated receptor (PPAR)γ, AR, and glucose transporter (GLUT) 4, and the mitochondrial mRNAs cytochrome c oxidase (COX) subunit 3, and COX4 (18). RNA was extracted from the skeletal muscle of individual subjects by the TRIzol method (Life Technologies, Gaithersburg, MD), treated with DNase (Life Technologies), and then reverse transcribed using the TaqMan Reverse Transcription Reagents (PE Biosystems). Results are expressed as arbitrary units relative to the housekeeping gene, 28S rRNA.
Statistical analyses
Baseline differences between groups were analyzed by one-way ANOVA, using the Bonferroni correction factor. Changes in outcome measures after 6-month hormone treatment between groups were analyzed by analysis of covariance adjusted for the value of the dependent variable at baseline and treatment group. Analysis of covariance was also used for within-group comparisons. Linear regression analysis was used to assess relationships between variables. P values less than 0.05 were considered significant. Results are expressed as mean ± se. Data were analyzed using the StataTM 6.0 computer program (StataCorp LP, College Station, TX).
Results
Subjects characteristics
Three of the 24 subjects who volunteered to participate in the measurement of leucine kinetics did not complete the study. Subjects were well matched in terms of anthropometric and hormonal data. Baseline characteristics are shown in Table 1. Subject characteristics, body composition, and hormone levels in the total study group and this subgroup have been reported previously (14,17).
Table 1.
Baseline characteristics of the subjects
| Placebo (n = 5) | GH (n = 5) | T (n = 6) | GHT (n = 5) | All (n = 21) | P value | |
|---|---|---|---|---|---|---|
| Age (yr) | 69.8 (1.5) | 70.6 (1.3) | 70.3 (0.9) | 70.4 (1.8) | 70.2 (0.6) | 0.98 |
| Weight (kg) | 77.2 (5.6) | 78.3 (4.3) | 77.2 (3.8) | 83.9 (5.3) | 80 (2.3) | 0.68 |
| Body mass index (kg/m2) | 24.8 (1) | 26.3 (1.3) | 26.1 (0.9) | 27.5 (1.6) | 26.1 (0.5) | 0.54 |
| Waist to hip ratio | 0.95 (0.01) | 0.95 (0.01) | 0.9 (0.01) | 0.94 (0.02) | 0.93 (0.009) | 0.16 |
| IGF-I (ng/ml) | 103.8 (6.9) | 104.5 (12.9) | 118.3 (12.9) | 109.1 (11.4) | 109.1 (5.3) | 0.80 |
| T (ng/dl) | 507.2 (80.6) | 440.9 (63.4) | 495.6 (63.4) | 512.9 (83.5) | 489.9 (33.4) | 0.88 |
| FT index | 0.22 (0.04) | 0.14 (0.01) | 0.18 (0.03) | 0.14 (0.02) | 0.17 (0.01) | 0.18 |
| FT pg/ml | 121 (18) | 110 (16.4) | 121 (14.6) | 125 (22.1) | 119 (8.3) | 0.94 |
All values are presented as mean (se). The P value is for the overall comparison between groups. To convert T to nmol/liter, multiply values by 0.0347. To convert IGF-I to nmol/liter, multiply by 0.131.
Hormone profile (Table 2)
Table 2.
Effects of treatment on T, IGF-I, IGFBP-3, IGFBP-1, insulin, C peptide, and glucose before and after 6-month treatment
| Placebo (n = 5) | GH (n = 5) | T (n = 6) | GHT (n = 5) | |
|---|---|---|---|---|
| T (ng/dl) | ||||
| Baseline | 507.2 (80) | 440.9 (63) | 495.6 (63) | 512.9 (83) |
| 26 wk | 524.4 (69) | 536 (92) | 855.9 (181) | 665.5 (156) |
| Change | 17 (106) | 95 (112) | 357.3 (175) | 150.1 (160) |
| P value compared with placebo | 0.92 | 0.028 | 0.95 | |
| IGF-I (ng/ml) | ||||
| Baseline | 103.8 (6.9) | 104.5 (12) | 118.3 (12) | 109.1 (11) |
| 26 wk | 85.4 (6) | 179 (16) | 105 (9) | 194 (25) |
| Change | −17.5 (9) | 74 (21) | −12.9 (16) | 85.4 (27) |
| P value compared with placebo | 0.001 | 0.5 | <0.0001 | |
| IGFBP-3 (ng/ml) | ||||
| Baseline | 2993 (438) | 3498 (246) | 3571 (333) | 3254 (384) |
| 26 wk | 2625 (331) | 3526 (187) | 3210 (309) | 3495 (383) |
| Change | −368 (550) | +27 (309) | −360 (455) | +241 (543) |
| P value compared with placebo | 0.07 | 0.52 | 0.02 | |
| IGFBP-1 (ng/ml) | ||||
| Baseline | 26.4 (8.8) | 16.2 (4.1) | 23.9 (4.7) | 18.6 (8.5) |
| 26 wk | 24.2 (7) | 22.8 (6.4) | 31.5 (8.7) | 13.1 (3.1) |
| Change | −2.14 (11.3) | +6.6 (7.6) | +7.5 (9.9) | −5.4 (9.1) |
| P value compared with placebo | 0.62 | 0.31 | 0.45 | |
| Insulin (pmol/liter) | ||||
| Baseline | 54.2 (7.8) | 52.4 (3.5) | 30.7 (13.4) | 62.2 (13.8) |
| 26 wk | 47.7 (7.5) | 68 (14.9) | 42.1 (5.8) | 52.2 (7.7) |
| Change | −6.7 (10.8) | +15.5 (6) | +11.3 (14.6) | −9.58 (15.8) |
| P value compared with placebo | 0.08 | 0.88 | 0.97 | |
| C peptide (pmol/liter) | ||||
| Baseline | 1215 (139) | 879 (144) | 1270 (186) | 1270 (186) |
| 26 wk | 492 (86) | 511 (65) | 700 (62) | 699 (62) |
| Change | −722 (164) | −368 (568) | −570 (196) | −570 (196) |
| P value compared with placebo | 0.64 | 0.34 | 0.16 | |
| Glucose (mmol/liter) | ||||
| Baseline | 5.8 (0.5) | 4.9 (0.2) | 4.8 (0.2) | 5.1 (0.1) |
| 26 wk | 5 (5) | 5.3 (0.3) | 4.8 (0.3) | 5.8 (0.2) |
| Change | −0.8 (0.6) | +0.37 (0.4) | +0.03 (0.3) | +0.7 (0.3) |
| P value compared with placebo | 0.54 | 0.64 | 0.08 |
All values are presented as mean (se). n, Number of participants who completed 6 months.
T levels increased significantly only in the T group (P = 0.02) but did not change in the GHT group. IGF-I levels increased significantly with administration of GH alone (P < 0.001) and GHT (P < 0.0001) when compared with placebo. IGFBP-3 also increased significantly after GHT (P < 0.03) and with a trend toward a significant increase in the GH group (P = 0.07). There were no significant changes in plasma insulin or glucose levels in any treatment group.
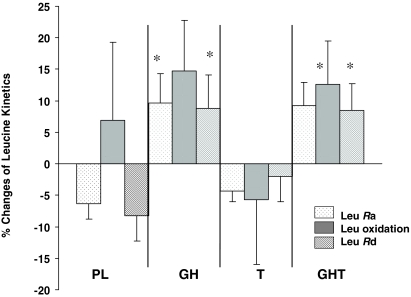
Whole body protein kinetics (WBPK) (Fig. 1 and Table 3)
Figure 1.
Effects of hormone administration on WBPK in healthy older men. *, P = 0.02. Leu, Leucine.
Table 3.
WBPK per kg body weight (μmol/min/kg BW)
| Placebo (n = 5) | GH (n = 5) | T (n = 6) | GHT (n = 5) | |
|---|---|---|---|---|
| Leucine Ra | ||||
| Baseline | 1.73 (0.1) | 1.69 (0.04) | 1.63 (0.02) | 1.56 (0.06) |
| 26 wk | 1.6 (0.03) | 1.85 (0.09) | 1.56 (0.03) | 1.71 (0.09) |
| Change | −1.67 (0.06) | +0.16 (0.1) | −0.07 (0.04) | +0.14 (0.1) |
| P value compared with placebo | 0.01 | 0.99 | 0.09 | |
| Leucine oxidation rate | ||||
| Baseline | 0.43 (0.07) | 0.41 (0.05) | 0.35 (0.04) | 0.37 (0.02) |
| 26 wk | 0.41 (0.02) | 0.46 (0.07) | 0.32 (0.02) | 0.42 (0.05) |
| Change | −0.01 (0.08) | +0.05 (0.09) | −0.03 (0.04) | +0.05 (0.05) |
| P value compared with placebo | 0.21 | 0.25 | 0.56 | |
| Leucine NOLD | ||||
| Baseline | 1.3 (0.06) | 1.28 (0.05) | 1.27 (0.05) | 1.18 (0.05) |
| 26 wk | 1.19 (0.03) | 1.38 (0.05) | 1.23 (0.03) | 1.28 (0.05) |
| Change | −0.1 (0.07) | +0.1 (0.07) | −0.03 (0.06) | +0.9 (0.07) |
| P value compared with placebo | 0.003 | 0.29 | 0.05 |
All values are presented as mean (se). n, Number of participants who completed 6 months.
Leucine Ra and NOLD increased significantly by 16 ± 5.9% (P = 0.015) and 17.1 ± 6.5% (P = 0.019) after 6-month GH administration, respectively. Similarly, leucine Ra and NOLD increased significantly by 15.5 ± 5.9% (P = 0.017) and 16.7 ± 6.5% (P = 0.021), respectively, in the GHT group. There was no change in leucine Ra or NOLD in the T group. Leucine oxidation rate did not change significantly in any treatment group compared with placebo. T administration had no effect on leucine Ra or NOLD.
A positive correlation was found between leucine NOLD and LBM (r = 0.47; P < 0.0001). At the end of the treatment period, changes in IGF-I levels were directly related to changes in leucine Ra (r = 0.19; P = 0.04) and leucine NOLD (r = 0.4; P = 0.002).
Body composition and middle thigh CSA (Table 4)
Table 4.
LBM, TFM, and VO2max before and after treatment
| Placebo (n = 5) | GH (n = 5) | T (n = 6) | GHT (n = 5) | |
|---|---|---|---|---|
| LBM (kg) | ||||
| Baseline | 53.2 (2.8) | 55.7 (2) | 53.8 (1.9) | 58 (2.1) |
| 26 wk | 53.2 (2.9) | 57.2 (2.7) | 54.4 (2.3) | 59.3 (2) |
| Change | 0.0 | +1.4 (3.4) | +0.6 (3) | +1.3 (3) |
| P value compared with placebo | 0.37 | 0.64 | 0.52 | |
| TFM (kg) | ||||
| Baseline | 20.8 (2.7) | 19.4 (2.3) | 20.1 (2) | 22.2 (4) |
| 26 wk | 20.4 (2.7) | 18.2 (1.4) | 19.4 (1.8) | 18.6 (4.3) |
| Change | −0.3 (3.9) | −1.1 (2.7) | −0.6 (2.7) | −3.6 (5.9) |
| P value compared with placebo | 0.46 | 0.81 | 0.01 | |
| VO2max (ml/kg·min−1) | ||||
| Baseline | 23.5 (4) | 26.1 (1.3) | 24.6 (1.2) | 23.1 (1.5) |
| 26 wk | 25.6 (4) | 28.1 (2.4) | 26 (1.2) | 29.4 (2.5) |
| Change | +2 (6) | +1.9 (2) | +1.4 (1.7) | +6.3 (3) |
| P value compared with placebo | 0.97 | 0.81 | 0.03 |
All values are presented as mean (se). n, Number of participants who completed 6 months.
Although a trend of increasing LBM was observed after GH (1.4 ± 3.4 kg) and after GHT (1.3 ± 3 kg), this did not reach statistical significance (P = 0.35). Total fat mass (TFM) decreased by 3.6 ± 5.9 kg (P = 0.015) only with GHT. The midthigh muscle area as assessed by computed tomography scan increased significantly by 7% (P = 0.05) only after GHT.
Aerobic capacity (VO2max) and muscle strength
VO2max increased significantly (P = 0.038) compared with placebo only after GHT administration (Table 4). No significant changes were recorded in any treatment group in either isokinetic or isometric strength measurements (data not shown).
Muscle gene transcript levels (Table 5)
Table 5.
Effects of treatment on skeletal muscle gene expression (MHC I, MHC IIa, MHC IIx COX3, COX4, AR, IGF-I, IGF-II, IGFBP-5, GLUT4, and PPARγ)
| Placebo (n = 5) | GH (n = 5) | T (n = 6) | GHT (n = 5) | |
|---|---|---|---|---|
| MHC I | ||||
| Baseline | 0.59 (0.1) | 0.33 (0.08) | 0.5 (0.1) | 0.39 (0.1) |
| 26 wk | 0.52 (0.2) | 0.34 (0.1) | 0.44 (0.1) | 0.57 (0.2) |
| P value compared with placebo | 0.49 | 0.74 | 0.94 | |
| MHC IIa | ||||
| Baseline | 0.33 (0.1) | 0.29 (0.05) | 0.45 (0.06) | 0.28 (0.09) |
| 26 wk | 0.29 (0.1) | 0.36 (0.1) | 0.33 (0.1) | 0.45 (0.1) |
| P value compared with placebo | 0.64 | 0.88 | 0.31 | |
| MHC IIx | ||||
| Baseline | 0.09 (0.03) | 0.33 (0.08) | 0.18 (0.05) | 0.2 (0.1) |
| 26 wk | 0.12 (0.07) | 0.18 (0.05) | 0.2 (0.1) | 0.32 (0.16) |
| P value compared with placebo | 0.71 | 0.86 | 0.47 | |
| COX3 | ||||
| Baseline | 0.54 (0.5) | 0.04 (0.01) | 0.03 (0.01) | 0.12 (0.1) |
| 26 wk | 0.02 (0.01) | 0.03 (0.01) | 0.05 (0.03) | 0.19 (0.1) |
| P value compared with placebo | 0.85 | 0.66 | 0.12 | |
| COX4 | ||||
| Baseline | 0.65 (0.1) | 0.49 (0.1) | 0.78 (0.1) | 0.33 (0.08) |
| 26 wk | 0.61 (0.1) | 0.43 (0.1) | 0.44 (0.1) | 0.66 (0.2) |
| P value compared with placebo | 0.54 | 0.48 | 0.79 | |
| IGF-I | ||||
| Baseline | 0.62 (0.1) | 0.41 (0.1) | 0.49 (0.06) | 0.49 (0.2) |
| 26 wk | 0.45 (0.1) | 0.38 (0.07) | 0.37 (0.1) | 0.44 (0.1) |
| P value compared with placebo | 0.9 | 0.77 | 0.88 | |
| IGF-II | ||||
| Baseline | 0.48 (0.1) | 0.5 (0.1) | 0.84 (0.1) | 0.47 (0.1) |
| 26 wk | 0.59 (0.1) | 0.51 (0.1) | 0.46 (0.2) | 0.55 (0.2) |
| P value compared with placebo | 0.72 | 0.28 | 0.88 | |
| IGFBP-5 | ||||
| Baseline | 1.1 (0.2) | 1 (0.1) | 1.5 (0.1) | 0.9 (0.1) |
| 26 wk | 0.9 (0.1) | 1.2 (0.1) | 0.9 (0.2) | 1.1 (0.2) |
| P value compared with placebo | 0.38 | 0.93 | 0.4 | |
| AR | ||||
| Baseline | 0.81 (0.1) | 0.7 (0.09) | 1.0 (0.06) | 0.6 (0.1) |
| 26 wk | 0.82 (0.2) | 0.8 (0.2) | 0.7 (0.1) | 1.1 (0.1)a |
| P value compared with placebo | 0.71 | 0.54 | 0.25 | |
| PPARγ | ||||
| Baseline | 1.1 (0.1) | 1 (0.3) | 1.8 (0.5) | 0.9 (0.5) |
| 26 wk | 1.2 (0.5) | 1.2 (0.2) | 1 (0.3) | 0.9 (0.1) |
| P value compared with placebo | 0.96 | 0.32 | 0.35 | |
| GLUT4 | ||||
| Baseline | 0.73 (0.1) | 0.7 (0.1) | 1.0 (0.1) | 0.6 (0.1) |
| 26 wk | 0.78 (0.1) | 0.88 (0.1) | 0.8 (0.2) | 0.76 (0.2) |
| P value compared with placebo | 0.38 | 0.93 | 0.4 |
P = 0.04 compared with baseline.
There were no changes in skeletal muscle gene expression for MHC I, MHC IIa, MHC IIx, IGF-I, IGF-II, IGFBP-5, PPARγ, and GLUT4, or the mitochondrial mRNAs COX3 and COX4. The only significant change was in AR levels, which increased significantly after GHT in a within-group comparison (P = 0.04).
Adverse events
Mild joint stiffness and muscular pain attributed to GH administration, and skin irritation and nipple tenderness attributed to T administration were observed with the same frequency as reported previously (14).
Discussion
The current study demonstrated that near-physiological dose coadministration of GHT and of GH alone for 6 months in healthy elderly men increased both the leucine appearance rate from protein breakdown and nonoxidative leucine disposal, a measure of protein synthesis. These results represent the first demonstration of GHT on an important physiological measurement affecting body protein stores.
There is controversy as to whether WBPS rate declines with aging (6,19). In one report, older adults’ WBPS expressed in terms of body weight was lower than in young adults, however, when the data were normalized to LBM, no difference was found (20). A recent study in 78 subjects across the adult age span demonstrated that the rates of whole body protein turnover and WBPS declined with aging, with no age effect on amino acid oxidation, even after adjustment for LBM (21). The differences between these studies may reflect study conditions and methodologies used.
Administration of GH to GHD patients for 1–8 wk has resulted in a greater increase in WBPS than proteolysis due to an inhibition of leucine oxidation, resulting in an improvement in protein balance (4,22,23). This improvement in protein balance can account for the increase in LBM reported with GH treatment (4,24). Similarly, in young healthy subjects, a high dose of GH (67 μg/kg·d−1 for 4 wk) increased protein synthesis and protein breakdown with a decrease in leucine oxidation and improvement in protein balance (25). In elderly men GH treatment for 16 wk combined with resistance exercise increased WBPK with no effect on oxidation (26). The finding in the current study of increased protein synthesis and proteolysis with no effect on oxidation with GH treatment suggests that a new steady state has been reached with an increase in protein turnover. In the short term, GH would be expected to have a greater effect on protein synthesis than on proteolysis, resulting in an increase in LBM. The subjects in this study were a subgroup of a larger study of 80 subjects. Although the increase in LBM with GH alone or GHT was not significant in the subgroup, in the total group, a significant increase in LBM was found (14). A reduction in both protein turnover and synthesis in elderly people represents a reduced capacity to remodel tissues (20). The current study shows that GH enhances the remodeling process in older people.
In our study physiological doses of T administered transcutaneously failed to produce any significant changes in protein turnover. T administration at a high unphysiological dose (biweekly injections of 3 mg/kg) for 6 months has increased WBPS and degradation, as well as muscle protein synthesis, with no effect on leucine oxidation in hypogonadal men (5). In addition, im administration of T for 6 months at a higher dose than used in this study has increased skeletal muscle protein synthesis in elderly men (10). The failure of T to produce any significant changes in our study is most likely due to administration of T via a patch, which results in a lower steady (and more physiological) T concentration than weekly (or biweekly) im injections that increases peak T to supraphysiological levels. Huang et al. (27) also reported no changes in whole body protein turnover in a study in which T was administered im at a much lower dose (100 mg every 2 weeks) for a 6-month period in elderly men.
Mauras et al. (28) have shown that coadministration of GH and T to prepubertal GHD boys markedly increases WBPS more than when each compound was given separately. GH and T are thought to elicit an anabolic effect on skeletal muscle by binding to their respective receptors and increasing muscle gene transcription, including that of IGF-I (29). The synergistic effect of GH and T on muscle protein synthesis reported by Mauras et al. (28) suggests that GH may augment the effect of T on mRNA levels of IGF-I and vice versa (30). In the current study, combined GHT had a similar effect as GH alone on protein kinetics, although middle thigh (CSA) muscle mass increased significantly only after GHT. GH and T regulate protein synthesis through different signaling pathways, and it is to be anticipated that their effects would be additive, if not synergistic.
No changes were found in our study in IGF-I, IGFBP-5, and AR mRNA levels when compared with placebo. The only significant change was a within-group increase of AR after GHT administration.
Muscle protein synthesis has been shown to increase by the first week after administration of GH or T and this effect peaks by 1 month. It has been shown that GH and T contribute to muscle protein accretion by activating muscle satellite cells, possibly by increasing im mRNA of IGF-I (30,31). Thus, it is reasonable to hypothesize that the increment of muscle IGF-I mRNA would occur early after the administration of GH or T. Indeed, administration of high doses of T im in healthy elderly men was found to increase expression of AR at 1 month, but not at 6 months (10). Of note, coadministration of GH and T, the latter in the form of a transdermal patch, has increased muscle mRNA of IGF-I after 1-month treatment (32). Thus, the timing of biopsy in the current study may well explain the failure to demonstrate an increase in muscle AR and IGF-I mRNA, despite an increase in middle thigh CSA.
An inherent limitation of human studies involving muscle biopsies is the small number prepared to undergo the procedure. In the T group, only six subjects, and in the GH, PL, and GHT group, only five subjects underwent muscle biopsies. Thus, it is possible that small changes in muscle gene transcript levels evaluated in the current study, persisting even at 6 months after hormone treatment, could have been missed due to type 2 errors.
In the present study, combined GHT administration resulted in a significant increase of protein synthesis and proteolysis, with no effect on leucine oxidation. To our knowledge, the combined effect of GH (0.025 mg/kg three times per week) and T (100 mg every 2 weeks im) on WBPK has been investigated by only one previous study, which reported similar findings (27) apart from the fact that lower and less frequent GH dosage alone had no significant effect. These differences may also be methodological, as in the study by Huang et al. (27), in which protein kinetics was measured using plasma leucine enrichment, which overestimates the intracellular leucine enrichment and may lead to errors in the measurement of leucine kinetics. In the current study, αKIC enrichment was used, which provides a more robust measure of intracellular leucine enrichment. Daily GH may also be more effective than three injections per week.
It has been suggested that the increase in proteolysis observed after GH administration may be due to the effect of GH to increase insulin resistance because insulin has inhibited proteolysis (33). In our study the increase in protein degradation, observed after GH and GHT, was not associated with increased insulin or C-peptide levels. Thus, increased insulin resistance seems an unlikely explanation for this.
There were no changes in muscle strength in any of the treatment groups in our study in accordance with previous similar studies (34,35). MHC protein is critical for muscle contractile functions and has declined with aging (6). The same investigators also reported an age-related lowering of the transcript levels of MHC IIa and MHC IIx but not of MHCI mRNA levels, and most importantly, this could not be reversed by 3-month resistance exercise (36). GHT treatment had no effect on any MHC isoform gene expression in our study.
Maximal aerobic capacity (VO2max) increased in the GHT group only. A decline in VO2max with aging has been shown in both cross-sectional and longitudinal studies, and has been attributed mainly to the decline of muscle mass (37). However, the latter could not entirely explain the decline in VO2max observed in the elderly because this decline persists even after VO2max is normalized to muscle mass (38). Aerobic performance capacity is determined from functional integrity in respiration, circulation, and muscle oxidative capacity. Reduced muscle oxidative capacity has been implicated in the decline of VO2max observed in the elderly. Indeed, the rates of mitochondrial protein synthesis also decrease with aging, and this in turn has been associated with a decline in the expression of COX, a flux-generating enzyme with a key role in the electron transfer chain in muscle fibers (38,39). Despite a significant increase in VO2max in our study in the GHT group, no changes were observed in the mitochondria COX3 and COX4 gene expression at 6 months. There are two possible interpretations of this observation. First, because we performed biopsies at a single time point 6 months after hormone treatment, transient changes of COX3 and COX4 mRNA would not be detected. In addition, a central action of GHT by increasing cardiac performance could not be excluded (40,41).
There is mounting evidence that suggests that GH exerts its anabolic effect mainly by locally produced IGF-I rather than liver-derived circulating IGF-I (13). Of major importance is the identification of a unique local IGF-I isoform, known as mechanogrowth factor, which is specifically expressed in response only to changes in the loading state of skeletal muscles (13,30). The importance of exercise on muscle strength can be further substantiated from observations that administration of T or GH could not prevent the decline of muscle strength after prolonged bed rest (42). Exercise also increases AR expression and the generalized IGF-I isoform in muscle (13,30), both of which are further up-regulated by the increased levels of GH secreted in response to exercise in normal young adults (43). The aforementioned findings support the role of exercise and hormonal integrity in muscle strength and protein metabolism, and may explain the failure of GH or T to increase muscle strength in short-tem interventional studies. We might expect that through the coadministration of GHT, it is possible to increase exercise capacity, which in turn improves (or sustains) physical performance, thus preventing the decline of muscle mass and strength.
In conclusion, administration of GH alone and GHT in the healthy elderly increased whole body protein turnover, and the latter had the additional benefit of increasing VO2max and, thus, muscle aerobic performance capacity. Most likely, the beneficial effects of exercise would be made more robust by replacing the anabolic hormonal milieu, which is deficient in the elderly. Trials looking at the effect of hormone administration on specific muscle (myofibrillar, mitochondrial) proteins and, more importantly, longer-term trials with the power to define surrogate endpoints of frailty such as falls or dependency are urgently needed.
Acknowledgments
We thank Derek Knowlden (Novo Nordisk, Princeton, NJ) for providing recombinant human GH and placebo, Norman Mazer (Watson Laboratories, Corona, CA) for providing testosterone and placebo patches, Jill Schimke for skilled technical assistance, Richard Savine for assistance with outcome assessments, and Paul Seed and Massoud Boroujerdi for statistical advice. Finally, we are grateful to all the volunteers for their enthusiastic participation.
Footnotes
This work was supported by a research fellowship grant (to M.G.G.) from the Guy’s and St. Thomas’ Hospital Charitable Foundation (now the Guy’s and St. Thomas’ Charity) and by National Institutes of Health Grant RO1 AG 09531.
Disclosure Information: The authors have declared that no conflict of interest exists.
First Published Online May 13, 2008
Abbreviations: AR, Androgen receptor; COX, cytochrome c oxidase; CSA, cross-sectional area; CV, coefficient of variation; FT, free testosterone; GHD, GH deficient; GHT, rhGH and testosterone; GLUT, glucose transporter; IGFBP, IGF binding protein; KIC, ketoisocaproic acid; LBM, lean body mass; MHC, myosin heavy chain; NOLD, nonoxidative leucine disposal rate of disappearance; PL, placebo GH and placebo testosterone; PPAR, peroxisome proliferator-activated receptor; Ra, rate of appearance; Rd, rate of disappearance; rhGH, recombinant human GH; T, testosterone; TFM, total fat mass; VO2max, maximal oxygen capacity; WBPK, whole body protein kinetics; WBPS, whole body protein synthesis.
References
- Doherty TJ 2003 Aging and sarcopenia. J Appl Physiol 95:1717–1727 [DOI] [PubMed] [Google Scholar]
- Iranmanesh A, Lizzarlde G, Veldhuis JD 1991 Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory burst and half-life of endogenous GH in healthy men. J Clin Endocrinol Metab 73:1081–1088 [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR 2001 Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 86:724–731 [DOI] [PubMed] [Google Scholar]
- Russell-Jones DL, Weissberger AJ, Bowes SB, Kelly JM, Thomason M, Umpleby AM, Jones RH, Sonksen PH 1993 The effects of growth hormone on protein metabolism in adult growth hormone deficient patients. Clin Endocrinol (Oxf) 38:427–431 [DOI] [PubMed] [Google Scholar]
- Brodsky IG, Balagopal P, Nair KS 1996 Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men. J Clin Endocrinol Metab 81:3469–3475 [DOI] [PubMed] [Google Scholar]
- Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS 1997 Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol 273(4 Pt 1):E790–E800 [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C 1993 Low plasma insulin-like growth factor-1 (IGF-I) is associated with reduced myofibrillar protein synthesis in men over 60. Gerontologist 33:43 (Abstract) [Google Scholar]
- Butterfield GE, Thompson J, Rennie MJ, Marcus R, Hintz RL, Hoffman AR 1997 Effect of rhGH and rhIGF-I treatment on protein utilization in elderly women. Am J Physiol 272(1 Pt 1):E94–E99 [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Statt M, McHenry B 1996 Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab 81:3239–3243 [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ 2002 Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282:E601–E607 [DOI] [PubMed] [Google Scholar]
- Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton 3rd LJ, Smith GE, Khosla S, Jensen MD 2006 DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355:1647–1659 [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C 1997 Insulin-like growth factor-I, actin, and myosin heavy chain messenger RNAs in skeletal muscle after an injection of growth hormone in subjects over 60 years old. J Endocrinol 155:93–97 [DOI] [PubMed] [Google Scholar]
- Bamman MM, Shipp JR, Jiang J, Gower BA, Hunter GR, Goodman A, McLafferty CL, Urban RJ 2001 Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab 280:E383–E390 [DOI] [PubMed] [Google Scholar]
- Giannoulis MG, Sonksen PH, Umpleby M, Breen L, Pentecost C, Whyte M, McMillan CV, Bradley C, Martin FC 2006 The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab 91:477–484 [DOI] [PubMed] [Google Scholar]
- Ford GC, Cheng KN, Halliday D 1985 Analysis of 1-13C leucine and 13C KIC in plasma by capillary gas chromatography/mass spectrometry in protein turnover studies. Biomed Mass Spectrom 128:432–436 [DOI] [PubMed] [Google Scholar]
- Matthews DE, Schwarz HP, Yang RD, Motil KJ, Young VR, Bier DM 1982 Relationship of plasma leucine and -ketoisocaproate during a L-[1–13C]leucine infusion in man: a method for measuring human intracellular leucine tracer environment. Metabolism 31:1105–1112 [DOI] [PubMed] [Google Scholar]
- Giannoulis MG, Jackson N, Shojaee-Moradie F, Sonksen PH, Martin FC, Umpleby M 2006 Effects of growth hormone and/or testosterone on very low density lipoprotein apolipoprotein B100 kinetics and plasma lipids in healthy elderly men: a randomised controlled trial. Growth Horm IGF Res 16:308–317 [DOI] [PubMed] [Google Scholar]
- Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS 2003 Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 100:7996–8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR 2001 Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286:1206–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S, Thornton C, Jozefowicz R, Statt M 1993 Myofibrillar protein synthesis in young and old men. Am J Physiol 264(5 Pt 1):E693–E698 [DOI] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS 2004 Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab 286:E92–E101 [DOI] [PubMed] [Google Scholar]
- Shi J, Sekhar RV, Balasubramanyam A, Ellis K, Reeds PJ, Jahoor F, Sharma MD 2003 Short- and long-term effects of growth hormone (GH) replacement on protein metabolism in GH-deficient adults. J Clin Endocrinol Metab 88:5827–5833 [DOI] [PubMed] [Google Scholar]
- Russell-Jones DL, Bowes SB, Rees SE, Jackson NC, Weissberger AJ, Hovorka R, Sonksen PH, Umpleby AM 1998 Effect of growth hormone treatment on postprandial protein metabolism in growth hormone-deficient adults. Am J Physiol 274(6 Pt 1):E1050–E1056 [DOI] [PubMed] [Google Scholar]
- Salomon F, Cuneo RC, Hesp R, Sonksen PH 1989 The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med 321:1797–1803 [DOI] [PubMed] [Google Scholar]
- Healy ML, Gibney J, Russell-Jones DL, Pentecost C, Croos P, Sonksen PH, Umpleby AM 2003 High dose growth hormone exerts an anabolic effect at rest and during exercise in endurance-trained athletes. J Clin Endocrinol Metab 88:5221–5226 [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Campbell JA, Bier DM 1995 Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol 268(2 Pt 1):E268–E276 [DOI] [PubMed] [Google Scholar]
- Huang X, Blackman MR, Herreman K, Pabst KM, Harman SM Caballero B 2005 Effects of growth hormone and/or sex steroid administration on whole-body protein turnover in healthy aged women and men. Metabolism 54:1162–1167 [DOI] [PubMed] [Google Scholar]
- Mauras N, Rini A, Welch S, Sager B, Murphy SP 2003 Synergistic effects of testosterone and growth hormone on protein metabolism and body composition in prepubertal boys. Metabolism 52:964–969 [DOI] [PubMed] [Google Scholar]
- Argetsinger LS, Carter-Su C 1996 Mechanism of signaling by growth hormone receptor. Physiol Rev 76:1089–1107 [DOI] [PubMed] [Google Scholar]
- Hameed M, Lange KHW, Andersen JL, Schjerling P, Kjaer M, Harridge SDR, Goldspink G 2004 The effect of recombinant human growth hormone and resistance training on IGF-I mRNA expression in the muscles of elderly men. J Physiol 555(Pt 1):231–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S 2006 Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab 91: 3024–3033 [DOI] [PubMed] [Google Scholar]
- Brill KT, Weltman AL, Gentili A, Patrie JT, Fryburg DA, Hanks JB, Urban RJ, Veldhuis JD 2002 Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab 87:5649–5657 [DOI] [PubMed] [Google Scholar]
- Umpleby AM, Boroujerdi MA, Brown PM, Carson ER, Sonksen PH 1986 The effect of metabolic control on leucine metabolism in type 1 (insulin-dependent) diabetic patients. Diabetologia 29:131–141 [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL 1999 Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 84:2647–2653 [DOI] [PubMed] [Google Scholar]
- Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O'Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St Clair C, Pabst KM, Harman SM 2002 Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA 288:2282–2292 [DOI] [PubMed] [Google Scholar]
- Balagopal P, Schimke JC, Ades P, Adey D, Nair KS 2001 Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am J Physiol Endocrinol Metab 280:E203–E208 [DOI] [PubMed] [Google Scholar]
- Stathokostas L, Jacob-Johnson S, Petrella RJ, Paterson DH 2004 Longitudinal changes in aerobic power in older men and women. J Appl Physiol 97:781–789 [DOI] [PubMed] [Google Scholar]
- Rooyakers O, Adey DB, Ades PA, Nair KS 1996 Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA 93:15364–15369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S, Bhatt K, Shah B, Needler N, Delehanty JM, Thornton CA 2003 Reduced amount of mitochondrial DNA in aged human muscle. J Appl Physiol 94:1479–1484 [DOI] [PubMed] [Google Scholar]
- Pugh PJ, Jones TH, Channer KS, 2003 Acute haemodynamic effects of testosterone in men with chronic heart failure. Eur Heart J 24:909–915 [DOI] [PubMed] [Google Scholar]
- Cittadini A, Berggren A, Longobardi S, Ehrnborg C, Napoli R, Rosen T, Fazio S, Caidahl K, Bengtsson B-A, Sacca L 2002 Supraphysiological doses of GH induce rapid changes in cardiac morphology and function. J Clin Endocrinol Metab 87:1654–1659 [DOI] [PubMed] [Google Scholar]
- Zachwieja JJ, Smith SR, Lovejoy JC, Rood JC, Windhauser MM, Bray GA 1999 Testosterone administration preserves protein balance but not muscle strength during 28 days of bed rest. J Clin Endocrinol Metab 84:207–212 [DOI] [PubMed] [Google Scholar]
- Giannoulis MG, Boroujerdi MA, Powrie J, Dall R, Napoli R, Ehrnborg C, Pentecost C, Cittadini A, Jørgensen JOL, Sonksen PH 2005 Gender differences in growth hormone response to exercise before and after rhGH administration and the effect of rhGH on the hormone profile of fit normal adults. Clin Endocrinol (Oxf) 62:315–322 [DOI] [PubMed] [Google Scholar]