Abstract
Context: Precise regulation of the neuroendocrine components of the female reproductive axis involves both negative and positive feedback of estrogen on gonadotropin secretion.
Objective: Our objective was to determine the hypothalamic and/or pituitary sites of estrogen negative and positive feedback using neuroimaging techniques.
Design and Setting: A graded estrogen infusion protocol was administered at a General Clinical Research Center in an academic medical center.
Subjects: Healthy postmenopausal women (n = 11) were recruited for study.
Interventions: Serum samples were measured every 4 h. A structural magnetic resonance imaging was performed at baseline, and [18F]2-fluoro-2-deoxy-d-glucose (18FDG) positron emission tomography was performed at baseline and 24 and 72 h. FDG positron emission tomography was co-registered with magnetic resonance imaging scans, and region of interest analysis was performed.
Main Outcome Measures: Serum LH and estradiol were assessed. Normalized values for glucose uptake were extracted from each region of interest for each subject at each time point.
Results: A decrease in normalized 18FDG uptake was apparent in the hypothalamus at 24 h (P < 0.02) associated with decreased LH (P < 0.0005). The increase in LH at 72 h (P < 0.0005) was associated with increased pituitary 18FDG uptake (P < 0.02) but no change in hypothalamic uptake.
Conclusions: Changes in 18FDG uptake as a measure of metabolic activity can be demonstrated in the hypothalamus and pituitary in association with discrete hormonal events. Results are consistent with mediation of estrogen negative feedback on LH at the hypothalamus, whereas estrogen positive feedback occurs at the pituitary with no evidence of increased hypothalamic activity in women.
In postmenopausal women receiving a graded estrogen infusion, fused FDG-PET and MR scans reveal decreased metabolic activity in the medial basal hypothalamus in conjunction with estrogen negative feedback on LH and increased metabolic activity in the pituitary, but not the hypothalamus, associated with estrogen positive feedback on LH.
The mechanisms underlying the biphasic effects of estrogen on gonadotropin secretion in women have long been the subject of intense scientific interest due to the critical roles of both negative and positive feedback in normal female reproductive cycles across all mammalian species (1). The hypothalamic-pituitary responses to estrogen feedback are both dose and time dependent. Acute or chronic exposure to low concentrations of estrogen inhibits LH and FSH secretion (2,3,4), whereas a progressive increase in estrogen over a period of several days stimulates LH secretion (2,5). What is less clear is how these disparate effects are mediated and specifically, whether they are exerted through modification of hypothalamic GnRH secretion and/or directly at the pituitary.
Studies in animal models, in which invasive and in vitro techniques permit the separation of hypothalamic and pituitary responses to steroid feedback, suggest that estrogen negative feedback is secondary to decreased hypothalamic GnRH secretion (6,7,8,9). Animal studies have provided strong evidence of a direct pituitary effect of estrogen positive feedback (10,11,12) but also show that the preovulatory LH surge is preceded by a dramatic increase in GnRH secretion in a number of species (13,14,15).
In women, studies that have used indirect techniques to assess the hypothalamic and pituitary responses to changes in gonadal steroids (3,4,16) combined with studies of the hypothalamus in postmortem samples (17) support the importance of the hypothalamus in mediating estrogen negative feedback and of the pituitary in mediating estrogen positive feedback on LH (18,19). However, studies do not support the presence of an increase in GnRH secretion at the time of the midcycle LH surge in normal women (20,21), nor do they indicate that an increase in GnRH is required to generate the midcycle LH surge in GnRH-deficient women receiving pulsatile GnRH (19).
In recent years, neuroimaging modalities have greatly expanded our ability to investigate the functional physiology and pathophysiology of the brain (22,23,24,25,26,27). Of the neuroimaging modalities available, positron emission tomography (PET) offers an effective combination of resolution and ability to quantitatively compare scans collected at different scanning sessions that permits assessment of changes in metabolic activity of the hypothalamus and pituitary over the time period associated with the biphasic effects of estrogen on LH. To provide functional neuroanatomic information on the hypothalamic and/or pituitary sites of estrogen feedback, a controlled and graded estradiol (E2) infusion that is known to induce both negative and positive feedback on LH (2,5) was administered to postmenopausal women. [18F]2-fluoro-2deoxy-d-glucose (18FDG)-PET was used as a measure of metabolic activity and co-registered with structural magnetic resonance imaging (MRI) scans to facilitate region of interest (ROI) analyses of the hypothalamus and pituitary.
Subjects and Methods
Postmenopausal women were studied to permit precise control of estradiol exposure. Eleven healthy, normal-weight women aged 45–76 yr (58.5 ± 11.2 yr, mean ± sd) were studied after providing written informed consent for this Institutional Review Board- and U.S. Food and Drug Administration-approved protocol. All women were at least 1 yr after their last menstrual period and on no hormonal therapy. Nine of the subjects had undergone a natural menopause, whereas two had a history of bilateral oophorectomy.
Protocol
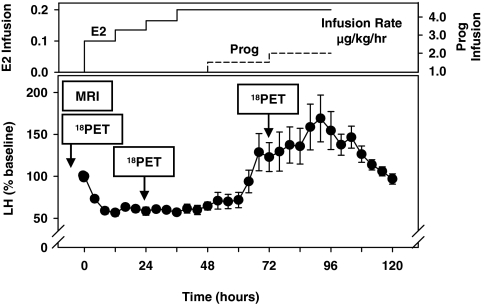
Graded doses of E2 were infused over 96 h and combined with a low-dose progesterone (P) infusion beginning at 48 h that recreates the very low serum levels of P that are present in the preovulatory period in normal women and has previously been shown to synchronize the timing of positive feedback across subjects. Blood was sampled at baseline, every 4 h during the 96-h infusion, and for 24 h thereafter and assayed for E2, P, and LH (Fig. 1). A single structural MRI was performed on each subject at baseline, and FDG-PET scans were performed at baseline and after 24 and 72 h. Prolactin (PRL), cortisol, and GH were measured at baseline and at 24 and 72 h.
Figure 1.
A graded E2 (left axis, top panel) infusion was administered over 96 h to mimic the increasing E2 levels of the follicular phase in normal women with addition of a very low dose of P (Prog, right axis, top panel) infusion at 48 h and resulted in inhibition followed by stimulation of LH secretion (bottom panel), presented as percentage of baseline (mean ± sem) for each subject. 18FDG-PET scanning was performed at baseline, in conjunction with estrogen negative feedback at 24 h and during estrogen positive feedback at 72 h. Structural MRI scans were obtained on each subject at baseline.
Hormone preparation/infusion
E2 was constituted from a stock of crystalline E2 dissolved in propylene glycol to produce a concentration of 0.5 mg/ml and passed through a 0.22-μm filter. Five hundred microliters of stock solution were mixed with 4.5 ml 25% human serum albumin and added to 500 ml normal saline in glass bottles. The P stock solution was similarly prepared using micronized P dissolved in propylene glycol, passed through a 0.22-μm filter, mixed with human serum albumin, and added to 500 ml normal saline in glass bottles. The final concentration of the solution was confirmed by assay of E2 or P using an aliquot obtained from each bottle.
The infusion began at 1500 h on the day of admission via a closed system, using non-PVC fluid-path iv tubing (Accuset; IMED Corp., San Diego, CA). A Micro 965 Volumetric Infusion Pump (IMED) was used to control the infusion rate. The infusion rate for each subject was adjusted for the measured concentration in each bottle. In the first three subjects, E2 was infused at a rate of 0.2 μg/kg·h for the first 12 h and progressively increased to 0.27, 0.33, and 0.4 μg/kg·h at 12, 24, and 36 h, respectively, and continued for a total of 96 h, based on the results of previous studies (2). However, because the serum E2 levels achieved were slightly higher than desired, the E2 infusion rate was adjusted to 0.1, 0.135, 0.165, and 0.2 μg/kg·h for the remaining subjects. P was infused beginning at 48 h at a rate of 1.5 μg/kg·h with an increase to 2.0 μg/kg·h at 72 h.
Hormone assays
Serum LH and E2 were measured using a two-site monoclonal nonisotopic system according to the manufacturer’s directions (Axsym; Abbott Laboratories, Abbott Park, IL), as previously described (16,28). P was analyzed using a sequential competitive immunoassay (Immulite 1000; Siemens, USA, Los Angeles, CA). LH is expressed in international units per liter, as equivalents of the Second International Reference Preparation 71/223 of human menopausal gonadotropins. For LH, the interassay coefficients of variation (CV) were 5.3, 5.5, and 7.4% for quality control sera containing 5.6, 26.2, and 69.0 IU/liter, respectively. The interassay CV for E2 were 9.2, 5.4, and 9.6% at E2 concentrations of 85, 300, and 700 pg/ml (312.0, 1101, and 2570 pmol/liter), respectively. For P, the interassay CV were 14.4, 10.6, and 10.8% at concentrations of 1.5, 3.2, and 14.3 ng/ml (4.8, 10.2, and 45.5 nmol/liter), respectively. PRL was measured using a microparticle enzyme immunoassay (Axsym), cortisol was analyzed using a competitive chemiluminescent enzyme immunoassay (Immulite 1000), and GH was measured using a solid-phase two-site chemiluminescent immunometric assay (Immulite 1000). PRL, cortisol, and GH were measured according to the manufacturer’s instructions, and CV were consistent with those reported by the manufacturers.
PET scanning and image reconstruction
After fasting for at least 6 h, subjects were injected with 5–6 mCi 18FDG in a dimly lighted room with eyes closed. Forty-five minutes after radiopharmaceutical administration, subjects were positioned supine on the PET scanner bed with their head aligned so that transverse section slices were obtained parallel to the canthomeatal line. A vacuum formable reusable polystyrene bead-filled pillow was used to minimize head movement. A single 20-min emission measurement was then acquired, followed by transmission imaging.
PET scanning was performed using a Siemens HR+, 32-ring, 63-slice body tomograph. The intrinsic spatial resolution of the scanner is 4.5 mm full width at half-maximum in the transverse and axial directions. The slice geometry consists of 63 contiguous slices with a center-to-center separation of 2.25 mm and a total axial field of 16.5 mm. Images were reconstructed using an iterative algorithm to an in-plane resolution of 4.5 mm full width at half-maximum. Projection data were corrected for nonuniformity of detector response, dead time, random coincidences, and scattered radiation. Using these methods, test-retest variability after scaling has previously been shown to be 1% or less (29).
MRI acquisition
High-resolution T1-weighted three-dimensional scans were acquired using a General Electric 1.5-T Signa scanner (Milwaukee, WI). Conventional sagittal scout images were followed by contiguous axial three-dimensional T1-weighted spoiled gradient echo pulse sequence, which included the following acquisition parameters: repetition time 30 msec, echo time 9 msec, flip angle 25°, band width 15.63, number of scan locs 124, field of view 22, matrix 256 × 192, number of excitations 1, frequency direction anteroposterior, phase direction left to right, phase field of view 0.75, and auto center frequency was water.
ROI analysis
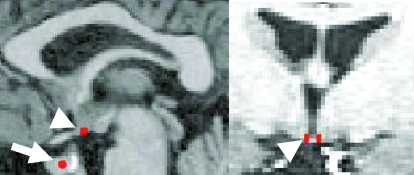
Analysis of PET images included SPM99 (Wellcome Department of Cognitive Neurology, London, UK) co-registration of each FDG-PET scan with that subject’s structural MRI. The MarsBaR toolbox of the SPM software package was used to outline a portion of the pituitary and the hypothalamus bilaterally on each subject’s MRI (Fig. 2) as our ROI, and normalized glucose uptake values were extracted from the ROI (30).
Figure 2.
The pituitary (arrow) and a rectangular portion of the medial basal hypothalamus bilaterally (arrowheads) were outlined as indicated in red in sagittal (left) and coronal (right) views on each subject’s MRI as the ROI and superimposed on the co-registered FDG-PET data for each subject. The hypothalamic ROI was standardized at 2 mm in width, 4 mm in length (anteroposterior dimension), and 2 mm in height, bilaterally. The anterior aspect was placed posterior to the optic chiasm, terminated anterior to the mammillary bodies, and was bounded on its medial border by the third ventricle. The pituitary ROI was standardized as a 2-mm radius in the center of the pituitary.
ROI dimensions used for assessment of the hypothalamus were standardized at 2 mm width (x-axis), 4 mm length (y-axis = anteroposterior dimension), and 3 mm height (z-axis) across all subjects to eliminate any variability in ROI counts that might be caused by variations in ROI volume. For all subjects, the anterior aspect of the ROI was placed at the posterior aspect of the optic chiasm, which resulted in the ROI volume terminating anterior to the mammillary bodies for all subjects. The ROI was bounded medially by the infundibular recess of the third ventricle. The 2-mm ROI width limited sampling of the hypothalamus to its medial aspect (Fig. 2). Visual comparison of ROI neuroanatomic placement with GnRH neuron topography from postmortem studies (31,32) confirmed that our ROI placement surrounded the median eminence region of the medial basal hypothalamus that is most densely populated with GnRH neurons in the human.
The pituitary ROI (Fig. 2) was determined by viewing each MRI slice in the coronal and transverse planes. The entire pituitary was visualized, and the height and width of the pituitary were determined. The apparent center was then determined, and the MarsBaR toolbox was used to create a 2-mm radius ROI around the center of the pituitary. The conservative ROI dimensions of the pituitary and hypothalamus were chosen to minimize any potential partial volume effects.
Data analysis
Normalized values for glucose uptake, which is tightly coupled to neuronal or cellular activity, were extracted from each ROI for each subject at each time point. Because values obtained in the right and left hypothalamic ROI were not statistically different, these values were averaged for each subject. Tests of normality were applied to each set of data and the data log-transformed where appropriate. Two-sided paired t tests were used for comparison of hormonal and imaging data between baseline and 24 h and 24 and 72 h. Regression analysis was used to determine the relationship of PRL to normalized glucose uptake at the hypothalamus and pituitary.
Results
The infusion protocol resulted in a progressive increase in E2 levels (Table 1). From the onset of P infusion at 48 h, P increased to the low preovulatory range at 72 h. LH reached its nadir between 12 and 40 h and was significantly lower at 24 h than at baseline (Table 1 and Figs. 1 and 3). The decrease in LH at 24 h was followed by an increase that was already apparent at 72 h but reached a maximum of 151 ± 23.3 IU/liter at 92 h (Table 1 and Figs. 1 and 3). The time of the LH peak varied from 68–112 h between individuals with an absolute peak of 195.8 ± 20.7 IU/liter (P < 0.0001 vs. baseline), a 100% increase from baseline and a 250% increase from the 24-h nadir.
Table 1.
Biphasic LH response to a graded steroid infusion
| Baseline | 24 h | 72 h | |
|---|---|---|---|
| E2 | |||
| pg/ml | 27.3 ± 2.5 | 336.2 ± 47.7a | 428.8 ± 60.4a |
| pmol/liter | 100.2 ± 9.2 | 1234.2 ± 175.1 | 1571.2 ± 221.7 |
| P | |||
| ng/dl | 0.39 ± 0.07b | 0.31 ± 0.03b | 0.98 ± 0.12 |
| nmol/liter | 1.2 ± 0.2 | 1.0 ± 0.1 | 3.1 ± 0.4 |
| LH (IU/liter) | 101.8 ± 10.5c | 58.1 ± 7.1 | 121.4 ± 20.5c |
P < 0.0001 vs. baseline.
P < 0.001 vs. 72 h.
P < 0.0005 vs. 24 h.
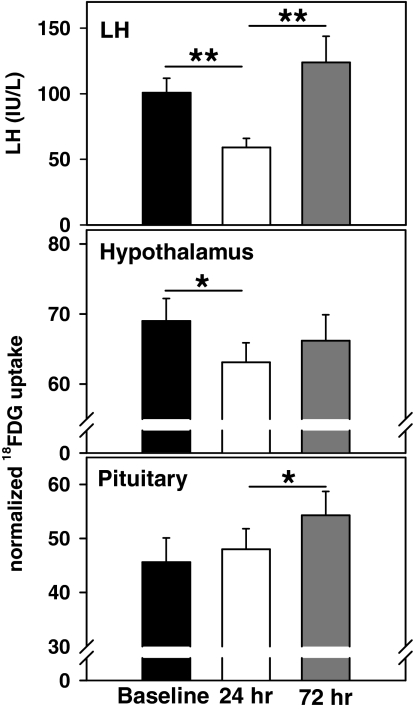
Figure 3.
The graded steroid infusion resulted in an initial decrease in serum LH levels at 24 h followed by a marked increase that was already apparent by 72 h (top panel). LH is expressed in international units per liter as equivalents of the Second International Reference Preparation of human menopausal gonadotropins. Inhibition of LH was associated with a decrease in normalized glucose uptake at the hypothalamus (middle panel), whereas the increase in LH was associated with a marked increase in normalized uptake at the pituitary (bottom panel) in the absence of a significant hypothalamic effect. One sem is indicated. *, P < 0.02; **, P < 0.001.
Normalized glucose uptake at the hypothalamus decreased from baseline to 24 h with no significant change at 72 h (69.0 ± 3.3, 63.1 ± 2.8, and 66.2 ± 3.7 counts at baseline and at 24 and 72 h, respectively; P < 0.02, baseline vs. 24 h; Fig. 3). There was no change in normalized glucose uptake at the pituitary from baseline to 24 h, but a significant increase was evident at 72 h (45.8 ± 4.5, 48.0 ± 4.0, and 54.3 ± 4.4 counts at baseline and 24 and 72 h, respectively; P < 0.02 for 24 vs. 72 h; Fig. 3). Thus, the early decrease in LH in response to the E2 infusion was associated with a concomitant decrease in normalized glucose uptake in the hypothalamus with no change in the pituitary. In contrast, the increase in LH at 72 h was associated with a marked increase in normalized glucose uptake in the pituitary but not in the hypothalamus.
There was a progressive increase in PRL with E2 administration (8.2 ± 1.1, 14.4 ± 2.4, and 27.5 ± 3.5 ng/ml at baseline and 24 and 72 h, respectively; P < 0.005 for baseline vs. 24 h and 24 vs. 72 h). However, PRL did not correlate with normalized glucose uptake at the pituitary or hypothalamus. Cortisol (0.54 ± 0.02, 1.51 ± 0.54, and 1.02 ± 0.24 μg/dl at baseline and 24 and 72 h, respectively) and GH (8.1 ± 1.1, 9.7 ± 1.5, and 8.8 ± 1.0 ng/ml at baseline and 24 and 72 h, respectively) were not significantly affected by hormone administration.
Discussion
In this study, we have used FDG-PET co-registered with structural MRI to investigate the sites of estrogen negative and positive feedback by relating hypothalamic and pituitary regional 18FDG uptake to changes in LH levels in response to a controlled steroid infusion paradigm. Both functional MRI (23,24) and PET (25,26,27) have been used to investigate the effects of estrogen on cortical areas involved in cognitive processing. PET has also been used in the clinical evaluation of the pituitary (33) and hypothalamus (34,35). However, neuroimaging techniques have not previously been applied to examine the hypothalamic and pituitary sites of steroid feedback that are so critical for normal reproductive function.
In conjunction with the decrease in LH levels that occurs with relatively short-term estradiol exposure, we have demonstrated a decrease in 18FDG uptake in the medial basal hypothalamus in the absence of a change in 18FDG uptake in the pituitary. These results provide evidence that estrogen exerts its negative feedback on LH predominately at the hypothalamic level in women. These findings are consistent with previous studies in women in which it was demonstrated that the quantity of GnRH secretion, estimated using a submaximal dose of a competitive GnRH receptor blocker, is decreased in postmenopausal women after exposure to low-dose estrogen replacement (3). The current studies are also consistent with studies in a variety of animal models (36), with the demonstration of direct inhibitory effects of estrogen on GnRH expression in GT1-7 cells, a GnRH-secreting hypothalamic cell line (9) and, importantly, with higher GnRH expression in the medial basal hypothalamus associated with the loss of estrogen negative feedback in postmenopausal compared with premenopausal women studied at autopsy (17). Although a transient inhibitory effect of estradiol has been demonstrated in pituitary cells in culture (37), the majority of studies across species in vivo demonstrate the absence of a persistent inhibitory effect of low-dose estrogen on LH secretion exerted directly at the pituitary (12), consistent with results in the current study.
In conjunction with steroid positive feedback on LH, we demonstrated increased 18FDG uptake in the pituitary, but not the hypothalamus. Animal and in vitro studies indicate that high levels of estrogen augment the pituitary response to GnRH, increase GnRH receptor number, impact the function of ion channels in the plasma membrane, and regulate both gene expression and second messenger systems within gonadotropes (12). In a variety of animal models, the preovulatory LH surge is also accompanied by an increase in hypothalamic GnRH secretion, although it is less clear whether this is achieved through an increase in GnRH pulse frequency, a signal pulse, or a surge of GnRH (12,15,38). In contrast, our previous studies demonstrate that the LH surge in women requires ongoing secretion of GnRH (21) but is otherwise mediated entirely at the pituitary. GnRH pulse frequency is not increased either preceding or in conjunction with the midcycle LH surge in normal women (20), and the quantity of GnRH secreted, estimated from the LH response to incomplete GnRH receptor blockade, is reduced rather than increased during the midcycle surge compared with other cycle stages (21). As predicted by our studies in normal women, in GnRH-deficient women receiving exogenous pulsatile GnRH, normal LH surges are achieved in association with the ovarian hormonal changes that occur with development of a single dominant follicle in the absence of an increase in the frequency or dose of GnRH (19). Moreover, a normal LH surge also occurs when the dose of GnRH is significantly reduced in the late follicular phase before the surge onset (39). Our current results, showing a marked increase in 18FDG uptake in the pituitary, but not the hypothalamus, in conjunction with estrogen positive feedback on LH provide functional neuroanatomic evidence in support of the overwhelming predominance of the direct effects of estrogen on the pituitary in generation of the midcycle LH surge in women. It is tempting to speculate that differences in the mechanisms underlying generation of the LH surge across species may be due to differences in the requirements for successful reproduction. For example, coordination of the precise timing of ovulation with circadian cues appears to be a critical feature of reproductive control in rodents (40) but is unlikely to be required in women.
The presence of low-dose P is not essential for generation of an LH surge in women (2,5) as it is in rodents (41). However, P reduces the variability between subjects in the timing of estrogen positive feedback (2) and was therefore used in the current study to ensure that the final PET scan was appropriately timed. In the presence of high-dose estrogen, P augments the LH response to GnRH in women (42), and thus, the addition of low-dose P in the current study may have contributed to the magnitude of the estradiol-induced increase in 18FDG uptake in the pituitary at 72 h.
We have assumed that the changes in 18FDG uptake at the hypothalamus and pituitary in response to E2 and P relate to changes in synthesis or secretion of reproductive hormones. Studies in the rat indicate that estrogen stimulates PRL levels directly at the pituitary as well as by decreasing dopamine levels (43). However, changes in PRL in the current study were not consistent with changes in 18FDG uptake in the hypothalamus or pituitary, and PRL levels did not correlate with 18FDG uptake. Although estrogen administration is associated with increases in GH in some settings (44), GH levels were not increased in the current study, possibly due to the overall decrease in GH secretion with aging (45) and/or the estrogen deprivation of menopause combined with the short duration of higher-dose estrogen exposure.
Detecting changes in 18FDG uptake in the hypothalamus and pituitary in response to physiological stimuli is challenging due to the small size of the structures of interest. We have used an anatomically based ROI analysis in which FDG-PET scans were co-registered with MRI scans and have studied subjects over time in response to precisely controlled hormonal stimuli to further reduce variability. The dimensions and positioning of the hypothalamic ROI were based on studies in human hypothalami that delineate the medial basal hypothalamus as the sight of the highest concentration of GnRH neurons (31,32) and demonstrate changes consistent with inhibitory effects of estrogen (17). In rodents, the anteroventral periventricular nucleus has been implicated in estrogen positive feedback (46). However, the anatomic organization of the GnRH neuronal system in humans is quite different from that in rodents, and there is no clear evidence for the existence of a human equivalent of the anteroventral periventricular nucleus (47).
It has been suggested that deficient positive feedback occurs during the menopausal transition (48). Although we demonstrated robust positive feedback, it is possible that steroid-induced LH surges in postmenopausal subjects are less than or functionally different from spontaneous LH surges in young women. It is also possible that the technique lacked the sensitivity to detect small changes in hypothalamic activity that might be associated with increased GnRH secretion or that we inadvertently failed to sample a region that will ultimately be found to be critical for positive feedback in women. However, the concordance of results from this study with those in normal and GnRH-deficient young women discussed above using complementary approaches decreases these potential concerns.
Importantly, changes in 18FDG uptake in specific brain regions imply changes in metabolic activity within that area but cannot identify a specific cell type. For example, decreased activity in the medial basal hypothalamus associated with a decrease in LH may be due to direct effects on GnRH neurons or to effects on neurons that reside in the same area and may mediate the estrogen feedback signal to GnRH (47,49).
These data demonstrate changes in 18FDG uptake as a marker of cellular metabolic activity in the hypothalamus and pituitary in association with discrete hormonal events in women. This study supports a hypothalamic site of estrogen negative feedback on LH secretion and a pituitary, but not a hypothalamic, site of positive feedback in women. The absence of functional neuroanatomic evidence of a hypothalamic site of positive feedback provides strong support for our previous studies in women. Taken together, these studies indicate that although mechanisms of negative feedback in women appear to be similar to those in lower animal species, mechanisms underlying the preovulatory gonadotropin surge are not identical.
Acknowledgments
We thank Steve Weise and Mariko Jameson for technical assistance and Patrick Sluss, Ph.D., Director of the Reproductive Endocrine Unit Assay Laboratory.
Footnotes
This study is registered with ClinicalTrials.gov, ID no. NCT00455741.
Current address for W.E.O.: Graduate School of Arts and Sciences, Columbia University, Department of Statistics, New York, New York 10027.
W.E.O. and A.L.F. have nothing to declare. J.E.H. serves on an Advisory Board for Ferring Pharmaceuticals, Inc. D.D.D. received lecture fees from Cyberonics and McNeil.
This work was supported by National Institutes of Health Grants R01 AG13241, K24 HD01290, and M01 RR01066.
First Published Online June 3, 2008
Abbreviations: CV, Coefficients of variation; E2, estradiol; 18FDG, [18F]2-fluoro-2-deoxy-d-glucose; MRI, magnetic resonance imaging; P, progesterone; PET, positron emission tomography; PRL, prolactin; ROI, regions of interest.
References
- Hall JE 2004 Neuroendocrine control of the menstrual cycle. In: Strauss JF, Barbieri RL, eds. Yen and Jaffe’s reproductive endocrinology. 5th ed. Philadelphia: Elsevier; 195–211 [Google Scholar]
- Taylor AE, Whitney H, Hall JE, Martin K, Crowley Jr WF 1995 Midcycle levels of sex steroids are sufficient to recreate the follicle-stimulating hormone but not the luteinizing hormone midcycle surge: evidence for the contribution of other ovarian factors to the surge in normal women. J Clin Endocrinol Metab 80:1541–1547 [DOI] [PubMed] [Google Scholar]
- Gill S, Sharpless JL, Rado K, Hall JE 2002 Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J Clin Endocrinol Metab 87:2290–2296 [DOI] [PubMed] [Google Scholar]
- Gill S, Lavoie HB, Bo-Abbas Y, Hall JE 2002 Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J Clin Endocrinol Metab 87:2297–2302 [DOI] [PubMed] [Google Scholar]
- Liu JH, Yen SS 1983 Induction of midcycle gonadotropin surge by ovarian steroids in women: a critical evaluation. J Clin Endocrinol Metab 57:797–802 [DOI] [PubMed] [Google Scholar]
- Chongthammakun S, Terasawa E 1993 Negative feedback effects of estrogen on luteinizing hormone-releasing hormone release occur in pubertal, but not prepubertal, ovariectomized female rhesus monkeys. Endocrinology 132:735–743 [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Glover BH, Karsch FJ 1994 Central regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by estradiol during the period leading up to the preovulatory GnRH surge in the ewe. Endocrinology 134:1806–1811 [DOI] [PubMed] [Google Scholar]
- Wray S, Zoeller RT, Gainer H 1989 Differential effects of estrogen on luteinizing hormone-releasing hormone gene expression in slice explant cultures prepared from specific rat forebrain regions. Mol Endocrinol 3:1197–1206 [DOI] [PubMed] [Google Scholar]
- Roy D, Angelini NL, Belsham DD 1999 Estrogen directly represses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-α (ERα)- and ERβ-expressing GT1-7 GnRH neurons. Endocrinology 140:5045–5053 [DOI] [PubMed] [Google Scholar]
- Wildt L, Hausler A, Hutchison JS, Marshall G, Knobil E 1981 Estradiol as a gonadotropin releasing hormone in the rhesus monkey. Endocrinology 108:2011–2013 [DOI] [PubMed] [Google Scholar]
- Neill JD, Smith PF, Luque EH, Munoz de Toro M, Nagy G, Mulchahey JJ 1987 Detection and measurement of hormone secretion from individual pituitary cells. Recent Prog Horm Res 43:175–229 [DOI] [PubMed] [Google Scholar]
- Clarke IJ 2002 Two decades of measuring GnRH secretion. Reprod Suppl 59:1–13 [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A, Karsch FJ 1991 Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129:1175–1182 [DOI] [PubMed] [Google Scholar]
- Levine JE, Bauer-Dantoin AC, Besecke LM, Conaghan LA, Legan SJ, Meredith JM, Strobl FJ, Urban JH, Vogelsong KM, Wolfe AM 1991 Neuroendocrine regulation of the luteinizing hormone-releasing hormone pulse generator in the rat. Recent Prog Horm Res 47:97–151 [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM 1997 Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod 56:303–309 [DOI] [PubMed] [Google Scholar]
- Welt CK, Pagan YL, Smith PC, Rado KB, Hall JE 2003 Control of follicle-stimulating hormone by estradiol and the inhibins: critical role of estradiol at the hypothalamus during the luteal-follicular transition. J Clin Endocrinol Metab 88:1766–1771 [DOI] [PubMed] [Google Scholar]
- Rance NE, Uswandi SV 1996 Gonadotropin-releasing hormone gene expression is increased in the medial basal hypothalamus of postmenopausal women. J Clin Endocrinol Metab 81:3540–3546 [DOI] [PubMed] [Google Scholar]
- Wang CF, Lasley BL, Lein A, Yen SS 1976 The functional changes of the pituitary gonadotrophs during the menstrual cycle. J Clin Endocrinol Metab 42:718–728 [DOI] [PubMed] [Google Scholar]
- Martin K, Santoro N, Hall J, Filicori M, Wierman M, Crowley Jr WF 1990 Clinical review 15: management of ovulatory disorders with pulsatile gonadotropin-releasing hormone. J Clin Endocrinol Metab 71:1081A–1081G [DOI] [PubMed] [Google Scholar]
- Adams JM, Taylor AE, Schoenfeld DA, Crowley Jr WF, Hall JE 1994 The midcycle gonadotropin surge in normal women occurs in the face of an unchanging gonadotropin-releasing hormone pulse frequency. J Clin Endocrinol Metab 79:858–864 [DOI] [PubMed] [Google Scholar]
- Hall JE, Taylor AE, Martin KA, Rivier J, Schoenfeld DA, Crowley Jr WF 1994 Decreased release of gonadotropin-releasing hormone during the preovulatory midcycle luteinizing hormone surge in normal women. Proc Natl Acad Sci USA 91:6894–6898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte A, Halsband U 2006 Brain imaging tools in neurosciences. J Physiol Paris 99:281–292 [DOI] [PubMed] [Google Scholar]
- Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA 2006 Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause 13:411–422 [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Naftolin F, Palter SF, Marchione KE, Katz L, Shankweiler DP, Fletcher JM, Lacadie C, Keltz M, Gore JC 1999 Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA 281:1197–1202 [DOI] [PubMed] [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, Ostrem JL, Weinberger DR 1997 Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci USA 94:8836–8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Resnick SM 2000 Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging 21:373–383 [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Silverman D, Siddarth P, Miller K, Ercoli LM, Elman S, Lavretsky H, Huang SC, Phelps ME, Small GW 2005 Estrogen use and brain metabolic change in postmenopausal women. Neurobiol Aging 26:229–235 [DOI] [PubMed] [Google Scholar]
- Welt CK, McNicholl DJ, Taylor AE, Hall JE 1999 Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab 84:105–111 [DOI] [PubMed] [Google Scholar]
- Bartlett EJ, Brodie JD, Wolf AP, Christman DR, Laska E, Meissner M 1988 Reproducibility of cerebral glucose metabolic measurements in resting human subjects. J Cereb Blood Flow Metab 8:502–512 [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB Region of interest analysis using an SPM toolbox. 8th International Conferance on Functional Mapping of the Human Brain, Sendai, Japan, 2002. Available on CD-ROM in NeuroImage, Vol 16, No 2 (Abstract 497) [Google Scholar]
- Dudas B, Mihaly A, Merchenthaler I 2000 Topography and associations of luteinizing hormone-releasing hormone and neuropeptide Y-immunoreactive neuronal systems in the human diencephalon. J Comp Neurol 427:593–603 [DOI] [PubMed] [Google Scholar]
- Rance NE, Young III WS, McMullen NT 1994 Topography of neurons expressing luteinizing hormone-releasing hormone gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol 339:573–586 [DOI] [PubMed] [Google Scholar]
- Bombardieri E, Seregni E, Villano C, Chiti A, Bajetta E 2004 Position of nuclear medicine techniques in the diagnostic work-up of neuroendocrine tumors. Q J Nucl Med Mol Imaging 48:150–163 [PubMed] [Google Scholar]
- Ryvlin P, Ravier C, Bouvard S, Mauguire F, Le BD, Arzimanoglou A, Petit J, Kahane P 2003 Positron emission tomography in epileptogenic hypothalamic hamartomas. Epileptic Disord 5:219–227 [PubMed] [Google Scholar]
- Matharu MS, Bartsch T, Ward N, Frackowiak RS, Weiner R, Goadsby PJ 2004 Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain 127:220–230 [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS 1999 Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- Frawley LS, Neill JD 1984 Biphasic effects of estrogen on gonadotropin-releasing hormone-induced luteinizing hormone release in monolayer cultures of rat and monkey pituitary cells. Endocrinology 114:659–663 [DOI] [PubMed] [Google Scholar]
- Levine JE 1997 New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod 56:293–302 [DOI] [PubMed] [Google Scholar]
- Martin KA, Welt CK, Taylor AE, Smith JA, Crowley Jr WF, Hall JE 1998 Is GnRH reduced at the midcycle surge in the human? Evidence from a GnRH-deficient model. Neuroendocrinology 67:363–369 [DOI] [PubMed] [Google Scholar]
- Chappell PE 2005 Clocks and the black box: circadian influences on gonadotropin-releasing hormone secretion. J Neuroendocrinol 17:119–130 [DOI] [PubMed] [Google Scholar]
- Chappell PE, Schneider JS, Kim P, Xu M, Lydon JP, O'Malley BW, Levine JE 1999 Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology 140:3653–3658 [DOI] [PubMed] [Google Scholar]
- Chang RJ, Jaffe RB 1978 Progesterone effects on gonadotropin release in women pretreated with estradiol. J Clin Endocrinol Metab 47:119–125 [DOI] [PubMed] [Google Scholar]
- DeMaria JE, Livingstone JD, Freeman ME 2000 Ovarian steroids influence the activity of neuroendocrine dopaminergic neurons. Brain Res 879:139–147 [DOI] [PubMed] [Google Scholar]
- Chowen JA, Frago LM, Argente J 2004 The regulation of GH secretion by sex steroids. Eur J Endocrinol 151(Suppl 3):U95–U100 [DOI] [PubMed] [Google Scholar]
- Lieman HJ, Adel TE, Forst C, von Hagen S, Santoro N 2001 Effects of aging and estradiol supplementation on GH axis dynamics in women. J Clin Endocrinol Metab 86:3918–3923 [DOI] [PubMed] [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD 2003 Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod 69:1771–1778 [DOI] [PubMed] [Google Scholar]
- Dudas B, Merchenthaler I 2006 Three-dimensional representation of the neurotransmitter systems of the human hypothalamus: inputs of the gonadotrophin hormone-releasing hormone neuronal system. J Neuroendocrinol 18:79–95 [DOI] [PubMed] [Google Scholar]
- Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ 2004 Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA 292:2991–2996 [DOI] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, Rance NE 2007 Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92:2744–2750 [DOI] [PubMed] [Google Scholar]