Abstract
Context: The conjoint effects and relative importance of ghrelin, leptin, and soluble leptin receptor (sOB-R), adipokines involved in appetite control and energy expenditure in mediating cardiometabolic risk, is unknown.
Objective: The objective of the study was to study the cross-sectional relations of these adipokines to cardiometabolic risk factors in a community-based sample.
Design, Setting, and Participants: We measured circulating ghrelin, leptin, and sOB-R in 362 participants (mean age 45 yr; 54% women) of the Framingham Third Generation Cohort.
Main Outcome Measures: Body mass index, waist circumference (WC), blood pressure, lipid measures, fasting glucose, smoking, and metabolic syndrome (MetS) were measured.
Results: Ghrelin and leptin concentrations were significantly higher in women (P < 0.0001). In multivariable models, ghrelin was inversely associated with age and systolic blood pressure, and leptin was positively related to body mass index and WC. sOB-R was positively associated with age, total cholesterol, and fasting glucose and inversely with WC and high-density lipoprotein cholesterol. Ghrelin and sOB-R concentrations were significantly lower with number of MetS components (P for trend = 0.022 and < 0.0001, respectively), whereas leptin concentrations were higher (P for trend = 0.0001). Relating all adipokines to MetS conjointly, higher ghrelin and leptin concentrations were associated with decreased and increased odds of MetS (odds ratio 0.55, P < 0.0001; odds ratio 4.44, P = 0.0002, per 1 sd increase of respective log adipokine).
Conclusions: In our community-based sample, we observed a sexual dimorphism in circulating ghrelin and leptin concentrations. Ghrelin, leptin, and sOB-R were associated with number of MetS components cross-sectionally, consistent with the hypothesis that these adipokines may have a central role in cardiometabolic risk.
There was sexual dimorphism in the levels of ghrelin, leptin, soluble leptin receptors, and free leptin index in a community-based cohort, suggesting that appetite and energy expenditure control could be different in men and women. Furthermore, these adipokines were associated with metabolic syndrome components, further implying that these adipokines have a central role in cardiometabolic risk.
Recent research has highlighted the role of gut hormones and other neuroendocrine hormones that control appetite and energy expenditure in the development of obesity, insulin resistance, and other cardiometabolic risk factors (1,2). These hormones include leptin, ghrelin, peptide YY, cholecystokinin, and glucagon-like peptide 1, among others.
Ghrelin was first described as the endogenous ligand for a new GH secretagogue receptor (3). Additional research has elucidated that it is a powerful inducer of GH release and functions as an appetite-stimulating signal from the gut to the brain (4). It counters the anorexigenic effect of leptin and is an important coordinator of behavioral, metabolic, and gastrointestinal responses to fasting and eating. Low concentrations of circulating ghrelin have been associated with obesity, insulin resistance, and type 2 diabetes (5,6,7,8,9).
Leptin acts as a satiety signal, regulates appetite and energy expenditure, but has also a range of other physiological functions including regulation of puberty, placental function, peripheral insulin sensitivity, and interaction with other hormonal mediators and regulators (10). Leptin binds to its receptor, the leptin receptor. In addition to several membrane-bound isoforms, a soluble form of the leptin receptor (sOB-R) is generated by cleavage of the membrane-bound forms (11). sOB-R has been proposed to function as a binding protein and is therefore an important regulator of leptin activity (10,12). One way of characterizing the balance between leptin and sOB-R is the free leptin index (FLI), which is determined by calculating the ratio between the concentrations of leptin and sOB-R (13). Elevated concentrations of circulating leptin repeatedly have been associated with cardiometabolic risk factors, such as hypertension, obesity, insulin resistance, and type 2 diabetes (14,15,16,17,18,19,20,21), whereas data regarding the correlations of sOB-R and FLI with cardiometabolic risk factors are more limited (22,23,24).
Whereas some prior studies have evaluated these biomarkers individually, to our knowledge none has assessed their conjoint effects and relative importance in mediating cardiometabolic risk. The balance between these important adipokines that regulate appetite control and energy expenditure may determine such risk. Accordingly, the aims of our study were to test the hypothesis that abnormal circulating concentrations of ghrelin, leptin, sOB-R, and FLI are associated (individually and conjointly) with adverse levels of cardiometabolic risk factors in a community-based sample.
Subjects and Methods
Study sample
The Framingham Third Generation Cohort was recruited between 2002 and 2005. The design and selection criteria for this cohort have been described elsewhere (25). Briefly, 4095 adults (53% women; mean age 40 yr) who have at least one parent in the Framingham Offspring Study cohort were enrolled. At their first examination, participants underwent anthropometry, medical history, physical examination, and laboratory assessment of cardiovascular risk factors. Smoking was ascertained by self-reported cigarette use during the year preceding the examination. The study was approved by the Institutional Review Board at Boston University Medical Center, and all participants provided written informed consent.
The present study was performed in a subsample of 362 participants (54% women) in whom ghrelin, leptin, and sOB-R were assayed. We randomly selected these participants using a weighted sampling scheme with oversampling of the lowest and highest sex-specific quintiles of body mass index (BMI; ratio of 1.5:2:1.5 for the lowest, middle three, and upper quintiles). We chose this sampling strategy for cost efficiency and optimizing use of nonrenewable serological resources, given the novelty of the biomarkers evaluated.
Laboratory measurements and definition of metabolic syndrome
Serum and plasma were drawn after an overnight fast and stored at −80 C until assay without previous thawing. Ghrelin was assayed using a commercially available ELISA kit (Phoenix Pharmaceuticals Inc., Belmont, CA) in unextracted serum, and leptin and sOB-R were assayed using a commercially available ELISA kit (R&D Systems Inc., Minneapolis, MN) in EDTA plasma. The average intraassay coefficients of variation were: ghrelin, 2.3%; leptin, 2.1%; and sOB-R, 3.1%.
The metabolic syndrome (MetS) was defined according to the modified National Cholesterol Education Program Adult Treatment Panel III criteria (26), by the presence of three or more of the following: increased waist circumference [≥88 (women) or ≥102 cm (men)]; elevated blood pressure (≥130 mm Hg systolic or ≥85 mm Hg diastolic or treatment for hypertension); hyperglycemia [fasting blood glucose ≥100 mg/dl (5.6 mmol/liter) or treatment for elevated glucose]; hypertriglyceridemia [serum concentration ≥150 mg/dl (1.7 mmol/liter)]; or low high-density lipoprotein (HDL) cholesterol [<50 mg/dl (1.3 mmol/liter, women) or <40 mg/dl (1.03 mmol/liter, men)].
Statistical methods
Ghrelin, leptin, sOB-R, and FLI concentrations were logarithmically transformed to normalize their skewed distributions. Age- and sex-adjusted Spearman partial correlation coefficients were estimated for the pairwise correlations of the adipokines with each other and for the relations of the adipokines to cardiometabolic risk factors (27). We performed stepwise multivariable regressions with all cardiometabolic risk factors as the independent and the adipokines as the dependent variables to evaluate the clinical correlates of the adipokines. Age and sex were forced into these models, whereas P < 0.10 was used as the level for retention in the models for the other variables.
Age- and sex-adjusted least square means with 95% confidence intervals (CIs) of the adipokines were calculated stratifying by BMI category (normal, <25.0 kg/m2; overweight, 25.0 to <30.0 kg/m2; obesity, ≥30.0 kg/m2) and according to the number of MetS components present using logarithmically transformed biomarker concentrations. The values were then transformed back to original units to allow meaningful interpretation. Age-adjusted least square means of the adipokines in pre- and postmenopausal women were also estimated and compared.
Ghrelin, leptin, and sOB-R were related conjointly to MetS in an age- and sex-adjusted logistic regression model with MetS as the response variable and the log adipokines as the predictor variables. We examined effect modification by testing the statistical significance of the following first-order interaction terms in the multivariable models separately for each adipokine as dependent variables: BMI by sex, waist circumference by sex. Two-tailed 95% CIs and P values were estimated, with P < 0.05 considered statistically significant. Analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
Results
The characteristics of our study sample are shown in Table 1. The age- and sex-adjusted pairwise correlation was inverse between leptin and sOB-R (r = −0.43, P < 0.0001). Ghrelin was not significantly correlated with the other adipokines in our sample.
Table 1.
Characteristics of the study samplea
| Total sample (n = 362) | Men (n = 167) | Women (n = 195) | |
|---|---|---|---|
| Cardiometabolic risk factors | |||
| Age, yr | 45 (6) | 43 (6) | 46 (6) |
| BMI, kg/m² | 27.6 (6.3) | 28.3 (5.4) | 27.0 (6.9) |
| Waist circumference, cm | 95 (16) | 100 (14) | 91 (17) |
| Systolic blood pressure, mm Hg | 119 (15) | 121 (13) | 117 (16) |
| Diastolic blood pressure, mm Hg | 76 (10) | 79 (9) | 74 (10) |
| Antihypertensive treatment, % | 12 | 11 | 13 |
| Total cholesterol, mg/dl | 191 (34) | 195 (31) | 188 (37) |
| HDL, mg/dl | 55 (19) | 47 (13) | 62 (20) |
| Triglycerides, mg/dl | 118 (75) | 140 (86) | 100 (58) |
| Fasting plasma glucose, mg/dl | 98 (26) | 103 (30) | 94 (20) |
| Smoking, % | 14 | 16 | 12 |
| MetS, %b | 25 | 32 | 20 |
| Ghrelin, leptin, sOB-R, and FLI | |||
| Ghrelin, ng/ml | 2.6 (1.7, 4.0) | 2.2 (1.5, 3.3) | 2.8 (2.0, 4.4) |
| Leptin, ng/ml | 6.5 (2.9, 15.9) | 3.9 (2.0, 6.4) | 13.6 (5.7, 26.9) |
| sOB-R, ng/ml | 37 (30, 45) | 36 (30, 45) | 38 (30, 45) |
| FLIc | 0.18 (0.07,0.50) | 0.11 (0.05,0.22) | 0.34 (0.12,0.87) |
Values are means (sd) or proportions for cardiometabolic risk factors and medians (25th percentile, 75th percentile) for ghrelin, leptin, sOB-R, and FLI.
MetS was defined as described in Subjects and Methods.
FLI was determined by calculating the ratio between the concentrations of leptin and soluble leptin receptor.
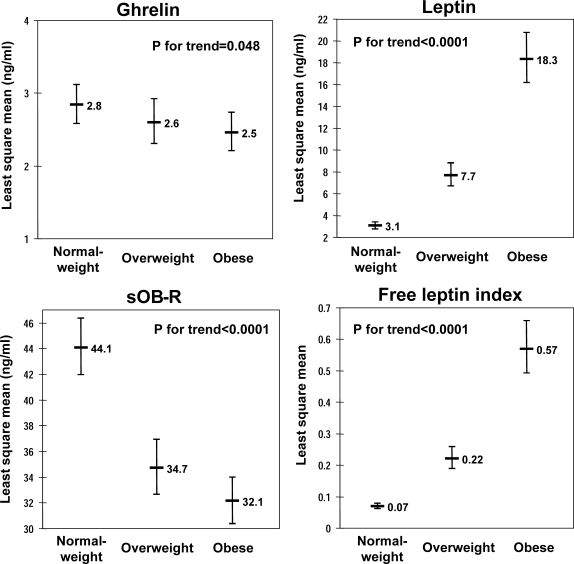
Ghrelin demonstrated inverse correlations with age, systolic, and diastolic blood pressure (Table 2). Leptin and FLI demonstrated significant positive correlations with all cardiometabolic risk factors but were inversely related to HDL cholesterol concentrations. sOB-R concentrations were inversely correlated with BMI, waist circumference, systolic and diastolic blood pressure, triglycerides, and fasting glucose and positively with HDL cholesterol. Smoking was not significantly correlated with any of the examined adipokines. The age- and sex-adjusted concentrations of ghrelin and sOB-R decreased, whereas leptin concentrations and FLI increased significantly across BMI categories (Fig. 1).
Table 2.
Correlations of ghrelin, leptin, sOB-R, and FLI and cardiometabolic risk factorsa
| Ghrelin
|
Leptin
|
sOB-R
|
FLI
|
|||||
|---|---|---|---|---|---|---|---|---|
| Correlation coefficient | P value | Correlation coefficient | P value | Correlation coefficient | P value | Correlation coefficient | P value | |
| Age | −0.12 | 0.020 | 0.18 | 0.0006 | 0.09 | 0.078 | 0.14 | 0.009 |
| Female sex | 0.23 | <0.0001 | 0.51 | <0.0001 | 0.03 | 0.60 | 0.43 | <0.0001 |
| BMI | −0.07 | 0.16 | 0.81 | <0.0001 | −0.43 | <0.0001 | 0.81 | <0.0001 |
| Waist circumference | −0.08 | 0.14 | 0.80 | <0.0001 | −0.41 | <0.0001 | 0.79 | <0.0001 |
| Systolic blood pressure | −0.11 | 0.036 | 0.37 | <0.0001 | −0.14 | 0.007 | 0.35 | <0.0001 |
| Diastolic blood pressure | −0.12 | 0.022 | 0.38 | <0.0001 | −0.16 | 0.002 | 0.37 | <0.0001 |
| Total cholesterol | −0.01 | 0.85 | 0.18 | 0.0008 | 0.08 | 0.13 | 0.13 | 0.013 |
| HDL | 0.06 | 0.25 | −0.34 | <0.0001 | 0.40 | <0.0001 | −0.40 | <0.0001 |
| Triglycerides | −0.07 | 0.20 | 0.46 | <0.0001 | −0.23 | <0.0001 | 0.46 | <0.0001 |
| Fasting plasma glucose | −0.06 | 0.29 | 0.38 | <0.0001 | −0.15 | 0.004 | 0.36 | <0.0001 |
| Smoking | −0.003 | 0.95 | 0.01 | 0.88 | −0.06 | 0.27 | 0.02 | 0.64 |
Values are age- and sex-adjusted Spearman partial correlation coefficients and P values for correlations of ghrelin, leptin, sOB-R, and FLI and cardiometabolic risk factors.
Figure 1.
Age- and sex-adjusted least square means of concentrations of ghrelin, leptin, sOB-R, and FLI in individuals with normal BMI (<25.0 kg/m2), overweight (BMI 25.0 to <30.0 kg/m2), and obesity (BMI ≥30.0 kg/m2).
In multivariable models examining the clinical correlates of adipokine concentrations, all adipokines were strongly correlated with sex (P ≤ 0.0009; Table 3). Concentrations of ghrelin, leptin, and FLI were higher in women, whereas sOB-R was lower. The age-adjusted means in premenopausal and postmenopausal women differed significantly for ghrelin (2.8 vs. 3.7 ng/ml, respectively; P = 0.024) but not for the other adipokines (leptin, 10.9 vs. 15.8 ng/ml; P = 0.10; sOB-R, 38.1 vs. 36.2 ng/ml; P = 0.47; and FLI, 0.29 vs. 0.44; P = 0.10).
Table 3.
Clinical correlates of ghrelin, leptin, sOB-R, and FLIa
| Log ghrelin
|
Log leptin
|
Log sOB-R
|
Log FLI
|
|||||
|---|---|---|---|---|---|---|---|---|
| β-coefficient (se) | P value | β-coefficient (se) | P value | β-coefficient (se) | P value | β-coefficient (se) | P value | |
| Age | −0.09 (0.03) | 0.004 | −0.03 (0.03) | 0.27 | 0.05 (0.02) | 0.0009 | −0.08 (0.04) | 0.029 |
| Female sex | 0.26 (0.06) | <0.0001 | 1.54 (0.07) | <0.0001 | −0.11 (0.03) | 0.0009 | 1.62 (0.09) | <0.0001 |
| BMI | 0.42 (0.08) | <0.0001 | 0.47 (0.10) | <0.0001 | ||||
| Waist circumference | 0.41 (0.09) | <0.0001 | −0.10 (0.02) | <0.0001 | 0.47 (0.11) | <0.0001 | ||
| Systolic blood pressure | −0.07 (0.03) | 0.032 | ||||||
| Diastolic blood pressure | ||||||||
| Total cholesterol | 0.06 (0.03) | 0.055 | ||||||
| HDL | 0.10 (0.02) | <0.0001 | −0.08 (0.05) | 0.076 | ||||
| Triglycerides | 0.07 (0.04) | 0.062 | 0.10 (0.04) | 0.024 | ||||
| Fasting plasma glucose | 0.07 (0.02) | <0.0001 | −0.10 (0.04) | 0.01 | ||||
| Smoking | ||||||||
| Model R2 | 0.09 | 0.76 | 0.27 | 0.729 | ||||
Values are from stepwise multivariable models with all cardiometabolic risk factors as predictor variables and ghrelin, leptin, sOB-R, and FLI as response variables. Values shown are for variables retained in the model. Age and sex were forced into these models, whereas a P < 0.10 was used as level for retention for the other variables. β-Coefficients are for a 1 sd increase of the predictor variable for continuous variables and represent an eβ-fold change in biomarker concentrations.
In the multivariable models, age, sex, and systolic blood pressure were significantly associated with ghrelin concentrations (Table 3). The strongest correlate in this multivariable model was sex (partial R2 = 0.04). Sex, BMI, and waist circumference were significantly associated with leptin concentrations, whereas total cholesterol and triglycerides were retained as borderline significant correlates. The strongest correlate was BMI (partial R2 = 0.48). The significant correlates of sOB-R concentrations were age, sex, waist circumference, HDL cholesterol, and fasting glucose, whereas FLI concentrations were determined by age, sex, BMI, waist circumference, triglycerides, and fasting glucose. HDL cholesterol was retained as a borderline significant correlate in this multivariable model. The strongest correlate with sOB-R was waist circumference (partial R2 = 0.043) and the strongest with FLI was BMI (partial R2 = 0.50).
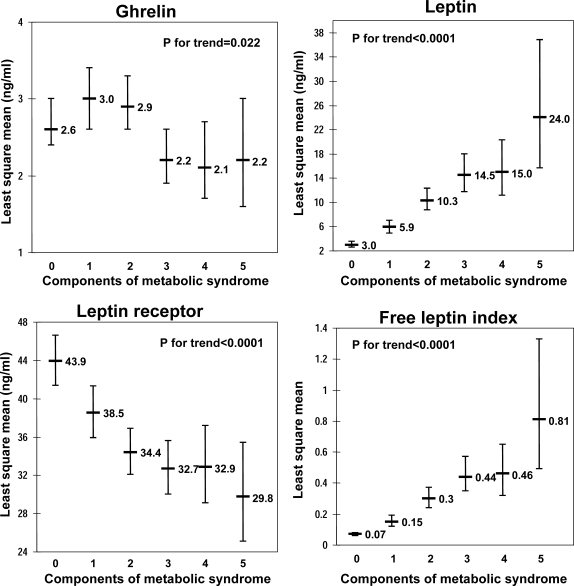
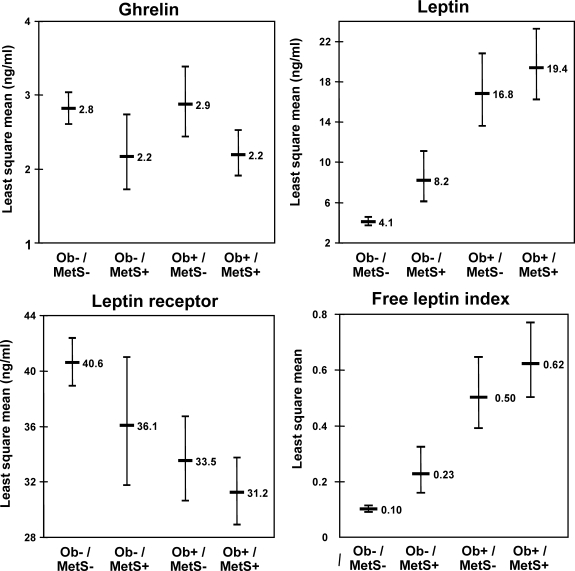
All adipokines were significantly associated with the number of components of MetS (Fig. 2). Ghrelin and sOB-R demonstrated decreasing concentrations with increase in the number of MetS components, whereas leptin concentrations and FLI increased. The age- and sex-adjusted least square means by presence vs. absence of obesity, and/or MetS are shown in Fig. 3. Ghrelin concentrations were lower in individuals with MetS, independently of obesity, whereas leptin, sOB-R and FLI concentrations differed across categories of obesity and MetS status in a linear fashion.
Figure 2.
Age- and sex-adjusted least square means of concentrations of ghrelin, leptin, sOB-R, and FLI according to the number of components of the MetS. The numbers of participants per group were 111, 80, 79, 53, 26, and 13 (for none, one, two, three, four, and five components of MetS).
Figure 3.
Age- and sex-adjusted least square means of concentrations of ghrelin, leptin, sOB-R, and FLI in nonobese individuals without MetS (Ob−/MetS−), nonobese individuals with MetS (Ob−/MetS+), obese individuals without MetS (Ob+/MetS−), and obese individuals with MetS (Ob+/MetS+).
When relating ghrelin, leptin, and sOB-R conjointly to MetS in age- and sex-adjusted analyses, higher ghrelin concentrations were associated with a decreased, and higher leptin concentrations were associated with an increased odds of MetS (Table 4). sOB-R was not significantly associated with MetS when modeled with ghrelin and leptin. In a multivariable model without leptin, log sOB-R [odds ratio (OR), 0.51; 95% CI 0.39–0.68; P < 0.0001 for a 1 sd increase] was associated with a decreased odds of MetS. Log ghrelin remained inversely related to MetS in this model (OR 0.57; 95% CI 0.43–0.76; P = 0.0001 for a 1 sd increase).
Table 4.
Conjoint consideration of the ghrelin, leptin, and sOB-R in relation to MetSa
| OR (95% CI) for MetS | P value | |
|---|---|---|
| Age | 1.04 (0.99–1.09) | 0.11 |
| Female sex | 0.39 (0.22–0.71) | 0.0021 |
| Log ghrelin | 0.55 (0.40–0.76) | 0.0002 |
| Log leptin | 4.44 (2.91–6.77) | <0.0001 |
| Log sOB-R | 0.82 (0.60–1.12) | 0.22 |
Values are ORs (95% CIs) and P values from a multivariable model including age, sex, and ghrelin, leptin, and sOB-R standardized within sex (mean 0, sd 1) conjointly as predictor variables, and MetS as response variable.
None of the interaction terms was statistically significant (all P > 0.10). With our sample size, we had 80% power to detect an increment to the model R2 of 0.021 and to observe a partial correlation coefficient of 0.15 or greater (at α = 0.05).
Discussion
Principal findings
In this study, we comprehensively characterized the cross-sectional relations of ghrelin, leptin, sOB-R concentrations, and the FLI with cardiometabolic risk factors in a community-based sample. Several interesting observations should be noted. First, we observed higher ghrelin, leptin, and FLI in women, whereas sOB-R concentrations were lower (in multivariable models simultaneously adjusting for other correlates), consistent with prior studies (28,29,30,31,32). The reasons for this sexual dimorphism are unknown but may include sex steroid effects, differential body fat distribution resulting in different secretion patterns of these adipokines, or other unknown factors. Second, the concentrations of all examined adipokines were associated with MetS, indicating that they may have a central role in mediating obesity, insulin resistance, and cardiovascular risk. Interestingly, presence or absence of MetS influenced adipokine concentrations independently of degree of obesity. This supports the notion of heterogeneity of risk by metabolic status among obese individuals (33). Third, ghrelin concentrations were not significantly correlated with leptin, sOB-R or FLI and demonstrated weaker associations with most of the cardiometabolic risk factors than the other biomarkers. Also, the model R2 in the multivariable model of clinical correlates of ghrelin concentrations was low, indicating that there may be other important factors determining ghrelin concentrations than those examined in the present study. Yet ghrelin was associated with MetS, even after adjustment for leptin and sOB-R. This could have been driven by a strong association with blood pressure and slight, but nonsignificant, associations with some of the other components of MetS. In contrast, leptin concentrations and FLI demonstrated a very high correlation, and they shared the same clinical correlates to a large degree. Also, sOB-R was correlated with similar cardiometabolic risk factors as leptin, although we observed some differences in their clinical correlates, a finding that would be interesting to pursue in a longitudinal study. However, when relating ghrelin, leptin, and sOB-R to MetS in a conjoint model, sOB-R was not significantly associated with MetS, presumably due to its high correlation with leptin.
Relations between ghrelin concentrations and cardiometabolic risk factors
In our study, ghrelin concentrations were inversely associated with blood pressure but not related to other cardiometabolic risk factors examined. Inverse relations between systolic and diastolic blood pressure and ghrelin have been previously reported in a case-control study of hypertensives and matched controls from the community (8). Furthermore, injection of ghrelin in healthy humans has been shown to decrease mean arterial pressure and increase cardiac output (34), and there is support for a vasodilating action of ghrelin, possibly through direct effects on the GH secretagogue receptor (also called ghrelin receptor) in the myocardium and aorta (34,35).
Ghrelin is a stimulator of GH release, which in turn has been proposed to be related to myocardial growth and improved cardiac function. In addition to these GH-dependent actions, a direct effect of ghrelin on the cardiovascular system has been proposed because mRNA for both the peptide and its receptor have been detected in the heart (36). Taken together, these observations are consistent with a role for ghrelin in the regulation of blood pressure and vasomotor function.
Furthermore, mean ghrelin concentrations were significantly lower with increasing number of MetS components and across categories of increasing BMI in our study. These findings are consistent with prior studies that have reported lower concentrations of ghrelin in individuals with MetS (7,9), insulin resistance (6), and obesity (5,6). Meal-related ghrelin and insulin concentrations are reciprocal, and it has been suggested that ghrelin concentrations may be secondary to changes in glucose and insulin (37). Conversely, ghrelin deficiency could theoretically promote insulin resistance by different mechanisms including somatotrophic effects (38) and modulation of insulin signaling in peripheral tissues (39). The complex relations between ghrelin, insulin, and associated pathways are still largely unknown and merits further study.
Leptin, leptin receptor, and cardiometabolic risk factors
In our investigation, leptin concentrations were positively correlated with all of the examined correlates, except for smoking. Also, the concentrations increased with the number of MetS components present. These findings concur with prior studies that demonstrated that higher leptin concentrations are associated with elevated blood pressure (14), type 2 diabetes (15), obesity (16,17), insulin resistance (17,18), and the MetS (19,20,21). A recent study in midlife women demonstrated that increases of leptin over the menopause transition period were associated with decreases in HDL cholesterol and increases in diastolic blood pressure, glucose, and insulin resistance, whereas increases in ghrelin concentrations over the same period were associated with an increase of low-density lipoprotein cholesterol (40). In another longitudinal study, both leptin and ghrelin were associated with most of the MetS components at baseline, but they did not seem to mediate the relations of MetS with coronary heart disease mortality (41).
The adipocyte-derived hormone leptin functions as a satiety signal, reduces the appetite and increases the metabolism (10,42). However, obese individuals have unusually high circulating concentrations of leptin, and the failure of leptin to suppress eating and promote weight loss in common forms of obesity have been termed leptin resistance (42). There is evidence that the association of leptin with insulin resistance may be independent of fat mass (17,20), and hyperleptinemia has even been proposed as a component of the MetS (20). The exact mechanisms behind the relations of leptin and insulin resistance and the complex interactions with other cardiometabolic risk factors are not entirely known. Leptin functions as a growth factor in a range of cell types as a mediator of energy expenditure and by interaction with other hormonal mediators and regulators of energy status and metabolism such as insulin, glucagon, IGFs, GH, and glucocorticoids (10).
Data on the associations between sOB-R and cardiometabolic risk factors are sparse and confined to selective study samples. One previous smaller study in overweight or obese men noted that lower sOB-R concentrations were associated with insulin resistance, abdominal obesity, and MetS (22), whereas sOB-R concentrations were not significantly correlated with insulin or glucose in a smaller study of women with polycystic ovary syndrome (23). A study in healthy Greek adolescents showed that sOB-R correlated inversely with body fat mass (24). It is plausible that the mechanisms by which sOB-R is associated with clinical correlates are shared with leptin because sOB-R is the main leptin-binding molecule in plasma (43) and thus an important modulator of leptin action.
Strengths and limitations
The strengths of our study include the community-based sample and the comprehensive assessment of cardiometabolic risk factors in relation to three different adipokines individually and conjointly. There were several limitations of our study. We had limited statistical power to detect modest associations because our study was based on a subsample (9%) of the whole cohort and had a low prevalence of diabetes (5%). Second, we did not relate the adipokines to measures of insulin sensitivity/secretion. Third, our sample consisted of middle-aged, white individuals of European decent, limiting the generalizability of our findings to other age groups and ethnicities. Fourth, because our study was cross-sectional, we cannot assess causality or determine whether adipokine concentrations are related to longitudinal tracking of cardiometabolic risk factors. Fifth, ghrelin was measured in serum in the present study, and although this has been done in some prior reports (7,41,44,45), it would have been preferable to use plasma and incorporate steps with addition of acid and protease inhibitors because ghrelin is known to be easily desacylated and/or degradated. However, a prior study demonstrated ghrelin concentrations in serum and four different plasma samples were very similar when samples were stored under optimal conditions (46).
Conclusions
In our community-based sample, ghrelin, leptin, sOB-R, and FLI demonstrated a sexual dimorphism, suggesting that appetite and energy expenditure control could be different in men and women. Furthermore, all examined adipokines were associated with the number of MetS components present. This finding is consistent with the hypothesis that these important regulators of appetite control and energy expenditure may have a central role in mediating cardiometabolic risk.
Footnotes
This work was supported by the Swedish Heart-Lung Foundation and the Swedish Society of Medicine (to E.I.), and the National Heart, Lung, and Blood Institute’s Framingham Heart Study (Contract N01-HC-25195) and Grants K23-HL-074077 (to T.J.W.), RO1-HL-076784, 1R01 AG028321 (to E.J.B.), and 1RO1 DK 080739 and 2K24HL4334 (to R.S.V.). J.B.M. was supported by a Career Development Award from the American Diabetes Association and National Institute of Diabetes and Digestive and Kidney Diseases Grant K24 DK080140. The funding sources had no role in the study design, analyses, or drafting of the manuscript.
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 20, 2008
Abbreviations: BMI, Body mass index; CI, confidence interval; FLI, free leptin index; HDL, high-density lipoprotein; MetS, metabolic syndrome; OR, odds ratio; sOB-R, soluble leptin receptor.
References
- Huda MS, Wilding JP, Pinkney JH 2006 Gut peptides and the regulation of appetite. Obes Rev 7:163–182 [DOI] [PubMed] [Google Scholar]
- Murphy KG, Bloom SR 2006 Gut hormones and the regulation of energy homeostasis. Nature 444:854–859 [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Pinkney J, Williams G 2002 Ghrelin gets hungry. Lancet 359:1360–1361 [DOI] [PubMed] [Google Scholar]
- Choi KM, Lee J, Lee KW, Seo JA, Oh JH, Kim SG, Kim NH, Choi DS, Baik SH 2004 The associations between plasma adiponectin, ghrelin levels and cardiovascular risk factors. Eur J Endocrinol 150:715–718 [DOI] [PubMed] [Google Scholar]
- Katsuki A, Urakawa H, Gabazza EC, Murashima S, Nakatani K, Togashi K, Yano Y, Adachi Y, Sumida Y 2004 Circulating levels of active ghrelin is associated with abdominal adiposity, hyperinsulinemia and insulin resistance in patients with type 2 diabetes mellitus. Eur J Endocrinol 151:573–577 [DOI] [PubMed] [Google Scholar]
- Langenberg C, Bergstrom J, Laughlin GA, Barrett-Connor E 2005 Ghrelin and the metabolic syndrome in older adults. J Clin Endocrinol Metab 90:6448–6453 [DOI] [PubMed] [Google Scholar]
- Poykko SM, Kellokoski E, Horkko S, Kauma H, Kesaniemi YA, Ukkola O 2003 Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes 52:2546–2553 [DOI] [PubMed] [Google Scholar]
- Ukkola O, Poykko SM, Antero KY 2006 Low plasma ghrelin concentration is an indicator of the metabolic syndrome. Ann Med 38:274–279 [DOI] [PubMed] [Google Scholar]
- Margetic S, Gazzola C, Pegg GG, Hill RA 2002 Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord 26:1407–1433 [DOI] [PubMed] [Google Scholar]
- Maamra M, Bidlingmaier M, Postel-Vinay MC, Wu Z, Strasburger CJ, Ross RJ 2001 Generation of human soluble leptin receptor by proteolytic cleavage of membrane-anchored receptors. Endocrinology 142:4389–4393 [DOI] [PubMed] [Google Scholar]
- Zastrow O, Seidel B, Kiess W, Thiery J, Keller E, Bottner A, Kratzsch J 2003 The soluble leptin receptor is crucial for leptin action: evidence from clinical and experimental data. Int J Obes Relat Metab Disord 27:1472–1478 [DOI] [PubMed] [Google Scholar]
- Kratzsch J, Lammert A, Bottner A, Seidel B, Mueller G, Thiery J, Hebebrand J, Kiess W 2002 Circulating soluble leptin receptor and free leptin index during childhood, puberty, and adolescence. J Clin Endocrinol Metab 87:4587–4594 [DOI] [PubMed] [Google Scholar]
- Beltowski J 2006 Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens 24:789–801 [DOI] [PubMed] [Google Scholar]
- Söderberg S, Zimmet P, Tuomilehto J, Chitson P, Gareeboo H, Alberti KG, Shaw JE 2007 Leptin predicts the development of diabetes in Mauritian men, but not women: a population-based study. Int J Obes (Lond) 31:1126–1133 [DOI] [PubMed] [Google Scholar]
- Ruhl CE, Everhart JE 2001 Leptin concentrations in the United States: relations with demographic and anthropometric measures. Am J Clin Nutr 74:295–301 [DOI] [PubMed] [Google Scholar]
- Zimmet P, Hodge A, Nicolson M, Staten M, de Courten M, Moore J, Morawiecki A, Lubina J, Collier G, Alberti G, Dowse G 1996 Serum leptin concentration, obesity, and insulin resistance in Western Samoans: cross sectional study. BMJ 313:965–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjaja A, Stratton IM, Horn R, Holman RR, Turner R, Brabant G 1997 UKPDS 20: plasma leptin, obesity, and plasma insulin in type 2 diabetic subjects. J Clin Endocrinol Metab 82:654–657 [DOI] [PubMed] [Google Scholar]
- Franks PW, Brage S, Luan J, Ekelund U, Rahman M, Farooqi IS, Halsall I, O'Rahilly S, Wareham NJ 2005 Leptin predicts a worsening of the features of the metabolic syndrome independently of obesity. Obes Res 13:1476–1484 [DOI] [PubMed] [Google Scholar]
- Leyva F, Godsland IF, Ghatei M, Proudler AJ, Aldis S, Walton C, Bloom S, Stevenson JC 1998 Hyperleptinemia as a component of a metabolic syndrome of cardiovascular risk. Arterioscler Thromb Vasc Biol 18:928–933 [DOI] [PubMed] [Google Scholar]
- Valle M, Gascon F, Martos R, Bermudo F, Ceballos P, Suanes A 2003 Relationship between high plasma leptin concentrations and metabolic syndrome in obese pre-pubertal children. Int J Obes Relat Metab Disord 27:13–18 [DOI] [PubMed] [Google Scholar]
- Sandhofer A, Laimer M, Ebenbichler CF, Kaser S, Paulweber B, Patsch JR 2003 Soluble leptin receptor and soluble receptor-bound fraction of leptin in the metabolic syndrome. Obes Res 11:760–768 [DOI] [PubMed] [Google Scholar]
- Sepilian VP, Crochet JR, Nagamani M 2006 Serum soluble leptin receptor levels and free leptin index in women with polycystic ovary syndrome: relationship to insulin resistance and androgens. Fertil Steril 85:1441–1447 [DOI] [PubMed] [Google Scholar]
- Yannakoulia M, Yiannakouris N, Bluher S, Matalas AL, Klimis-Zacas D, Mantzoros CS 2003 Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrinol Metab 88:1730–1736 [DOI] [PubMed] [Google Scholar]
- Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino Sr RB, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D 2007 The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 165:1328–1335 [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith Jr SC, Spertus JA, Costa F 2005 Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 1999 SAS Institute Inc., SASr procedures guide, version 8. Cary, NC: SAS Institute Inc.; 291–293 [Google Scholar]
- Chan JL, Bluher S, Yiannakouris N, Suchard MA, Kratzsch J, Mantzoros CS 2002 Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes 51:2105–2112 [DOI] [PubMed] [Google Scholar]
- Makovey J, Naganathan V, Seibel M, Sambrook P 2007 Gender differences in plasma ghrelin and its relations to body composition and bone—an opposite-sex twin study. Clin Endocrinol (Oxf) 66:530–537 [DOI] [PubMed] [Google Scholar]
- Ogawa T, Hirose H, Yamamoto Y, Nishikai K, Miyashita K, Nakamura H, Saito I, Saruta T 2004 Relationships between serum soluble leptin receptor level and serum leptin and adiponectin levels, insulin resistance index, lipid profile, and leptin receptor gene polymorphisms in the Japanese population. Metabolism 53:879–885 [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL 1996 Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab 81:3424–3427 [DOI] [PubMed] [Google Scholar]
- Tonstad S, Thorsrud H, Torjesen PA, Seljeflot I 2007 Do novel risk factors differ between men and women aged 18 to 39 years with a high risk of coronary heart disease? Metabolism 56:260–266 [DOI] [PubMed] [Google Scholar]
- Karelis AD, St. Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET 2004 Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab 89:2569–2575 [DOI] [PubMed] [Google Scholar]
- Nagaya N, Kojima M, Uematsu M, Yamagishi M, Hosoda H, Oya H, Hayashi Y, Kangawa K 2001 Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol Regul Integr Comp Physiol 280:R1483–R1487 [DOI] [PubMed] [Google Scholar]
- Okumura H, Nagaya N, Enomoto M, Nakagawa E, Oya H, Kangawa K 2002 Vasodilatory effect of ghrelin, an endogenous peptide from the stomach. J Cardiovasc Pharmacol 39:779–783 [DOI] [PubMed] [Google Scholar]
- Katugampola S, Davenport A 2003 Emerging roles for orphan G-protein-coupled receptors in the cardiovascular system. Trends Pharmacol Sci 24:30–35 [DOI] [PubMed] [Google Scholar]
- Murdolo G, Lucidi P, Di Loreto C, Parlanti N, De Cicco A, Fatone C, Fanelli CG, Bolli GB, Santeusanio F, De Feo P 2003 Insulin is required for prandial ghrelin suppression in humans. Diabetes 52:2923–2927 [DOI] [PubMed] [Google Scholar]
- Gil-Campos M, Aguilera CM, Canete R, Gil A 2006 Ghrelin: a hormone regulating food intake and energy homeostasis. Br J Nutr 96:201–226 [DOI] [PubMed] [Google Scholar]
- Murata M, Okimura Y, Iida K, Matsumoto M, Sowa H, Kaji H, Kojima M, Kangawa K, Chihara K 2002 Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. J Biol Chem 277:5667–5674 [DOI] [PubMed] [Google Scholar]
- Wildman RP, Mancuso P, Wang C, Kim M, Scherer PE, Sowers MR Adipocytokine and ghrelin levels in relation to cardiovascular disease risk factors in women at midlife: longitudinal associations. Int J Obes (Lond) 32:740–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg C, Bergstrom J, Scheidt-Nave C, Pfeilschifter J, Barrett-Connor E 2006 Cardiovascular death and the metabolic syndrome: role of adiposity-signaling hormones and inflammatory markers. Diabetes Care 29:1363–1369 [DOI] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Munzberg H Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70:537–556 [DOI] [PubMed] [Google Scholar]
- Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J 2001 Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun 283:982–988 [DOI] [PubMed] [Google Scholar]
- Kotani K, Sakane N, Saiga K, Adachi S, Mu H, Kurozawa Y, Kawano M 2006 Serum ghrelin and carotid atherosclerosis in older Japanese people with metabolic syndrome. Arch Med Res 37:903–906 [DOI] [PubMed] [Google Scholar]
- Siahanidou T, Mandyla H, Vounatsou M, Anagnostakis D, Papassotiriou I, Chrousos GP 2005 Circulating peptide YY concentrations are higher in preterm than full-term infants and correlate negatively with body weight and positively with serum ghrelin concentrations. Clin Chem 51: 2131–2137 [DOI] [PubMed] [Google Scholar]
- Groschl M, Wagner R, Dotsch J, Rascher W, Rauh M 2002 Preanalytical influences on the measurement of ghrelin. Clin Chem 48:1114–1116 [PubMed] [Google Scholar]