Abstract
Context: Treatment of pituitary gigantism is complex and the results are usually unsatisfactory.
Objective: The objective of the study was to describe the results of therapy of three children with pituitary gigantism by a GH receptor antagonist, pegvisomant.
Design: This was a descriptive case series of up to 3.5 yr duration.
Setting: The study was conducted at a university hospital.
Patients: Patients included three children (one female, two males) with pituitary gigantism whose GH hypersecretion was incompletely controlled by surgery, somatostatin analog, and dopamine agonist.
Intervention: The intervention was administration of pegvisomant.
Main Outcome Measures: Plasma IGF-I and growth velocity were measured.
Results: In all three children, pegvisomant rapidly decreased plasma IGF-I concentrations. Growth velocity declined to subnormal or normal values. Statural growth fell into lower growth percentiles and acromegalic features resolved. Pituitary tumor size did not change in two children but increased in one boy despite concomitant therapy with a somatostatin analog.
Conclusions: Pegvisomant may be an effective modality for the therapy of pituitary gigantism in children. Titration of the dose is necessary for optimal efficacy, and regular surveillance of tumor size is mandatory.
Pegvisomant may be an effective treatment strategy in children with pituitary gigantism who do not respond to other modalities, including somatostatin analogs.
Pituitary gigantism most frequently results from excess GH secretion by a somatotroph pituitary tumor with onset during childhood, before epiphyseal closure (1). Due to the irreversible effect of GH excess on stature rapid abolition of excessive somatic growth in children is required. Unfortunately, the existing therapies for pituitary gigantism are unsatisfactory. Surgery alone is rarely effective and can lead to multiple hormone deficiencies (2). Radiotherapy has delayed action (1), cannot prevent accelerated somatic growth, and may cause significant central nervous system morbidity as well as hypopituitarism. Somatostatin analogs are effective only in a proportion of patients with acromegaly (3) and have not been adequately studied in children with gigantism. The introduction of the GH receptor antagonist pegvisomant has offered a novel approach to the treatment of acromegaly (4), but experience with this drug in pituitary gigantism is limited (5,6,7). We present three children with pituitary gigantism treated with pegvisomant for up to 3.5 yr.
Patients and Methods
The requirement for signed informed consent to report the data were waived by the University of Michigan Institutional Review Board for all three patients.
Patients
Patient 1 is a 9-yr-old girl who surpassed the 95th percentile for length at 9 months of age. Her plasma GH and IGF-I were elevated for age, and brain magnetic resonance imaging (MRI) at 10 months demonstrated an intra- and suprasellar pituitary macroadenoma measuring 1.8 cm. She underwent unsuccessful tumor resection by subfrontal approach at 11 months of age. Postoperatively her plasma GH and IGF-I remained elevated at 134 μg/liter and 1419 μg/liter (normal 17–248), respectively. Serum prolactin was 265 μg/liter (normal 1–23), and accelerated linear growth continued. At 36 months of age, her tumor was debulked by transsphenoidal approach. Immunohistochemical staining of tumor tissue confirmed GH-secreting adenoma.
The patient presented to us at age 3 yr 2 months, after her second surgery, with clinical features of gigantism [height 110.8 cm, +3.68 sd score (SDS)], hyperhydrosis, snoring, and coarse facial features. Laboratory evaluation revealed plasma GH 8.9 μg/liter, which decreased to 2.7 μg/liter after 50 g of oral glucose, IGF-I 390 μg/liter (74–202), and prolactin of 230 μg/liter. Cabergoline (0.25 mg twice weekly) did not suppress GH or IGF-I levels, and her annualized growth velocity was 10 cm/yr. At 3 yr 9 months of age, octreotide long-acting release (LAR) was started at 10 mg im every 4 wk. The dose was gradually increased to 30 mg every 4 wk; however, the patient’s GH (8.2 μg/liter), IGF-I (1243 μg/liter), and annualized growth velocity (10.3 cm/yr) remained elevated (Fig. 1A). At the age of 5 yr 6 months, pegvisomant 10 mg sc daily was added.
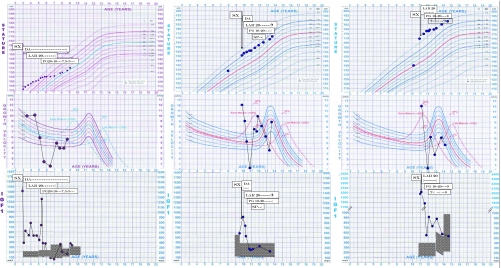
Figure 1.
Clinical course of three patients with gigantism. Upper row, Actual growth curves. Middle row, Annualized GVs. Lower row, Plasma IGF-I concentrations (shaded areas represent normal ranges). Left column, Patient 1. Center column, Patient 2. Right column, Patient 3. Sx, Surgery; DA, dopamine agonist; LAR, octreotide LAR; Pg, pegvisomant; SP, spontaneous puberty; T, exogenous testosterone. Arrows mark the time of surgery.
Patient 2 is a boy 13 yr 7 months old who exhibited accelerated linear growth beginning at approximately 8 yr of age. Pituitary MRI revealed a 2.5-cm macroadenoma, and visual field examination showed bitemporal hemianopsia. His IGF-I was 577 μg/liter (110–565), and GH was 70.4 μg/liter. At 9 yr 11 months, he underwent transsphenoidal tumor debulking, which led to normalization of visual fields. Pathology revealed a GH producing adenoma. He presented to us at 10 yr of age, measuring 153 cm (+2.09 SDS) and exhibiting excess perspiration and coarsening of facial features. His IGF-I level was 923 μg/liter and GH was 12.7 μg/liter. Prolactin was 100.1 μg/liter. Cabergoline 0.5 mg twice weekly was ineffective in lowering GH and IGF-1 concentrations and octreotide LAR 20 mg im every 4 wk was added. His IGF-I declined to 504 μg/liter, but plasma GH remained high at 20.7 μg/liter. His annualized growth velocity was 9 cm/yr (>97th percentile for age) despite drug therapy. At the age of 10 yr 7 months, pegvisomant 20 mg/day sc was added.
Patient 3 is a boy 14 yr 7 months old who developed accelerated growth and headaches at age 9 yr. Between 10 and 11 yr of age, his height increased by almost 30 cm. A pituitary MRI revealed a 2-cm pituitary adenoma. He presented to us at 11 1 months yr of age. His height was 170 cm (+3.49 SDS) and he had excess perspiration, meaty hands, and coarse facial features. His GH was 126 μg/liter, IGF-I was 1963 μg/liter (normal 110–395), and his prolactin was less than 1 μg/liter.
His tumor was debulked transsphenoidally, and immunohistochemical staining was positive for GH. Neuroophthalmologic examination was normal before and after the surgery. Postoperatively his GH was 37.2 μg/liter, IGF-I was 2023 μg/liter, and accelerated growth continued. After three monthly injections of octreotide LAR 20 mg, his GH and IGF-I remained elevated (32.9 and 1760 μg/liter, respectively), and annualized growth velocity was 21 cm/yr. Pegvisomant 20 mg/d was added at age 11 yr 7 months.
Postoperatively, all three patients were found to have ACTH and TSH deficiency and were given replacement therapy with hydrocortisone and l-thyroxine in standard doses. Free T4 concentrations have remained normal.
Methods
Plasma IGF-I was measured by immunoluminometric assay kit (Diagnostic Systems Laboratories, Webster, TX) in patients 1 and 3 and by Esoterix (Calabasas Hills, CA) in patient 2. Manufacturer-provided age/gender-adjusted normative data were used for comparisons. Patients’ heights were regularly recorded using the same stadiometer and annualized growth velocity (GV; centimeters per year) was calculated. Pituitary dedicated MRI studies were performed using pre- and postgadolinium contrast images. Skeletal ages were determined by analysis of x-ray films of the left wrist and hand, using the method of Greulich and Pyle (8).
Results
IGF-I and growth velocity
After the initiation of pegvisomant, linear growth virtually ceased for 6 months in subjects 2 (height 157 cm between ages 10.5 and 11 yr) and 3 (177 cm between 11 yr 7 months and 12 yr 2 months) and for 1 yr in subject 1 (125 cm between 5.5 and 6.5 yr). In patients 1 and 2, this was accompanied by subnormal and normal IGF-I levels, respectively; in patient 3 plasma IGF-I levels remained elevated for almost 2 yr after pegvisomant was started before falling into the normal range (Fig. 1). Administration of pegvisomant promptly and durably abolished excessive perspiration in all children. Within several months, diminution of soft tissue hypertrophy occurred, and within 1 yr of therapy, the facial features of acromegaly resolved completely. Throughout the follow-up period, liver function remained normal in all three patients, and there were no other noticeable side effects.
In patient 1, octreotide LAR therapy was eventually terminated. Her stature followed the 75th percentile for age until the age of 8 yr 8 months when it fell to the 50th percentile. Her IGF-I was then 154 μg/liter (−1 SDS) and the pegvisomant dose was decreased further to 5 mg daily.
Subject 2 went into spontaneous puberty as was evident by 120 ng/dl testosterone at age 11 yr 5 months, but by 12 yr 7 months, his testosterone decreased to subnormal levels. The patient and his parents declined testosterone replacement until further consideration. Subject 3 was started on testosterone therapy after his testosterone was unmeasurable at age 12 yr. With spontaneous or testosterone-induced puberty, GV rapidly and temporarily increased in both boys on the same dose of pegvisomant and then decreased spontaneously within 6–12 months. Octreotide LAR was stopped in patient 3 at the age of 13 yr 11 months but continued in patient 2 because of the larger size of the tumor remnant.
Statural growth
In patient 1 skeletal and chronological ages were synchronous before initiation of pegvisomant (5 yr 2 months at 5 yr of age). Due to her early bone age, calculations of predicted height were unreliable. At the age of 7 yr, after 1.5 yr of pegvisomant therapy, her skeletal age was 6 yr 10 months and remained similar to chronological age at 8 yr 1 months. Her predicted adult height by bone age determination (9,10) at 8 yr 1 month was 168.9 cm. The midparental predicted target height was calculated at 155.8 ± 9 cm (11).
The skeletal age of patient 2, after 6 months of pegvisomant treatment, matched the chronological age (11 yr for both). His predicted adult height by bone age was 193 cm. His midparental target height was calculated at 180.5 ± 9 cm.
In patient 3, after 10 months of pegvisomant and 2 months of testosterone therapy, skeletal age was 13 yr vs. 12 yr 4 months of chronological age. At the age of 13 yr 11 months, spontaneous puberty occurred and the skeletal age advanced to 15 yr. His calculated predicted height decreased from 205.4 (as calculated before the introduction of pegvisomant) to 194.8 cm. His midparental target height was 177.4 ± 9 cm.
Tumor size monitoring
Repeat pituitary MRI studies showed stable size of the pituitary tumor remnant throughout the treatment period in patients 1 and 3. In patient 2, despite continued somatostatin LAR therapy, tumor size increased between 13 and 14 yr of age and pegvisomant was stopped. Three months after the discontinuation of pegvisomant, tumor size did not increase further. The patient stopped octreotide LAR therapy as well and underwent a second transsphenoidal surgery at the age of 14 yr 2 months. After surgery and on no medications, he still had a small residual tumor within the sella, his random GH values were 6.6 and 9 μg/liter, his plasma IGF-1 was 490 μg/liter (normal < 540), and his growth velocity was 9.4 cm/yr (at or > 97th percentile). His parents decided not to use pharmacological therapy but to pursue radiation therapy instead.
Discussion
Pegvisomant is a novel therapeutic agent for the treatment of acromegaly (4,12). It binds to the GH receptor and prevents receptor dimerization and subsequent receptor-mediated activity, thus impeding GH-dependent production of IGF-I. Administration of pegvisomant to patients with acromegaly results in elevation of circulating GH levels by removing the negative feedback influence of circulating IGF-I (12). This and the cross-reactivity of pegvisomant in many GH assays make GH unsuitable as a marker of therapeutic efficacy. However, in acromegaly and pituitary gigantism, the decline in IGF-I generally correlates with the degree of clinical improvement (4,5,6,7,12).
In this series, administration of pegvisomant resulted in significant reduction of IGF-I, prompt improvement in soft tissue hypertrophy and resolution of acromegalic features in all three children. Most dramatic was the effect on growth velocity. Documentation of clinical efficacy of therapeutic modalities in adults with acromegaly is often difficult due to irreversible changes brought about by years of active disease. In contrast, accelerated growth is a cardinal and easily quantifiable feature of gigantism. In three previously reported cases of gigantism treated with pegvisomant (5,6,7), patients also showed decreases in growth velocity. In all three cases reported here, pegvisomant treatment was followed by an immediate cessation of somatic growth. Titration of the pegvisomant dose in patient 1 allowed us to maintain growth velocity at desired rate. In patients 2 and 3, GV was kept within the expected range to maintain statures within acceptable limits. Most importantly, disease control with pegvisomant afforded an opportunity to delay considerations of radiotherapy until all three patients had matured further and the dangers of central nervous system toxicity diminished.
In the two boys, spontaneous or medical increase in testosterone was followed by a brief period of accelerated statural growth in parallel with temporary increases in plasma IGF-I concentrations. This is reminiscent of a similar phenomenon in individuals with Laron’s type dwarfism (congenital insensitivity to GH) (13) in whom linear growth is accelerated at the time of pubarche, despite absent GH action (14,15). We can speculate that growth acceleration in our patients was shorter than expected due to only transient pubertal testosterone increase in subject 2 and intermittent testosterone administration in subject 3.
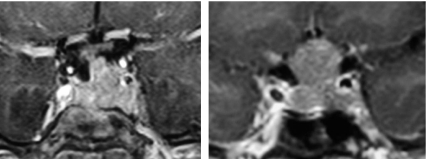
Several cases of tumor expansion have been described in patients with acromegaly treated with pegvisomant (12,16,17). Whether the increase in tumor size is due to the removal of the negative IGF-I feedback or is a manifestation of spontaneous progression of inherently aggressive tumors is uncertain. We initially continued therapy with octreotide LAR in these patients despite its lack of biochemical efficacy in the hope that it might attenuate growth potential of the tumors. Subsequently when the stability of tumor size during combined pegvisomant/octreotide LAR was documented, the latter was stopped in patients 1 and 3, and no further tumor growth occurred. However, in patient 2, tumor size has expanded despite persistent octreotide LAR administration, perhaps as a reflection of intrinsically higher aggressiveness of the tumor (Fig. 2B). It is obvious that these patients require continuous follow-up, and the decision to use pegvisomant alone or in combination with a somatostatin analog needs to be considered on an individual basis.
Figure 2.
Expansion of pituitary tumor size in patient 2. Left panel, Coronal view of the sellar area after about 2 yr of combined octreotide LAR (20 mg every month) and pegvisomant (20 mg every day) therapy. Right panel, Same view after another year of therapy with octreotide LAR and pegvisomant in the same doses.
In conclusion, pegvisomant may be an effective therapy for pituitary gigantism in children who do not respond to other modalities, including somatostatin analogs. It fulfills the demand for rapid and durable reduction in growth velocity and prevention of gigantism in children. Titration of pegvisomant dose allows progression of somatic growth at a normal rate. Caution should be exercised when using pegvisomant and follow-up of pituitary tumor size is mandatory.
Footnotes
This work was supported by the Department of Veterans Affairs Medical Research Service and National Institutes of Health Grant R01 DK071955 (to A.B.) and Genentech Fellowship (to N.G.).
Disclosure Statement: The authors have nothing to declare.
First Published Online May 20, 2008
Abbreviations: GV, Growth velocity; LAR, long-acting release; MRI, magnetic resonance imaging; SDS, sd score.
References
- Eugster EA, Pescovitz OH, Gigantism J 1999. Clin Endocrinol Metab 84:4379–4384 [DOI] [PubMed] [Google Scholar]
- Zampieri P, Scanarini M, Sicolo N, Andrioli G, Mingrino S 1983 The acromegaly gigantism syndrome. Report of four cases treated surgically. Surg Neurol 20:498–503 [DOI] [PubMed] [Google Scholar]
- Cozzi R, Montini M, Attanasio R, Albizzi M, Lasio G, Lodrini S, Doneda P, Cortesi L, Pagani G 2006 Primary treatment of acromegaly with octreotide LAR: a long-term (up to nine years) prospective study of its efficacy in the control of disease activity and tumor shrinkage. J Clin Endocrinol Metab 91:1397–1403 [DOI] [PubMed] [Google Scholar]
- Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, Dimaraki EV, Stewart PM, Friend KE, Vance ML, Besser GM, Scarlett JA, Thorner MO, Parkinson C, Klibanski A, Powell JS, Barkan AL, Sheppard MC, Malsonado M, Rose DR, Clemmons DR, Johannsson G, Bengtsson BA, Stavrou S, Kleinberg DL, Cook DM, Phillips LS, Bidlingmaier M, Strasburger CJ, Hackett S, Zib K, Bennett WF, Davis RJ 2000 Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med 342:1171–1177 [DOI] [PubMed] [Google Scholar]
- Rix M, Laurberg P, Hoejberg AS, Brock-Jacobsen B 2005 Pegvisomant therapy in pituitary gigantism: successful treatment in a 12 year old girl. Eur J Endocrinol 153:195–201 [DOI] [PubMed] [Google Scholar]
- Müssig K, Gallwitz B, Honegger J, Strasbuerg CJ, Bidlingmaier M, Machicao F, Bornemann A, Ranke MB, Häring HU, Petersenn S 2007 Pegvisomant treatment in gigantism caused by a growth hormone-secreting giant pituitary adenoma. Exp Clin Endocrinol Diabetes 115:198–202 [DOI] [PubMed] [Google Scholar]
- Ali O, Banerjee S, Kelly DF, Lee PDK 2007 Management of type 2 diabetes mellitus associated with pituitary gigantism. Pituitary 10:359–364 [DOI] [PubMed] [Google Scholar]
- Greulich W, Pyle S 1999 Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Palo Alto, CA: Stanford University Press [Google Scholar]
- Tanner JM, Landt KW, Cameron N, Carter BS, Patel J 1983 Prediction of adult height from height and bone age in childhood. A new system of equations (TW Mark II) based on a sample including very tall and very short children. Arch Dis Child 58:767–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM, Whitehouse RH, Marshall WA, Carter BS 1975 Prediction of adult height from height, bone age, and occurrence of menarche, at ages 4 to 16 with allowance for midparent height. Arch Dis Child 50:14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wales JKH, Wit J-M, Rogol A 2003 Pediatric endocrinology and growth. 2nd ed. Philadelphia: Saunders [Google Scholar]
- Parkinson C, Trainer PJ 1999 Growth hormone receptor antagonists therapy for acromegaly. Bailleres Best Pract Res Clin Endocrinol Metab 13:419–430 [DOI] [PubMed] [Google Scholar]
- Laron Z, Sarel R, Pertzelan A 1980 Puberty in Laron type dwarfism. Eur J Pediatr 134:79–83 [DOI] [PubMed] [Google Scholar]
- Aynsley-Green A, Zachmann M, Prader A 1976 Interrelation of the therapeutic effects of growth hormone and testosterone on growth in hypopituitarism. J Pediatr 89:992–999 [DOI] [PubMed] [Google Scholar]
- Borski RJ, Tsai W, DeMott-Friberg R, Barkan AL 1996 Regulation of somatic growth and the somatotropic axis by gonadal steroids: primary effect on insulin-like growth factor 1 gene expression and secretion. Endocrinology 137:3253–3259 [DOI] [PubMed] [Google Scholar]
- Trainer PJ 2003 Lessons from 6 years of GH receptor antagonist therapy for acromegaly. J Endocrinol Invest 26:44–52 [PubMed] [Google Scholar]
- Colao A, Pivonello R, Auriemma RS, De Martino MC, Bidlingmaier M, Briganti F, Tortora F, Burman P, Kourides IA, Strasburger CJ, Lombardi G 2006 Efficacy of 12-month treatment with the GH receptor antagonist pegvisomant in patients with acromegaly resistant to long-term, high-dose somatostatin analog treatment: effect of IGF-1 levels, tumor mass, hypertension and glucose tolerance. Eur J Endocrinol 154:467–477 [DOI] [PubMed] [Google Scholar]