Abstract
Context: Adipose tissue-derived adipokines may contribute to insulin resistance.
Objective: We tested the hypothesis that adipokines are associated with insulin resistance in a community-based cohort and that associations are maintained in people with and without the metabolic syndrome (high vs. low risk of diabetes).
Design, Setting, and Participants: We studied a cross-sectional sample of 2356 individuals attending the seventh examination (1998–2001) of the Framingham Offspring Study. We measured levels of glucose, insulin, adiponectin, resistin, and TNFα in fasting blood samples and defined metabolic syndrome by updated National Cholesterol Education Program criteria. We used ANOVA to test associations of adipokines with insulin resistance and multivariable logistic regression models to assess joint associations of adipokines and metabolic syndrome with insulin resistance.
Main Outcome Measure: Homeostasis model (HOMA-IR), with insulin resistance defined by HOMA-IR greater than the 75th percentile, was measured.
Results: Age- and sex-adjusted HOMA-IR levels were inversely related to adiponectin (r = −0.40, P < 0.0001) and positively related to resistin (r = 0.13, P < 0.0001) and TNFα (r = 0.12, P < 0.0001). The prevalence of insulin resistance increased with decreasing tertiles of adiponectin (from 10.9% in the third to 42.5% in the first tertile; P < 0.0001) and increasing tertiles of resistin (from 19.3 to 30.9%; P < 0.0001) and TNFα (from 18.8 to 32.0%; P < 0.0001). Results were similar after adjustment for body mass index. These associations were present in individuals with or without the metabolic syndrome. In multivariable regression models, metabolic syndrome and adipokines individually and jointly were significantly associated with insulin resistance.
Conclusion: Adverse levels of adipokines are associated with insulin resistance in individuals at low or high diabetes risk.
Insulin resistance, measured by HOMA-IR, is inversely associated with plasma adiponectin levels, but positively associated with resistin and TNFα levels in individuals at low or high risk of future type 2 diabetes, even considering their adiposity.
Obesity and diabetes are epidemic worldwide (1). Adipose tissue is now recognized as an endocrine organ that contributes to the physiopathology of type 2 diabetes. Adipokines, proteins produced by adipose tissue, have been identified as potential contributors to insulin resistance in humans (2). Over the past few years, emerging evidence has shown that adipokines are produced both by adipocytes and macrophages in human adipose tissue and that diverse paracrine and autocrine pathways are involved in their regulation (2). To capture these interrelations, we selected a set of key adipokines representing these adipose tissue cell types, including adipokines produced primarily by adipocytes (adiponectin), macrophages (resistin), or both (TNFα). Plasma levels of adiponectin, an antiinflammatory, antidiabetic hormone, are inversely related to central adiposity and insulin resistance (3,4). Adiponectin is produced by differentiated adipocytes and circulates at high levels in the bloodstream (2). Low levels of circulating adiponectin levels are associated with a higher risk of future type 2 diabetes (5,6). Resistin is a 12-Da polypeptide that was initially linked to insulin resistance in animal models (7). Results in human have been inconsistent, showing positive associations in some studies (8,9) but not in others (10,11). In human adipose tissue, resistin seems to be produced mainly by infiltrating macrophages (12). Circulating resistin is positively related to adiposity and may be implicated in proinflammatory signaling associated with excess adiposity (13). Within the adipose tissue, TNFα is a cytokine secreted by both adipocytes and macrophages (2). It may participate in the inflammatory reaction that links central adiposity to insulin resistance (14). Controversy about the role of TNFα in insulin resistance has been raised by inconsistent results in human studies, some finding no association (15,16) whereas others have (17,18).
Inconsistency in human data may be due in part to study of small samples of highly selected subjects and lack of assessment of several adipokines simultaneously. Therefore, the role and interrelations of adipokines in the pathophysiology of insulin resistance in community-based samples has not been fully characterized. It is unknown whether associations of adipokines with insulin resistance in humans are modified by the presence or absence of prediabetes defined by conditions such as metabolic syndrome or impaired fasting glucose (IFG). Comprehensive, simultaneous analysis of several adipokines among individuals at low or high risk for type 2 diabetes is needed to clarify the magnitude and significance of the role of adipokines in human insulin resistance.
Therefore, the aim of this study was to test in a large unselected sample the following hypotheses: 1) levels of adiponectin, resistin, and TNFα are associated with insulin resistance measured by the homeostasis model insulin resistance (HOMA-IR) without and with adjustment for body mass index (BMI); 2) adipokine-insulin resistance associations are maintained in individuals at low or high risk of developing type 2 diabetes (defined here by the absence or presence the metabolic syndrome or IFG); and 3) adipokine levels are associated with insulin resistance individually and simultaneously in multivariable models including prediabetes status.
Subjects and Methods
Study participants
The Framingham Offspring Study is a community-based study of cardiovascular disease risk factors (19). The study began in 1971 with the enrollment of 5124 people (mainly Caucasian) who were the children of the original Framingham Heart Study cohort, and the spouses of the children. During the seventh examination cycle (1998–2001; n = 3539), participants provided fasting blood samples, and had a standardized medical examination. A total of 2356 subjects provided data for the present analysis, after exclusion of those with prevalent diabetes (n = 389), missing values for the diagnosis of metabolic syndrome (n = 311), or levels of adipokines (n = 483). Individuals with vs. without missing values were similar in age, sex, and mean BMI distribution. The study protocol was approved by the Institutional Review Boards of the Boston University School of Medicine and the Massachusetts General Hospital; all the participants provided written informed consent.
Exposure and outcome definitions
The primary dependent variable was insulin resistance, measured using the homeostasis model [HOMA-IR, calculated by (fasting glucose × fasting insulin)/22.5] (20). We assessed insulin resistance both as a continuous and a categorical outcome, defining the state of insulin resistance for the individuals in the top quartile of the HOMA-IR distribution (21). The primary independent variables were fasting plasma levels of adiponectin, resistin, and TNFα. Total adiponectin, resistin, and high-sensitivity TNFα were measured by ELISA (R&D Systems, Minneapolis, MN). Laboratory methods for glucose, insulin, and lipid assays have been published (22). Fasting plasma glucose (FPG) was measured immediately with a hexokinase reagent kit (A-gent glucose test, Abbott, South Pasadena, CA), and other plasma samples were frozen at −80 C until assay. Fasting plasma insulin was measured with a human-specific insulin assay having essentially no cross-reactivity to proinsulin or insulin split-products (Linco Inc., St. Louis, MO). Intraassay coefficients of variation were less than 3% for glucose, 6.1% for insulin, 5.8% for adiponectin, 9.0% for resistin, and 6.6% for TNFα.
Other covariates included standardized measurements cardiovascular risk factors. We measured height, weight, and waist circumference (at the umbilicus) with the subject standing. We calculated BMI as weight in kilograms divided by the square of height in meters. We used blood pressure as the mean of two measurements after the subject had been seated for at least 5 min. We classified people with prediabetes as having metabolic syndrome, or alternatively, with IFG. Metabolic syndrome was defined using the 2005 updated Third Report of the National Cholesterol Education Program’s Adult Treatment Panel criteria, as any three or more of: FPG, 5.6–6.9 mmol/liter; waist circumference, 102 cm or greater (in men) or 88 cm or greater (in women); fasting triglycerides 1.7 mmol/liter or greater; high-density lipoprotein-cholesterol less than 1.0 mmol/liter (in men) or less than 1.3 mmol/liter (in women); and blood pressure 130/85 mm Hg or greater or treatment for hypertension (23). IFG was defined as FPG from 5.6 to 6.9 mmol/liter inclusively, and diabetes was defined by a FPG level above 7.0 mmol/liter or current use of hypoglycemic drugs (24).
Statistical analysis
Means and sds are presented for continuous percentages for categorical characteristics. The t tests and χ2 tests were used to compare continuous and categorical baseline characteristics of individuals with and without metabolic syndrome. To compare adipokine levels with HOMA-IR as continuously distributed covariates, Spearman correlations, age-sex-adjusted linear regression, and scatter plot analysis were used. To test associations with prevalence of insulin resistance, we categorized participants into sex-specific tertiles of the distribution of each adipokine. The χ2 tests of trend were used to assess differences in insulin resistance prevalence across adipokine tertiles. We used metabolic syndrome as the primary prediabetes phenotype and used the same approach for association analyses stratified by presence or absence of metabolic syndrome. We tested the interaction of metabolic syndrome on the association of adipokine level with insulin resistance using first-order (metabolic syndrome by adipokine) interaction terms in linear or logistic regression models for the continuous and dichotomous parameterizations of insulin resistance. Logistic regression analysis related insulin resistance to metabolic syndrome (presence vs. absence) and/or tertiles of each adipokine modeled as ordinal (0, 1, 2) variables. We developed models by considering first the association of metabolic syndrome and adipokines individually and then in combination, with the fully specified model including metabolic syndrome and all three adipokines. Analyses were age-sex adjusted and then further adjusted for BMI.
We performed subsidiary analyses to assess assumptions made in the main analyses. Insulin resistance was defined, by convention, using the 75th percentile. We recently reported that using a HOMA-IR threshold around the 92nd percentile provides better specificity (>90%) than the 75th percentile to predict type 2 diabetes (25). Therefore, we repeated the analyses using the 90th percentile of HOMA-IR to define insulin resistance. Prediabetes may be defined in several ways. Prior data show metabolic syndrome to be a powerful predictor of diabetes beyond IFG alone (26). Therefore, we used metabolic syndrome to define the prediabetic state in our main analysis, but we also conducted the analysis using IFG. In another subsidiary analysis, we used waist circumference instead of BMI to adjust for adiposity. Next, because adipokine levels differ between male and female, interactions between sex and each individual adipokine on the level of insulin resistance were tested in the age-sex-BMI adjusted models using first-order sex-by-adipokine interaction terms. Finally, we conducted the analysis for the original individual adipokine models adjusting further for hypertensive and dyslipidemic medications. We considered P < 0.05 to indicate statistical significance. We performed all analyses using SAS software (version 8.1; SAS Institute, Cary, NC).
Results
Characteristics of the study subjects are displayed in Table 1. By definition, 25% of subjects were classified as having insulin resistance. Metabolic syndrome criteria were fulfilled by 42% of subjects; those with metabolic syndrome were older, had a higher mean BMI, were more likely to be men, had higher HOMA-IR values, and were more likely to be classified as insulin resistant. Among the individuals with metabolic syndrome, 47.5% were classified as insulin resistant. In those with insulin resistance, 79.6% fulfilled the metabolic syndrome criteria. In the overall cohort, 31% were using antihypertensive medication, whereas 17% were taking drugs for lipid disorders; in individuals with metabolic syndrome the proportions were 50 and 32%, respectively. Subjects with metabolic syndrome had lower mean levels of adiponectin and higher levels of resistin and TNFα than subjects without metabolic syndrome.
Table 1.
Characteristics of 2356 Framingham Offspring Study participants, overall and stratified by metabolic syndrome status
| Overall | No metabolic syndrome | Metabolic syndrome | P valuea | ||||
|---|---|---|---|---|---|---|---|
| n (%) | 2356 | 1373 (58%) | 983 (42%) | ||||
| Age (mean yr, sd) | 60 | 9.5 | 59 | 9.6 | 62 | 9.0 | <0.0001 |
| Women, % | 55.0 | 58.1 | 49.8 | <0.0001 | |||
| BMI (mean kg/m2, sd) | 27.8 | 5.0 | 25.9 | 4.1 | 30.4 | 5.0 | <0.0001 |
| HOMA-IR (mean U, sd) | 3.3 | 1.8 | 2.6 | 1.6 | 4.6 | 1.7 | <0.0001 |
| Insulin resistance (%) | 25.0 | 8.8 | 47.5 | <0.0001 | |||
| Adiponectin (mean μg/ml, sd) | 8.75 | 1.85 | 10.2 | 1.79 | 7.04 | 1.82 | <0.0001 |
| Resistin (mean ng/ml, sd) | 12.8 | 1.49 | 12.3 | 1.48 | 13.6 | 1.50 | <0.0001 |
| TNFα (mean pg/ml, sd) | 1.25 | 1.63 | 1.18 | 1.61 | 1.34 | 1.65 | <0.0001 |
P values are for comparisons between groups with and without metabolic syndrome.
Each component of the metabolic syndrome, age, and BMI were all significantly associated with HOMA-IR in Spearman correlations (all P < 0.0001). In age-sex adjusted analysis, with variables as continuous, adiponectin was inversely related to HOMA-IR (r = −0.40, P < 0.0001), whereas resistin (r = 0.13, P < 0.0001) and TNFα (r = 0.12, P < 0.0001) were positively related.
We tested associations between increasing tertile of each adipokine and the prevalence of insulin resistance. The prevalence of insulin resistance decreased with increasing level of adiponectin, with a prevalence of 43.8% in the lowest tertile, 22.0% in the middle, and 10.1% in the highest tertile (P < 0.0001 for trend). The prevalence of insulin resistance increased positively with increasing level of resistin (18.7, 26.3, and 29.8% in ascending tertiles, P < 0.0001) and TNF-α (18.7, 25.7, and 31.5% in ascending tertiles, P < 0.0001). The same analyses were carried out with age, sex, and BMI adjustment with similar results (P values for trends < 0.0001 for adiponectin, 0.03 for resistin, and 0.0002 for TNFα). Adjusting for adiposity with waist circumference instead of BMI resulted in similar trends and P values (P values for trends < 0.0001 for adiponectin, 0.03 for resistin, and 0.0001 for TNFα).
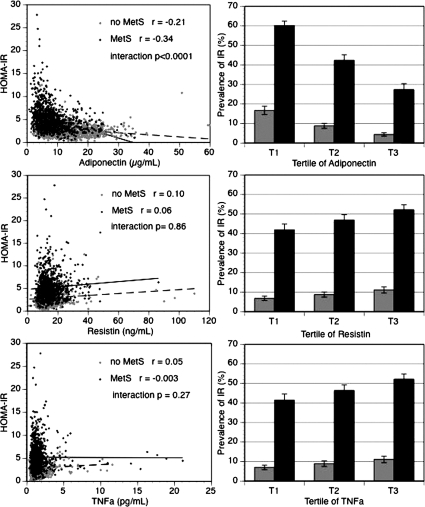
The distributions of HOMA-IR and each adipokine according to the presence or absence of metabolic syndrome are illustrated in Fig. 1 (left hand column). In the adiponectin scatter plot in particular, we noticed that few individuals without the metabolic syndrome appear in the high range of HOMA-IR; likewise, few individuals with the metabolic syndrome are in the high range of adiponectin. The negative correlation between adiponectin levels and HOMA-IR was stronger in the group with metabolic syndrome (r = −0.34 with metabolic syndrome vs. r = −0.21 without metabolic syndrome, interaction P < 0.0001). In the resistin and TNFα scatter plots, stratifying the group with and without the metabolic syndrome reduced the correlations between adipokines and insulin resistance, but in both cases, there was no significant interaction by metabolic syndrome on the adipokine-insulin resistance association (P > 0.2 for interaction).
Figure 1.
Adverse adipokine levels are associated with insulin resistance in individuals with metabolic syndrome (MetS) and without (no MetS). The left-hand column shows scatter plots (crude data) of plasma adiponectin (top), resistin (middle), and TNF-α (bottom) in relation to insulin resistance measured by HOMA-IR according to metabolic syndrome status (gray circle, no MetS; black circle, MetS; dashed line, no MetS; solid line, MetS). The right-hand column shows the prevalence (and ses) of insulin resistance (IR) (age-sex adjusted) defined by HOMA-IR greater than 75th percentile in relation to adipokines grouped into tertiles of increasing plasma concentration, according to metabolic syndrome status (light gray, no metabolic syndrome; dark gray, metabolic syndrome). For trends within metabolic syndrome categories, P < 0.0001 for decreasing prevalence of insulin resistance across adiponectin tertiles in both groups; for resistin (middle) and TNF-α (bottom), all P < 0.01 for trends in insulin resistance prevalence across tertiles in both groups.
The prevalence of insulin resistance by level of adipokines and stratified by presence or absence of the metabolic syndrome is shown in Fig. 1 (right hand column). In each panel, the presence of metabolic syndrome was associated with a higher prevalence of insulin resistance. The prevalence of insulin resistance decreased with increasing level of adiponectin in individual with or without metabolic syndrome (P < 0.0001 for trends in both groups). In the resistin and TNFα panels, the prevalence of insulin resistance increased with increasing adipokine level in people with or without metabolic syndrome (P < 0.01 for trends in all categories). For all three adipokines, these associations were similar comparing groups with vs. without metabolic syndrome (interaction P > 0.3).
Results of multivariable logistic regression modeling testing the hypothesis that adipokines levels are associated with insulin resistance are shown in Table 2. In individual age-sex adjusted models, metabolic syndrome and levels of each adipokine were significantly associated with insulin resistance. Further adjustment for BMI substantially decreased the magnitude of the insulin resistance association for metabolic syndrome (25% decrease in the size of the β-coefficient), resistin (52% decrease), and TNFα (23% decrease). The insulin resistance-adiponectin association appeared less affected by BMI adjustment (14% decrease). In models including the metabolic syndrome plus one adipokine, each adipokine remained significantly associated with insulin resistance, even when further adjusted for BMI. In the age-sex adjusted full model including metabolic syndrome and all three adipokines, all showed significant association with insulin resistance. When this last model was further adjusted for BMI, resistin lost significant association with insulin resistance, whereas the associations with metabolic syndrome, adiponectin, and TNFα all remained statistically significant.
Table 2.
Logistic regression models predicting insulin
| Adjusted for sex and age
|
Adjusted for sex, age, and BMI
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Metabolic syndrome | Adiponectin | Resistin | TNFα | Metabolic syndrome | Adiponectin | Resistin | TNFα | |
| (yes vs. no)a | (per tertile)b | (per tertile) | (per tertile) | (yes vs. no) | (per tertile) | (per tertile) | (per tertile) | |
| Individual models (metabolic syndrome or adipokines)c | Individual models (metabolic syndrome or adipokines)c | |||||||
| Coefficientd | 2.26 | 1.69 | ||||||
| OR (95% CI) | 9.62 (7.62–12.15) | 5.43 (4.23–6.98) | ||||||
| P value | <0.0001 | <0.0001 | ||||||
| Coefficient | −1.01 | −0.87 | ||||||
| OR (95% CI) | 0.36 (0.32–0.42) | 0.42 (0.36–0.49) | ||||||
| P value | <0.0001 | <0.0001 | ||||||
| Coefficient | 0.31 | 0.15 | ||||||
| OR (95% CI) | 1.36 (1.21–1.53) | 1.16 (1.02–1.33) | ||||||
| P value | <0.0001 | 0.03 | ||||||
| Coefficient | 0.35 | 0.27 | ||||||
| OR (95% CI) | 1.42 (1.25–1.61) | 1.32 (1.14–1.52) | ||||||
| P value | <0.0001 | 0.0002 | ||||||
| Metabolic syndrome + one adipokine model | Metabolic syndrome + one adipokine model | |||||||
| Coefficient | 2.00 | −0.75 | 1.49 | −0.72 | ||||
| OR (95% CI) | 7.41 (5.82–9.43) | 0.47 (0.41–0.55) | 4.42 (3.41–5.72) | 0.49 (0.42–0.57) | ||||
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Coefficient | 2.25 | 0.26 | 1.69 | 0.16 | ||||
| OR (95% CI) | 9.49 (7.51–12.00) | 1.30 (1.14–1.48) | 5.44 (4.23–6.99) | 1.17 (1.02–1.35) | ||||
| P value | <0.0001 | 0.0002 | <0.0001 | 0.03 | ||||
| Coefficient | 2.22 | 0.28 | 1.67 | 0.24 | ||||
| OR (95% CI) | 9.24 (7.22–11.81) | 1.32 (1.15–1.52) | 5.32 (4.09–6.92) | 1.27 (1.09–1.48) | ||||
| P value | <0.0001 | 0.001 | 0.002 | |||||
| Full model | Full model | |||||||
| Coefficient | 1.97 | −0.73 | 0.23 | 0.19 | 1.48 | −0.70 | 0.13 | 0.17 |
| OR (95% CI) | 7.19 (5.57–9.27) | 0.48 (0.41–0.56) | 1.25 (1.09–1.45) | 1.21 (1.04–1.40) | 4.39 (3.35–5.76) | 0.50 (0.42–0.58) | 1.14 (0.98–1.33) | 1.19 (1.01–1.39) |
| P value | <0.0001 | <0.0001 | 0.002 | 0.01 | <0.0001 | <0.0001 | 0.09 | 0.03 |
Age, sex, and BMI were modeled as continuously distributed covariates.
Odds ratios (OR) for metabolic syndrome are for subjects with metabolic syndrome relative to those without.
Adipokine levels are modeled as ordinal variables (0, 1, 2) with regression coefficients and associated odds ratios (OR) and 95% confidence intervals (CI) expressed as risk per tertile increase. Age-sex adjusted mean adipokine values for each tertile were: adiponectin = 4.8, 8.9, and 15.8 μg/ml; resistin = 8.5, 12.6, and 19.7 ng/ml; TNFα = 0.78, 1.2 and 2.1 pg/ml.
Individuals models are using each variable (metabolic syndrome or each adipokine levels by tertile) by itself to predict prevalence of insulin resistance, adjusted for age and sex, or age, sex, and BMI according to the models.
Coefficients are the β-coefficients for each variable when included in the multivariable logistic regression analysis.
Subsidiary analyses
By definition, when using the 90th percentile, the prevalence of insulin resistance was lower overall and in adipokine categories, but the significance of associations of adipokines and insulin resistance were the same as in the main analysis (see online supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://jcem.endojournals.org).
We also conducted the analysis using IFG (40% of the study sample) vs. normal glucose tolerance (NGT) to define prediabetes (see online supplemental Table 2 for complete results). Individuals with IFG were older (62 vs. 59 yr) and more likely to be male (58 vs. 37%). IFG presented lower adiponectin levels and higher resistin and TNFα levels (all P < 0.01). The trends in HOMA-IR and in prevalence of insulin resistance across tertiles of adipokines were similar in individuals with NGT or with IFG.
We also conducted all multivariable models analysis using waist circumference to adjust for adiposity. Substituting waist circumference for BMI in all the models did not alter the results (see online supplemental Table 3). We also tested the presence of a sex interaction with each adipokine on risk of insulin resistance in the BMI-adjusted individual models; no significant interaction was detected (P = 0.09–0.5 for sex by adipokine interaction terms). Looking at the individual adipokine models, we further adjusted for hypertensive and dyslipidemic medications. The results remained very similar (adiponectin, P < 0.0001; resistin, P = 0.05; TNFα, P = 0.0002) once adjusted for age-sex-BMI plus medications for both hypertension and dyslipidemia.
Discussion
We have demonstrated that adverse levels of the adipokines adiponectin, resistin, and TNFα are associated with insulin resistance in a community-based cohort. This association persisted, albeit attenuated, after adjustment for BMI. Elevated levels of resistin and TNFα had an additive effect with metabolic syndrome, with higher levels of insulin resistance in those with metabolic syndrome at every level of adipokine. Metabolic syndrome appeared to modify the association of adiponectin with insulin resistance. Multivariable logistic regression analysis showed that metabolic syndrome and each of adiponectin, resistin, and TNFα made independent contributions to the presence of insulin resistance. By use of a large, unselected population sample and simultaneous measurement of type 2 diabetes risk factors and several major adipokines, we extend the current literature to show that multiple adipose-tissue derived signaling molecules are correlates of insulin resistance. Furthermore, these associations are not explained by the presence of other risk factors for type 2 diabetes and can be seen in groups at either low or high future risk of diabetes.
Our findings are consistent with the prior literature on adiponectin. Cross-sectional studies have shown that adiponectin was positively associated with insulin sensitivity measured by hyperinsulinemic clamp (4,27), whereas prospective studies demonstrated that hypoadiponectinemia was associated with an increase in insulin resistance (28) and higher risk of developing diabetes (5,6). Our data also confirm the association between low adiponectin and the presence of metabolic syndrome (29). We add to current knowledge by showing that adiponectin was associated with insulin resistance either in the presence or absence of elevated diabetes risk as embodied in metabolic syndrome. Furthermore, in individuals with metabolic syndrome, adiponectin seemed to have a stronger negative correlation with HOMA than in individuals without the metabolic syndrome. As we observed in Fig. 1 (right hand panel), the presence of metabolic syndrome was associated with a very high risk of insulin resistance, but being in the highest tertile of adiponectin decreased this risk by more than half, compared with the lowest tertile of adiponectinemia (prevalence of insulin resistance of 60.1% decreased to 27.3%). The multivariable models demonstrated that adiponectin was associated with insulin resistance, even when age, sex, BMI, metabolic syndrome, and the other adipokines were accounted for. Each increase in tertile of adiponectin is associated with a lower prevalence of insulin resistance by half (odds ratio 0.50). Those observations suggest that low adiponectin levels are related to insulin resistance above the usual clinical markers of prediabetes.
We demonstrated that TNFα is associated with insulin resistance in the community. TNFα has been suspected to be involved in the pathophysiology of obesity-induced insulin resistance based on animal models (30) and increased TNFα expression in obese human adipose tissue (14). Subsequently, conflicting results were reported relating circulating TNFα to insulin resistance in humans (15,16). The small number of subjects in those studies might have limited their power to detect an association. Also, previous immunoassays might not have been sensitive enough to measure adequately the usual low circulating TNFα levels in healthy individuals. We used a high-sensitive assay to measure TNFα in the very low range (limit of detection as low as 0.06 pg/ml), which might have helped to detect the association that others have not seen. Our results are in concordance with smaller studies reporting that TNFα was related to insulin resistance measured by HOMA-IR (18) or insulin clamp (17,31), and to metabolic syndrome status (29). We added to the current knowledge by demonstrating that TNFα remained significantly associated with insulin resistance above metabolic syndrome status and even when adjusted for adiponectin, resistin, and BMI.
The association between resistin and insulin resistance in humans has been controversial. Many studies did not find an association between resistin and measures of insulin resistance (10,11), whereas others found a relationship that was attenuated when adjusting for adiposity (8,32). A lot of those studies included small numbers of participants, which limited their power. Our finding is in accordance with small studies showing a significant association between resistin and HOMA-IR (33,34) and one recent large study in the general Japanese population (9). We extend these data by showing that elevated levels of resistin are associated with not only insulin resistance in the community but also remain associated with insulin resistance, even after accounting for metabolic syndrome and levels of adiponectin and TNFα. Only with simultaneous adjustment for all risk factors and BMI does the association weaken to not significant, although it can be argued that these fully specified models are overadjusted for adiposity because they include terms both for BMI and waist circumference (as part of the metabolic syndrome phenotype).
In all the models, having metabolic syndrome was associated with an increased prevalence of insulin resistance. Nevertheless, the crude data showed that more than half of the individuals with metabolic syndrome were not classified as insulin resistance. We hypothesized that this discrepancy might be partly explained by variability in adipokines. The full model suggests that even when identifying individuals at risk by the clinical definition of metabolic syndrome, the variability of insulin resistance can be explained by both antiinflammatory (adiponectin) and proinflammatory (TNFα) pathways. Some authors have suggested that a paracrine loop exists in the adipose tissue by demonstrating that adipocytes cocultured with macrophages up-regulate proinflammatory cytokines (TNFα) and in counterpart, those proinflammatory proteins down-regulate adiponectin expression (35). Some have proposed that the interrelations between the metabolic and immune pathways could be explained by resistin (13), whereas others have suggested TNF as the missing link (36). In adipose tissue, macrophages appear to be the main source of both resistin (37) and TNFα (35). Macrophages are primary mediators of immune response and represent up to 40% of infiltrating cells in adipose tissue of obese human (38). Also, both resistin and TNFα have been shown to impair insulin signaling in adipocytes and skeletal muscle (13,36). Our findings argue in favor of the hypothesis that resistin and TNFα are both part of the complex system of adiposity-induced proinflammatory pathways that contribute to insulin resistance.
Strengths of this study include a large sample size from a representative community. Clinical measurements were taken under standardized protocol and biomarkers were measured using assays with good precision. We had adequate sample size to observe effects of the adipokines in tertiles and subgroups with or without the metabolic syndrome. Limitations of the study include the fact that we used surrogate markers for insulin resistance (HOMA-IR) and prediabetes (metabolic syndrome or IFG). Also, we measured total adiponectin and not high molecular weight fraction, which has been proposed to have a stronger correlation with insulin resistance, compared with total adiponectin (39). All these limitations would have reduced our chance to observe significant associations. Furthermore, the cross-sectional design of our analyses does not allow us to make conclusions regarding causality. Finally, the Framingham cohort is largely white and middle-aged to elderly, so findings may have limited generalizability to other ethnic and age groups.
Conclusion
Adverse levels of several key adipokines representing different adipose tissue cellular components are associated with insulin resistance in free-living people at low or high risk of future type 2 diabetes. The joint combination of metabolic syndrome and low adiponectin levels is associated with especially high levels of insulin resistance. The information gained from adipokines levels explained part of the insulin resistance variability above metabolic syndrome, but a large part of the variability of this association remains unexplained. The observations made in the multivariable models points toward the complexity of the anti- and proinflammatory pathways related to excess adiposity in free-living humans and reinforce the importance of analyzing several adipokines in analyses of the physiopathology of insulin resistance in humans.
Supplementary Material
Acknowledgments
The authors thank Peter Shrader, M.S., for outstanding technical support.
Footnotes
This work was supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (Contract N01-HC-25195), the National Institutes of Health, National Center for Research Resources, General Clinical Research Centers Program (Grant M01-RR-01066), and a Career Development Award from the American Diabetes Association. J.B.M. was further supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant K24 DK080140. M.F.-H. was supported by the Centre de Recherche Medicale de l’Universite de Sherbrooke and a Canadian Institute of Health Research Fellowships Health Professional Award. J.B.M. currently has research grants from GlaxoSmithKline and Sanofi-Aventis and has consulting agreements with GlaxoSmithKline, Sanofi-Aventis, Interleukin Genetics, Kalypsis, and Outcomes Science.
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 20, 2008
Abbreviations: BMI, Body mass index; FPG, fasting plasma glucose; HOMA-IR, homeostasis model insulin resistance; IFG, impaired fasting glucose; NGT, normal glucose tolerance.
References
- James PT, Leach R, Kalamara E, Shayeghi M 2001 The worldwide obesity epidemic. Obes Res 9:228S–233S [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS 2004 Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89:2548–2556 [DOI] [PubMed] [Google Scholar]
- Hanley AJ, Bowden D, Wagenknecht LE, Balasubramanyam A, Langfeld C, Saad MF, Rotter JI, Guo X, Chen YD, Bryer-Ash M, Norris JM, Haffner SM 2007 Associations of adiponectin with body fat distribution and insulin sensitivity in nondiabetic Hispanics and African-Americans. J Clin Endocrinol Metab 92:2665–2671 [DOI] [PubMed] [Google Scholar]
- Tschritter O, Fritsche A, Thamer C, Haap M, Shirkavand F, Rahe S, Staiger H, Maerker E, Haring H, Stumvoll M 2003 Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes 52:239–243 [DOI] [PubMed] [Google Scholar]
- Duncan BB, Schmidt MI, Pankow JS, Bang H, Couper D, Ballantyne CM, Hoogeveen RC, Heiss G 2004 Adiponectin and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 53:2473–2478 [DOI] [PubMed] [Google Scholar]
- Snijder MB, Heine RJ, Seidell JC, Bouter LM, Stehouwer CD, Nijpels G, Funahashi T, Matsuzawa Y, Shimomura I, Dekker JM 2006 Associations of adiponectin levels with incident impaired glucose metabolism and type 2 diabetes in older men and women: the Hoorn study. Diabetes Care 29:2498–2503 [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA 2001 The hormone resistin links obesity to diabetes. Nature 409:307–312 [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Rood J, Janderova L, Albu JB, Kelley DE, Ravussin E, Smith SR 2004 Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J Clin Endocrinol Metab 89:1844–1848 [DOI] [PubMed] [Google Scholar]
- Osawa H, Tabara Y, Kawamoto R, Ohashi J, Ochi M, Onuma H, Nishida W, Yamada K, Nakura J, Kohara K, Miki T, Makino H 2007 Plasma resistin, associated with single nucleotide polymorphism-420, is correlated with insulin resistance, lower HDL cholesterol, and high-sensitivity C-reactive protein in the Japanese general population. Diabetes Care 30:1501–1506 [DOI] [PubMed] [Google Scholar]
- Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, Orlova C, Mantzoros CS 2003 Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab 88:4848–4856 [DOI] [PubMed] [Google Scholar]
- Vozarova de Court, Degawa-Yamauchi M, Considine RV, Tataranni PA 2004 High serum resistin is associated with an increase in adiposity but not a worsening of insulin resistance in Pima Indians. Diabetes 53:1279–1284 [DOI] [PubMed] [Google Scholar]
- Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A 2006 Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 49:744–747 [DOI] [PubMed] [Google Scholar]
- McTernan PG, Kusminski CM, Kumar S 2006 Resistin. Curr Opin Lipidol 17:170–175 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM 1995 Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J Clin Invest 95:2409–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush EC, Plank LD, Yajnik CS 2007 Interleukin-6, tumour necrosis factor-α and insulin relationships to body composition, metabolism and resting energy expenditure in a migrant Asian Indian population. Clin Endocrinol (Oxf) 66:684–690 [DOI] [PubMed] [Google Scholar]
- Zavaroni I, Numeroso F, Dongiovanni P, Ardigo D, Valenti L, Fracanzani A, Valtuena S, Delsignore R, Fargion S, Reaven GM 2003 What is the contribution of differences in three measures of tumor necrosis factor-α activity to insulin resistance in healthy volunteers? Metabolism 52:1593–1596 [DOI] [PubMed] [Google Scholar]
- Behre CJ, Fagerberg B, Hulten LM, Hulthe J 2005 The reciprocal association of adipocytokines with insulin resistance and C-reactive protein in clinically healthy men. Metabolism 54:439–444 [DOI] [PubMed] [Google Scholar]
- Zinman B, Hanley AJ, Harris SB, Kwan J, Fantus IG 1999 Circulating tumor necrosis factor-α concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab 84:272–278 [DOI] [PubMed] [Google Scholar]
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP 1979 An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 110:281–290 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Balkau B, Charles MA 1999 Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med 16:442–443 [DOI] [PubMed] [Google Scholar]
- Meigs JB, Mittleman MA, Nathan DM, Tofler GH, Singer DE, Murphy-Sheehy PM, Lipinska I, D'Agostino RB, Wilson PW 2000 Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the Framingham Offspring Study. JAMA 283:221–228 [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith Jr SC, Spertus JA, Costa F, American Heart Association, National Heart, Lung and Blood Institute 2005 Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752 [DOI] [PubMed] [Google Scholar]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus 2003 Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 26:S5–S20 [DOI] [PubMed] [Google Scholar]
- Rutter MK, Wilson PW, Sullivan L, D'Agostino RB, Fox CS, Meigs JB 2008 Use of alternative thresholds defining insulin resistance to predict incident type 2 diabetes and cardiovascular disease. Circulation 117:1003–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino Sr RB 2007 Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med 167:1068–1074 [DOI] [PubMed] [Google Scholar]
- Kantartzis K, Fritsche A, Tschritter O, Thamer C, Haap M, Schafer S, Stumvoll M, Haring HU, Stefan N 2005 The association between plasma adiponectin and insulin sensitivity in humans depends on obesity. Obes Res 13:1683–1691 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hirose H, Saito I, Nishikai K, Saruta T 2004 Adiponectin, an adipocyte-derived protein, predicts future insulin resistance: two-year follow-up study in Japanese population. J Clin Endocrinol Metab 89:87–90 [DOI] [PubMed] [Google Scholar]
- Matsushita K, Yatsuya H, Tamakoshi K, Wada K, Otsuka R, Takefuji S, Sugiura K, Kondo T, Murohara T, Toyoshima H 2006 Comparison of circulating adiponectin and proinflammatory markers regarding their association with metabolic syndrome in Japanese men. Arterioscler Thromb Vasc Biol 26:871–876 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM 1993 Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259:87–91 [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Pipek R, Mandarino LJ, DeFronzo RA 2003 Tumor necrosis factor α and insulin resistance in obese type 2 diabetic patients. Int J Obes Relat Metab Disord 27:88–94 [DOI] [PubMed] [Google Scholar]
- Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, Zhu Q, Considine RV 2003 Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab 88:5452–5455 [DOI] [PubMed] [Google Scholar]
- Ohmori R, Momiyama Y, Kato R, Taniguchi H, Ogura M, Ayaori M, Nakamura H, Ohsuzu F 2005 Associations between serum resistin levels and insulin resistance, inflammation, and coronary artery disease. J Am Coll Cardiol 46:379–380 [DOI] [PubMed] [Google Scholar]
- Silha JV, Krsek M, Skrha JV, Sucharda P, Nyomba BL, Murphy LJ 2003 Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur J Endocrinol 149:331–335 [DOI] [PubMed] [Google Scholar]
- Suganami T, Nishida J, Ogawa Y 2005 A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor α. Arterioscler Thromb Vasc Biol 25:2062–2068 [DOI] [PubMed] [Google Scholar]
- Ruan H, Lodish HF 2003 Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-α. Cytokine Growth Factor Rev 14:447–455 [DOI] [PubMed] [Google Scholar]
- Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA 2003 Resistin is expressed in human macrophages and directly regulated by PPARγ activators. Biochem Biophys Res Commun 300:472–476 [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW 2003 Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T 2006 Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care 29:1357–1362 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.