Abstract
BACKGROUND
The identification of polymorphisms associated with a disease can help to elucidate its pathogenesis, and this knowledge can be used to improve prognosis for women with a particular disorder, such as polycystic ovary syndrome (PCOS). Since an altered response to ovarian stimulation is also a characteristic of the disease, further knowledge about its aetiology could help in defining the parameters that determine the response of an individual to ovarian stimulation.
METHODS
PubMed and EMBASE databases were systematically searched for gene association studies published until the end of August 2007, using search criteria relevant to PCOS and ovarian response to stimulation. Data from additional papers identified through hand searches were also included; 139 publications were reviewed.
RESULTS
Several genes involved in ovarian function and metabolism are associated with increased susceptibility to PCOS, but none is strong enough to correlate alone with susceptibility to the disease, or response to therapy. A single-nucleotide polymorphism in exon 10 of the FSH receptor (FSHR) gene, FSHR p.N680S, was consistently identified as having a significant association with ovarian response to FSH.
CONCLUSIONS
No consistent association between gene polymorphism and PCOS could be identified. The FSHR gene may play a significant role in the success of ovarian stimulation, and can be used as a marker to predict differences in FSHR function and ovarian response to FSH. Genotyping the FSHR p.N680S polymorphism may provide a means of identifying a population of poor responders before in vitro fertilization procedures are initiated.
Keywords: female reproduction, genetic polymorphisms, polycystic ovary syndrome
Introduction
Genetic studies contribute towards our understanding of disease pathogenesis and hold the promise of improving our ability to individualize treatment for patients. In this review, we first explain the rationale and methodology of studies that identify gene–disease associations. We then review the extensive body of research aimed at identifying contributing genetic factors in polycystic ovary syndrome (PCOS) and assess genetic studies that have investigated patients' ovarian response to gonadotrophin treatment using assisted reproductive techniques. This provides the first systematically selected summary of published studies about all genes that have so far been investigated in these areas. We then discuss the limitations of such studies and provide recommendations for using this information in the future, with the aim of guiding the design and interpretation of future studies.
Genetic association studies
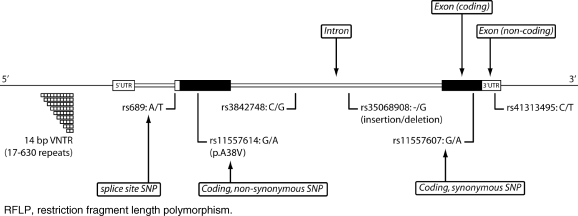
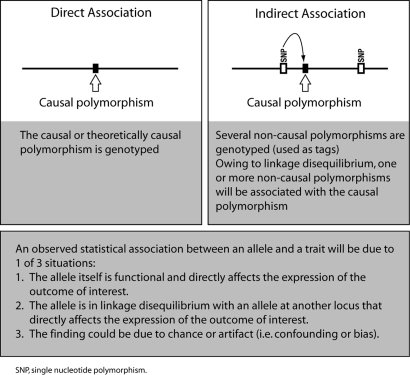
Genetic association studies are used to investigate genes or genetic markers that might be associated with a particular disease phenotype or trait. These studies rely on the identification and characterization of natural variants or polymorphisms in the DNA sequence among individuals. If an association is present, a particular variant will be seen more often than expected by chance in an individual carrying the trait. Variants fall into several categories, depending on where they occur relative to a gene. First, variants can occur within or outside a gene. Those that occur within a gene may occur within an exon, an intron or regulatory regions. Regulatory regions control gene transcription and include the promoter region upstream of the gene and the 3′-untranslated region downstream of the protein-coding region (Fig. 1). Variants within a gene can be functional or silent. Functional variation within a gene can be the direct cause of a phenotype abnormality or may increase susceptibility to a disease. Functional variants in coding regions can change the protein sequence; in non-coding regions they may have effects on RNA transcription and processing (Kim et al., 2005; Wang et al., 2005). Silent variants occur in protein-coding DNA but do not change the sequence of a gene product. In the majority of cases, variation that occurs outside a gene is used as a tag to identify nearby functional variation within a gene (Fig. 2). Such analyses are based on a chromosomal property called linkage disequilibrium. Linkage disequilibrium refers to the observation that in the general population, two DNA variants that are located close to each other tend to be observed together more frequently than two variants that are located further apart. Variants may be single-base changes, known as single-nucleotide polymorphisms (SNPs), or they may occur as insertions or deletions of one base or more.
Figure 1:
Graphic representation of types of genetic variants, showing insertion/deletion (ins/del) polymorphisms, both coding and non-coding SNPs, and repeat polymorphisms such as tandem repeats or VNTR. Variants are shown occurring within a gene (in this example the INS gene), but can also occur outside of genes. Other types of genetic variations that affect larger regions, such as copy number variations, are not shown. SNP, single-nucleotide polymorphism; VNTR, variable number of tandem repeats.
Figure 2:
Principles of genetic association, and possible explanations for an observed association.
Until recently, the high cost of testing for genetic variation has meant that most analyses have concentrated on the study of a limited number of functional genetic variants, primarily SNPs, in specific genes. Candidate genes for genotyping are selected according to their function: they encode proteins that are thought to have a role in the disease or response to treatment. Variants within the candidate genes are most often selected because they occur within exons and would result in a change in amino acid sequence in the protein. Alternatively, they are located in non-coding regions, but change a transcription factor-binding site or influence splicing efficiency, affecting the expression of a protein. The main advantage of the candidate SNP approach is that such studies are affordable, as only a limited number of variants are studied and a relatively small sample size can be used. In the context of genome-wide analyses, the main advantages of using SNPs are their abundance in the genome, and the possibility of conducting genotyping in a high-throughput manner. To date, a wealth of results has been obtained from studies addressing the problem of potential associations between genetic polymorphisms and PCOS or ovarian response to gonadotrophins.
Materials and Methods
We systematically searched the PubMed and EMBASE databases for gene association studies published until the end of August 2007, using the terms ‘PCOS’, ‘polycystic and (ovary or ovaries)’, ‘ovarian and response’, ‘OHSS’ or ‘ovarian and hyperstimulation’, combined with ‘polymorphism or polymorphisms’ or ‘mutation or mutations’. The search was not limited by language of publication. Two authors (B.C.J.M.F. and M.S.) then selected relevant studies using the following criteria: more than one patient, inclusion of a control group and with at least the abstract written in English. Also included were additional papers identified through hand searches carried out by the same authors.
All results of selected studies are comprehensively summarized in functional group-specific tables by gene of interest, with a brief description in the text. As the frequency of genetic differences varies between ethnic or geographic populations, each study is based on a specific patient population to minimize heterogeneity. Therefore, the tables also include critical details that give information about the context (ethnic background) and likely strength (based on sample size) of the study, to provide a source of reference.
Results
Polycystic ovary syndrome
PCOS affects about 1 in 10 women of reproductive age, and is the most common endocrine condition in this group. The syndrome is associated with multiple endocrinological and metabolic abnormalities, with hyperandrogenaemia and anovulation as the central hallmarks (The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004a, b). About 80% of women with PCOS have abnormal menstrual cycles (Conway et al., 1989), many are anovulatory (Laven et al., 2002) and, in those who manage to conceive successfully after treatment, there is an increased risk of complications during pregnancy as well as neonatal complications (Boomsma et al., 2006).
Twin and familial studies suggest that there is a genetic component to PCOS, with a polygenic pattern of inheritance (Jahanfar and Eden, 1996; Diamanti-Kandarakis and Piperi, 2005; Fratantonio et al., 2005). There is also a strong environmental component (e.g. diet and exercise) (Abbott et al., 2002). A series of familial studies conducted in the USA have demonstrated a strong linkage between susceptibility to PCOS and the dinucleotide marker D19S884 in the chromosomal region 19p13.2 (Urbanek et al., 2000a, 2005, 2003; Stewart et al., 2006). The identity of the gene or genetic element responsible for this effect is not yet known. As complex traits such as PCOS result from the interaction of several genetic variations with environmental factors, individual genetic variations have a modest effect that is more difficult to detect than for monogenic traits.
The search for PCOS susceptibility genes has focused mainly on genes involved in sex hormone regulation, insulin sensitivity, cardiovascular risk or steroid biosynthesis and detoxification (Ehrmann, 2005; Escobar-Morreale et al., 2005; Fratantonio et al., 2005). The results from these studies are summarized in Tables I–IV and are described below.
Table I.
Polymorphisms of genes encoding sex hormones and hormone regulators.
| Gene (locus, protein name and its function) | Variant |
Association with susceptibility |
Phenotype | ||
|---|---|---|---|---|---|
| Name | dbSNP ID | Positive (number of cases, number of controls) | Negative (number of cases, number of controls) | ||
| ADRB2 (5q31-q32, β2 adrenic receptor: calcium signalling, may play a role in insulin resistance) | p.R16G | rs1042713 | Japanese women (59, 97) (Kurabayashi et al., 2006) | ||
| p.Q27E | rs1042714 | Japanese women (59, 97) (Kurabayashi et al., 2006) | |||
| ADRB3 (8p12-p11.2, β3 adrenic receptor: hormone receptor) | p.W64R | rs4994 | Chilean women (106, 82) (Perez-Bravo et al., 2005) | Chilean women—triglyceride levels (106, 82) (Perez-Bravo et al., 2005) | |
| Japanese women (59, 97) (Kurabayashi et al., 2006) | |||||
| AR (Xq11.2-q12, androgen receptor: hormone receptor) | CAG repeat | rs5902610, rs25885096 | Australian women (122, 83) (Hickey et al., 2002) | Finnish women (106, 112) (Jaaskelainen et al., 2005) | Asian women—T levels (91, 12) (Mifsud et al., 2000) |
| DRD3 (3q13.3, dopamine D3 receptor: regulates sex hormone secretion) | MscI RFLP (p.S9G) | rs6280 | Caucasian women (152, 96) (Kahsar-Miller et al., 1999) | ||
| FSHB (11p13, follicle-stimulating hormone β: sex hormone) | AccI RFLP (1736T/C) | rs6169 | Chinese women (135, 105) (Tong et al., 2000) | ||
| FSHR (2p21-p16, follicle-stimulating hormone receptor: hormone receptor) | p.A307T | rs6165 | Japanese women (18, 258) (Sudo et al., 2002) | Chinese women (124, 236) (Tong et al., 2001) Caucasian women (93, 51) (Conway et al., 1999) | |
| p.N680S | rs6166 | Japanese women (18, 258) (Sudo et al., 2002) | Chinese women (124, 236) (Tong et al., 2001) | ||
| Caucasian women (93, 51) (Conway et al., 1999) | |||||
| FST (5q11.2, follistatin: inhibits follicle-stimulating hormone release) | 951G/A | rs1746136 | Spanish women (34, 15) (Calvo et al., 2001) | ||
| 343xT/A | rs722910 | Families, mixed descent (249a) (Urbanek et al., 2000b) | |||
| D5S474, D5S822, D5S628 | Families, mixed descent (249a) (Urbanek et al., 2000b) | ||||
| rs3797297 | Australian women (173, 107) (Jones et al., 2007) | Australian women—FAI and SHBG levels (173, 107) (Jones et al., 2007) | |||
| rs11745088 | Australian women (173, 107) (Jones et al., 2007) | Australian women—DHES-S level (173, 107) (Jones et al., 2007) | |||
| rs1423560, rs3203788, rs1062809, rs1127760, rs1127761 | Australian women (173, 107) (Jones et al., 2007) | ||||
| GNAS (20q13.32, guanine nucleotide-binding protein G(s) subunit alpha isoform: GPCR signal transduction) | 393T/C | rs7121 | German caucasian women (278, 209) (Hahn et al., 2006) | German caucasian women—BMI/weight, insulin resistance (278, 209) (Hahn et al., 2006) | |
| LEPR (1p31, leptin receptor: regulates hormone activity) | p.K109R | rs1137100 | Finnish women (38, 122) (Oksanen et al., 2000) | ||
| p.R223Q | rs1137101 | Finnish women (38, 122) (Oksanen et al., 2000) | |||
| p.N656K | rs8179183 | Finnish women (38, 122) (Oksanen et al., 2000) | |||
| ins/del CTTTA in 3′-UTR | Finnish women (38, 122) (Oksanen et al., 2000) | ||||
| LHB (19q13.32, luteinizing hormone: sex hormone) | p.W8R (p.W28R) | rs1800447 | Turkish women (30, 30) (Elter et al., 1999), | ||
| UK women (153, 212) (Rajkhowa et al., 1995) | |||||
| p.I15T (p.I35T) | rs3434826 | Turkish women (30, 30) (Elter et al., 1999), UK women (153, 212) (Rajkhowa et al., 1995) | |||
| SHBG (17p13-p12, sex hormone-binding globulin: mediates hormonal activity) | TAAAA repeat | rs35785886 | Greek women (185, 324) (Xita et al., 2003) | Slovenian women (123, 110) (Ferk et al., 2007) | Greek women—FAI and SHBG levels (185, 324) (Xita et al., 2003) |
| Sloven women—SHBG levels (123, 110) (Ferk et al., 2007) | |||||
| p.D327 N | rs6259 | Czech Republic women (248, 109) (Bendlova et al., 2007) | |||
| Slovenian women (123, 110) (Ferk et al., 2007) | |||||
The list (ordered alphabetically) shows genes that have been investigated for their role in polycystic ovary syndrome. Studies showing positive or no associations of these genes with disease susceptibility and positive associations with phenotype (clinical characteristics of the condition, e.g. endocrinological abnormalities) are presented. Genes are listed alphabetically. aNumber of PCOS families. BMI, body mass index; PCOS, polycystic ovary syndrome; RFLP, restriction fragment length polymorphism.
Table IV.
Polymorphisms of genes encoding proteins involved in tissue remodelling or inflammatory processes.
| Gene (locus, protein name and its function) | Variant |
Association with susceptibility |
Phenotype | ||
|---|---|---|---|---|---|
| Name | dbSNP ID | Positive (number of cases, number of controls) | Negative (number of cases, number of controls) | ||
| Genes involved in tissue remodeling | |||||
| MMP1 (11q22.3, matrix metalloproteinase-1: endometrial lesion formation) | 1607ins/delG | rs11292517 | Caucasian women (62, 94) (Walch et al., 2005b) | ||
| Inflammatory mediators | |||||
| HLA-A (6p21.3, major histocompatibility complex class I, A: antigen processing and presentation) | A11 | Japanese women (56, 237) (Kaibe et al., 2006) | |||
| HLA-B (6p21.3, major histocompatibility complex class I, B: antigen processing and presentation) | B39 | Protective effect in Japanese women (56, 237) (Kaibe et al., 2006) | |||
| HLA-C (6p21.3, major histocompatibility complex class I, C: antigen processing and presentation) | All alleles | Japanese women (56, 237) (Kaibe et al., 2006) | |||
| HLA-DRB1 (6p21.3, major histocompatibility complex class I, DR beta 1: antigen processing and presentation) | *0403 | Japanese women (68, 292) (Kaibe et al., 2006) | |||
| HFE (6p21.3, haemochromatosis protein (MHC class I family): antigen processing and presentation, iron binding) | p.H63D | rs1799945 | Spanish women (78, 43) (Botella-Carretero et al., 2006) | ||
| p.C282Y | rs1800562 | Spanish women (78, 43) (Botella-Carretero et al., 2006) | |||
| IL1A (2q14, interleukin-1 alpha: cytokine) | −889C/T | rs1800587 | Austrian caucasian women (105, 102) (Kolbus et al., 2007) | Austrian caucasian women—FSH serum level, LH/FSH ratio (105, 102) (Kolbus et al., 2007) | |
| IL1B (2q14, interleukin-1 beta: cytokine) | −511C/T, exon 5 | rs16944 | Austrian caucasian women (105, 102) (Kolbus et al., 2007) | ||
| 3953G/A (exon5 TaqI RFLP, E1/E2) | rs1143634 | Austrian caucasian women (105, 102) (Kolbus et al., 2007) | |||
| IL6 (7p21, interleukin-6: cytokine) | −597G/A | rs1800797 | Spanish caucasian women (85, 25) (Villuendas et al., 2002) | ||
| −572G/C | rs1800796 | Spanish caucasian women (85, 25) (Villuendas et al., 2002) | |||
| −174G/C | rs1800795 | Spanish caucasian women (85, 25) (Villuendas et al., 2002) | Austrian caucasian women (62, 94) (Walch et al., 2004) | Austrian caucasian women—BMI, T levels, OGTT results (62, 94) (Walch et al., 2004) | |
| German women—androstendione levels (50, 0) (Mohlig et al., 2004) | |||||
| IL6R (1q22, interleukin-6 repector alpha: cytokine receptor) | CA repeat | Spanish women (88, 45) (Escobar-Morreale et al., 2003) | |||
| IL6ST (5q11.2, gp130, interleukin-6 repector beta: cytokine receptor) | p.G148R | rs3729960 | Spanish women (88, 45) (Escobar-Morreale et al., 2003) | ||
| TNF (6p21.3, tumour necrosis factor alpha: cytokine) | −850C/T (−857C/T) | rs1799724 | Cauasian Finnish women (87, 115) (Korhonen et al., 2002) | Australian women—OGTT results (122, 28) (Milner et al., 1999) | |
| −308G/A | rs1800629 | Chinese women (118, 54) (Mao et al., 2000) | |||
| Australian women (122, 28) (Milner et al., 1999) | |||||
| TNFRSF1B (1p36.3-p36.2, tumour necrosis factor receptor 2: cytokine receptor) | p.M196R | rs1061622 | Spanish women (87, 36) and Italian women (64, 29) (76, 36) (Peral et al., 2002) | Spanish women (87, 36) and Italian women (64, 29)—hyperandrogenism (Peral et al., 2002) | |
| CA repeat | Spanish women (87, 36) and Italian women (64, 29) (76, 36) (Peral et al., 2002) | ||||
| 1663G/A | rs1061624 | Spanish women (87, 36) and Italian women (64, 29) (76, 36) (Peral et al., 2002) | |||
| 1668T/G | rs5030792 | Spanish women (87, 36) and Italian women (64, 29) (76, 36) (Peral et al., 2002) | |||
| 1690T/C | rs3397 | Spanish women (87, 36) and Italian women (64, 29) (76, 36) (Peral et al., 2002) | |||
The list shows genes that have been investigated for their role in polycystic ovary syndrome. Genes are grouped according to their function (and then alphabetically), and studies showing positive or no associations of these genes with disease susceptibility and positive associations with phenotype (clinical characteristics of the condition, e.g. endocrine abnormalities) are presented.
Sex hormones and hormone regulators
Because PCOS is characterized by endocrinological abnormalities, polymorphisms in genes encoding sex hormones or regulators of their activity have been investigated (Table I).
Hormone receptors
Two variants in the gene encoding the β2 adrenergic receptor (ADRB2) were studied in Japanese women: the p.Q27E variant was linked to PCOS susceptibility, but the p.R16G variant was not (Kurabayashi et al., 2006). Within the β3 adrenergic receptor (ADRB3) gene, the p.W64R polymorphism does not appear to confer an increased risk of PCOS (Perez-Bravo et al., 2005; Kurabayashi et al., 2006), although it may affect triglyceride regulation in women with this condition (Perez-Bravo et al., 2005). Conflicting findings have been reported from studies investigating the association between the length of the CAG repeat microsatellite in the androgen receptor (AR) gene and PCOS susceptibility (Hickey et al., 2002; Jaaskelainen et al., 2005); however, short alleles of this microsatellite appear to be associated with increased androgenic activity (Mifsud et al., 2000), and this may lead to PCOS. One study has shown that an AccI restriction fragment length polymorphism (RFLP) in the gene that encodes the beta subunit of the gonadotrophin follicle-stimulating hormone (FSHB) may be associated with PCOS susceptibility (Tong et al., 2000). A larger number of studies have investigated polymorphisms in the gene that encodes the receptor for FSH rather than the gene for the hormone itself. The FSHR gene contains two important SNPs in exon 10, which are in linkage disequilibrium and change two amino acids at positions 307 and 680. However, although the p.N680S genotype influences ovarian response (see section on ovarian response to gonadotrophins), this common polymorphism does not seem to play a major role in PCOS. In Japanese women with PCOS, the S allele was more prevalent than in normal subjects (Sudo et al., 2002), but no differential allelic distribution was found in Caucasian (Conway et al., 1999) or Chinese women (Tong et al., 2001).
Sex hormone-binding globulin
Women with PCOS have subnormal levels of SHBG, and a microsatellite repeat in the promoter of the SHBG gene has been linked to PCOS susceptibility in Greek (Xita et al., 2003) but not in Slovenian (Ferk et al., 2007) women, although in both populations, serum SHBG levels were strongly influenced by the genotype. The p.D327N polymorphism in this gene was not found to influence PCOS susceptibility in studies in Slovenia or the Czech Republic (Bendlova et al., 2007; Ferk et al., 2007).
Regulators of hormone activity
Studies of the following genes that also encode hormones or proteins regulating hormone activity have failed to show an association with PCOS susceptibility: Dopamine D3 receptor (DRD3) (Kahsar-Miller et al., 1999), follistatin (FST) (Urbanek et al., 2000b; Calvo et al., 2001; Jones et al., 2007), guanine nucleotide-binding protein G (s) subunit alpha (GNAS) (Hahn et al., 2006), leptin receptor (LEPR) (Oksanen et al., 2000) and luteinizing hormone (LHB) (Rajkhowa et al., 1995; Elter et al., 1999). However, there was an association between phenotype and two of the SNPs investigated in the FST gene (Jones et al., 2007). Furthermore, although not associated with susceptibility, the genotype distribution of the signal transduction protein gene GNAS was found to be associated with body mass index (BMI) and insulin resistance in women with PCOS (Hahn et al., 2006; Jones et al., 2007).
Steroid metabolism and biosynthesis
Several studies have investigated whether polymorphisms in enzymes involved in the biosynthesis and metabolism of sex steroids confer PCOS susceptibility (Table II).
Table II.
Polymorphisms of genes encoding enzymes involved in steroid metabolism and biosynthesis.
| Gene (locus, protein name and its function) | Variant |
Association with susceptibility |
Phenotype | Treatment response |
||
|---|---|---|---|---|---|---|
| Name | dbSNP ID | Positive (number of cases, number of controls) | Negative (number of cases, number of controls) | |||
| AKR1C3 (17q11-q21, 5 17β-hydroxysteroid dehydrogenase: testosterone biosynthesis, oestrogen metabolism) | −71G/A | rs3763676 | North American women (121, 128) (Qin et al., 2006) | |||
| CYP1A1 (15q22-q24, cytochrome P450 1A1 enzyme: steroid biosynthesis, oestrogen metabolism, Phase I detoxification) | MspI RFLP (6235T/C) (3801T/C) | rs4646903 | South Indian women (180, 72) (Babu et al., 2004) | |||
| CYP11A1 (15q23-q24, cytochrome P450 11A enzyme: steroid biosynthesis) | TTTTA VNTR (D15S520) | Greek women (80, 90) (Diamanti-Kandarakis et al., 2000) | Chinese women— BMI (201, 147) (Hahn et al., 2006; Wang et al., 2006a), | |||
| UK (371, 350) and Finnish women (1589a) (Gaasenbeek et al., 2004) | UK women—T levels (97, 51aPCO, 59) (Gharani et al., 1997) | |||||
| Chinese women (201, 147) (Hahn et al., 2006; Wang et al., 2006a) | ||||||
| UK women (97, 51aPCO, 59) (Gharani et al., 1997) | ||||||
| AAAT repeat (intron 1) | UK (371, 350) and Finnish women (1589a) (Gaasenbeek et al., 2004) | |||||
| CYP11B2 (8q21-q22, aldosterone synthetase: steroid biosynthesis, regulates ovarian renin–angiotensin system) | −344C/T | rs1799998 | Chinese women (92, 60) (Zhao et al., 2003) | |||
| CYP17A1 (10q24.3, 17α-hydroxylase: oestrogen biosynthesis) | MspA1 RFLP (−34T/C) | rs743572 | Polish women (55, 56) (Marszalek et al., 2001) | |||
| Greek women (50, 50) (Diamanti-Kandarakis et al., 1999) | ||||||
| UK women (69, 124) (Techatraisak et al., 1997) | ||||||
| CYP19A1 (15q21.1, aromatase: steroid biosynthesis) | TTTA VNTR (D15S103) | UK women (97, 59) (Gharani et al., 1997) | ||||
| SNP44 | rs12907866 | Spanish women (186, 71) (Petry et al., 2005) | ||||
| SNP50 | rs2414096 | Spanish women—‘PCOS score’ (186, 71) (Petry et al., 2005) | ||||
| SNP60 | rs17601241 | Spanish women (186, 71) (Petry et al., 2005) | ||||
| SNP64 | rs4646 | Spanish women (186, 71) (Petry et al., 2005) | ||||
| EPHX1 (1q42.1, microsomal epoxide hydrolase: detoxification, component of the anti-oestrogen binding site complex) | p.Y113H | rs1051740 | Finnish women (rs1051740, rs2234922 haplotype) (112, 115) (Korhonen et al., 2003b) | |||
| p.H139R | rs2234922 | Finnish women (rs1051740, rs2234922 haplotype) (112, 115) (Korhonen et al., 2003b) | ||||
| GATA6 (18q11.2, GATA binding protein 6: transcription factor) | rs7235350, rs9957475, rs9944560, rs3764962, rs1941083 | Australian women (173, 107) (Jones et al., 2006) | ||||
| GSTM1 (1p13.3, glutathione S-transferase M1: phase 2 detoxification) | Null deletion | South Indian women (180, 72) (Babu et al., 2004) | ||||
| GSTT1 (22q11.23, glutathione S-transferase T1: phase 2 detoxification) | Null deletion | South Indian women (180, 72) (Babu et al., 2004) | ||||
| H6PD (1p36, hexose-6 phosphate dehydrogenase: steroid biosynthesis) | p.R453Q | rs6688832 | Spanish women (116, 76) (San Millan et al., 2005) | UK women (191, 261) (Draper et al., 2006) | Spanish women—basal F and 17OHP levels (116, 76) (San Millan et al., 2005) | |
| HSD11B1 (1q32-q41, 11β-hydroxysteroid dehydrogenase: steroid biosynthesis) | 83557ins/delA | Spanish women (116, 76) (San Millan et al., 2005) | ||||
| UK women (202,263) (Draper et al., 2006) | ||||||
| 83597T/G | rs12086634 | UK women (202, 263) (Draper et al., 2006) | ||||
| HSD17B6 (12q13, 17β-hydroxysteroid dehydrogenase 6: steroid biosynthesis) | rs898611 | Australian women (173, 107) (Jones et al., 2006) | Australian women—fasting insulin and glucose levels, BMI (173, 107) (Jones et al., 2006) | |||
| rs2277339, | Australian women (173, 107) | |||||
| rs10459246 | (Jones et al., 2006) | |||||
| rs7967600 | Australian women (173, 107) (Jones et al., 2006) | Australian women—fasting insulin and glucose levels, BMI (173, 107) (Jones et al., 2006) | ||||
| rs1870673 | Australian women (173, 107) (Jones et al., 2006) | Australian women—BMI, T levels (173, 107) (Jones et al., 2006) | ||||
| rs1227117 | Australian women (173, 107) (Jones et al., 2006) | Australian women—BMI (173, 107) (Jones et al., 2006) | ||||
| SRD5A1 (5p15, 5 α-reductase isoform: steroid biosynthesis) | Haplotype:rs3797179,rs39848 | North American women (287, 187) (Goodarzi et al., 2006) | North American women—hirsutism, mFG score (287, 187) (Goodarzi et al., 2006) | |||
| Haplotype: rs472402, | North American women—hirsutism, mFG score (287, 187) (Goodarzi et al., 2006) | |||||
| rs2677933, | ||||||
| rs248805, | ||||||
| rs3822430, | ||||||
| rs10060745 | ||||||
| SRD5A2 (2p23, 5 α-reductase isoform: | Haplotype: | North American women (287, 187) (Goodarzi et al., 2006) | ||||
| steroid biosynthesis) | rs11889731, | |||||
| rs7571644, | ||||||
| rs12470143, | ||||||
| rs12467911, | ||||||
| rs2300697 | ||||||
| rs11675297, | North American women (287, 187) (Goodarzi et al., 2006) | |||||
| rs2754530 | ||||||
| p.V89L | rs523349 | North American women (287, 187) (Goodarzi et al., 2006) | ||||
The list (ordered alphabetically) shows genes that have been investigated for their role in polycystic ovary syndrome. Studies showing positive or no associations of these genes with disease susceptibility and positive associations with phenotype (clinical characteristics of the condition, e.g. endocrine abnormalities) and/or treatment response are presented. aNot a case–control study.
Sex steroid synthesis
Type 5 17β-hydroxysteroid dehydrogenase is an important enzyme for testosterone biosynthesis, and a polymorphism in the gene encoding this enzyme (AKR1C3) has been linked to PCOS susceptibility in North American women (Qin et al., 2006). A polymorphism in the CYP1A1 gene, which encodes the enzyme cytochrome P450 1A1, has shown an association with PCOS susceptibility in Indian women (Babu et al., 2004). Cytochrome P450 11A (CYP11A1) is a rate-limiting enzyme involved in the synthesis of androgens. Studies have indicated that a pentanucleotide repeat in the gene is associated with PCOS susceptibility (Gharani et al., 1997; Diamanti-Kandarakis et al., 2000; Gaasenbeek et al., 2004; Wang et al., 2006a; Jones et al., 2007). However, the large study of Gaasenbeek et al. found only a weak association between the pentanucleotide repeat and PCOS, and no association with another promoter microsatellite (Gaasenbeek et al., 2004). In Chinese women, a polymorphism in the promoter region of the aldosterone synthetase gene (CYP11B2), which affects the balance of the ovarian renin–angiotensin system, has been linked to PCOS susceptibility (Zhao et al., 2003). A polymorphism in the gene for 17α-hydroxylase (CYP17A1), which is active in estrogen biosynthesis, was not associated with PCOS susceptibility (Techatraisak et al., 1997; Diamanti-Kandarakis et al., 1999; Marszalek et al., 2001). The CYP19A1 gene encodes a key component of aromatase, which catalyses the production of estrogens from androgens. Although polymorphisms in this gene were not found to directly affect susceptibility to PCOS in women from the UK (Gharani et al., 1997) or Spain (Petry et al., 2005), the SNP50 polymorphism in this gene did influence PCOS severity in the Spanish study (Petry et al., 2005).
Sex steroid metabolism
The low activity haplotype (H113–R139) of the gene encoding the detoxification enzyme microsomal epoxide hydrolase (EPHX1) was significantly associated with PCOS susceptibility in Finnish women (Korhonen et al., 2003b). A variant of the hexose-6-phosphate dehydrogenase gene (H6PD), which has been implicated in a rare cortisone reductase deficiency that is characterized by a PCOS-like phenotype, has been linked to PCOS susceptibility in Spanish (San Millan et al., 2005), but not in UK women (Draper et al., 2006). Polymorphisms in the gene encoding 17β-hydroxysteroid dehydrogenase 6 (HSD17B6) have been found to be associated with either PCOS or with the clinical phenotype (Jones et al., 2006). A North American genetic association study of the two isoforms of the steroid biosynthesis enzyme 5α-reductase found that haplotypes in both SRD5A1 and SRD5A2 were associated with PCOS susceptibility, but that only variants in the SRD5A1 gene were associated with severity of hirsutism (Goodarzi et al., 2006).
Polymorphisms in genes encoding 11β-hydroxysteroid dehydrogenase (HSD11B1) (San Millan et al., 2005; Draper et al., 2006), the glutathione S-transferases M1 or T1 (GSTM1, GSTT1) (Babu et al., 2004) and the transcription factor GATA-binding protein 6 (GATA6) (Jones et al., 2006) have also been investigated but failed to show an association with PCOS.
Genes involved in type 2 diabetes and cardiovascular disease
The increased risk of type 2 diabetes and cardiovascular disease in women with PCOS has led to numerous association studies in genes related to these diseases (Table III).
Table III.
Polymorphisms of genes encoding proteins involved in type 2 diabetes and cardiovascular disease.
| Gene (locus, protein name and its function) | Variant |
Association with susceptibility |
Phenotype | Treatment response |
||
|---|---|---|---|---|---|---|
| Name | dbSNP ID | Positive (number of cases, number of controls) | Negative (number of cases, number of controls) | |||
| Genes encoding proteins involved in insulin pathways | ||||||
| ADIPOQ (3q27, adiponectin: mediates insulin resistance) | 45G/T | rs2241766 | German women (57, 567) (Haap et al., 2005) | Greek women (132, 100; 100, 140) (Panidis et al., 2004; Xita et al., 2005) Caucasian women (72, 42) (San Millan et al., 2004) | Greek women—insulin level (100, 140) (Panidis et al., 2004; Xita et al., 2005) | |
| Finnish women (143, 245) (Heinonen et al., 2005) | ||||||
| 276G/T | rs1501299 | Greek women (132, 100; 100, 140) (Panidis et al., 2004; Xita et al., 2005) | Greek women—insulin exposue, adiponectine levels, BMI (100, 140) (Panidis et al., 2004; Xita et al., 2005) | |||
| Caucasian women (72, 42) (San Millan et al., 2004) | ||||||
| Finnish women (143, 245) (Heinonen et al., 2005) | ||||||
| APOE (19q13.2, apolipoprotein E: mediates insulin resistance) | 3937C/T and 4075C/T (e2, e3 and e4 alleles) | rs429358+rs7412 | Finnish women (58, 91) (Heinonen et al., 2001) | |||
| CAPN10 (2q37.3, calpain-10: mediates insulin resistance) | 4841T/C (UCSNP44) | rs2975760 | Spanish women (148, 93) (Gonzalez et al., 2003) | German women (57, 567) (Haap et al., 2005) | Spanish women—hirsutism score (81, 37) (Escobar-Morreale et al., 2002) | |
| Spanish women (81, 37) (Escobar-Morreale et al., 2002) | ||||||
| 4852G/A (UCSNP43) | rs3792267 | German women (57, 567) (Haap et al., 2005) | Spanish women—hirsutism score (81, 37) (Escobar-Morreale et al., 2002) | |||
| Spanish women (148, 93; 81, 37) (Gonzalez et al., 2003) | ||||||
| North American women (181, 422) (Ehrmann et al., 2002a) | ||||||
| 7920ins/del32 bp (UCSNP19) | Spanish women (148, 93) (Gonzalez et al., 2003) | |||||
| North American women (181, 422) (Ehrmann et al., 2002a) | ||||||
| UCSNP45 | rs41266971 | Spanish women (81, 37) (Escobar-Morreale et al., 2002) | German women (57, 567) (Haap et al., 2005) | |||
| UCSNP63 | rs5030952 | Spanish women (148, 93) (Gonzalez et al., 2003) | Spanish women (148, 93)—cholesterol level (Gonzalez et al., 2003) | |||
| North American women (181, 422) (Ehrmann et al., 2002a) | ||||||
| ENPP1 (6q22-q23, plasma cell differentiation antigen glycoprotein (PC-1): mediates insulin resistance) | p.K121Q | rs1044498 | Finnish women (143, 115) (Heinonen et al., 2004) | Caucasian women (72, 42) (San Millan et al., 2004) | ||
| Japanese women (123, 180) (Baba et al., 2007) | ||||||
| FOXC2 (16q22-16q24, forkhead box C2: transcription factor, mediates insulin resistance) | −512C/T | rs34221221 | German women (57, 567) (Haap et al., 2005) | |||
| GYS1 (19q13.3, glycogen synthetase 1 (muscle): mediates insulin resistance) | Xbal RFLP (C/T) | rs8103451 | UK women (90, 62) (Rajkhowa et al., 1996) | |||
| IGF1 (12q22-q23, insulin-like growth factor-1: insulin signalling) | CA repeat (promoter) | Caucasian women (72, 42) (San Millan et al., 2004) | ||||
| IGF2 (11p15.5, insulin-like growth factor-2: insulin signalling) | ApaI RFLP (17200G/A) | rs680 | Caucasian women (72, 42) (San Millan et al., 2004) | |||
| IGF1R (15q26.3, insulin-like growth factor-1 receptor: insulin signalling) | AGG repeat at position 967 | Caucasian women (72, 42) (San Millan et al., 2004) | ||||
| IGF2R (6q26, insulin-like growth factor-2 receptor: insulin signalling) | ins/delACAA in 3′ UTR | Caucasian women (72, 42) (San Millan et al., 2004) | ||||
| INS (11p15.5, insulin: hormone) | VNTR | UK women, linkage studies (54, 78 and 2 family-based linkage studies; 74, 150) (Franks et al., 1999; Michelmore et al., 2001) | UK (255 families+185, 1062) and Finnish women (530 [72 confirmed by US], 1069) (Powell et al., 2005) | |||
| Spanish women (40, 38) (Calvo et al., 2002) | ||||||
| Czech women (38, 22) (Vankova et al., 2002) | ||||||
| INSR (19p13.3-p13.2, insulin receptor: hormone regulation) | p.H1058H T/C (3364T/C) | rs1799817 | Chinese women (120, 40) (Chen et al., 2004) Non-obese North American women (99, 136) (Siegel et al., 2002) |
Korean women (174, 93) (Lee et al., 2006) | Chinese women—weight/BMI (120, 40) (Chen et al., 2004) | |
| Korean women (134, 100) (Lee et al., 2007) | ||||||
| p.C1008C T/C (3128T/C) | No dbSNP ID | Chinese women (109, 107) (Jin et al., 2006) | Chinese women—insulin sensitivity (109, 107) (Jin et al., 2006) | |||
| 109482A/G,109665C/T, | No dbSNP ID, rs6510959, | Korean women (134, 100) (Lee et al., 2007) | ||||
| 125498A/G, | rs2303672, | |||||
| 127527G/A, | rs2059806, | |||||
| 143485G/C, | rs2252673, | |||||
| 161822G/A, | rs2860175, | |||||
| 168828T/A | No dbSNP ID | |||||
| 176477C/T | No dbSNP ID | Korean women (134, 100) (Lee et al., 2007) | ||||
| IRS1 (2q36, insulin receptor substrate-1: insulin signalling) | p.G972R | rs1801278 | Chilean women (146, 97) (Sir-Petermann et al., 2004) | German women (57, 567) (Haap et al., 2005) | Chilean women—insulin resistance (146, 97) (Sir-Petermann et al., 2004) | Metformin response in Turkish women (60a) (Ertunc et al., 2005) |
| Japanese women (123, 180) (Baba et al., 2007) | North American women (227a) (Ehrmann et al., 2002b) | North American women, in carriers of CYP21 heterozygous mutation (114, 95) (Witchel et al., 2005) | ||||
| Turkish women (60, 60) (Dilek et al., 2005) | Spanish caucasian women (103, 48) (Villuendas et al., 2005) | Spanish caucasian women—BMI, insulin resistance (103, 48) (Villuendas et al., 2005) | ||||
| Caucasian women (53, 102) (El Mkadem et al., 2001) | Caucasian women—insulin resistance (53, 102) (El Mkadem et al., 2001) | |||||
| IRS2 (13q34, insulin receptor substrate-2: insulin signalling) | p.G1057D | rs1805097 | German women (57, 567) (Haap et al., 2005) | Caucasian American women—blood glucose level (227a) (Ehrmann et al., 2002b) | ||
| Spanish caucasian women (103, 48) (Villuendas et al., 2005) | Caucasian women—blood glucose level (53, 102) (El Mkadem et al., 2001) | |||||
| Caucasian women (53, 102) (El Mkadem et al., 2001) | ||||||
| PON1 (7q21.3, paraoxonase 1: antioxidant enzyme, linked to insulin resistance and cardiovascular disease) | −108C/T | rs705379 | Caucasian women (72, 42) (San Millan et al., 2004) | |||
| p.L55M | rs854560 | Caucasian women (72, 42) (San Millan et al., 2004) | Caucasian women—BMI and insulin resistance (72, 42) (San Millan et al., 2004) | |||
| p.Q192R | rs662 | Caucasian women (72, 42) (San Millan et al., 2004) | ||||
| PPARG (3p25, peroxisome proliferator-activated receptor-γ: transcription factor, mediates insulin resistance, regulates CCL5 expression) | p.P12A | rs1801282 | Finnish women (135, 115) (Korhonen et al., 2003a) | German women (57, 567) (Haap et al., 2005) | Turkish women—glucose metabolism (60, 60) (Tok et al., 2005) | |
| Caucasian women (72, 42) (San Millan et al., 2004) | German women—insulin sensitivity; hirsutism score (102, 104) (Hahn et al., 2005) | |||||
| Chinese women (201, 147) (Wang et al., 2006b) | North American women—insulin sensitivity (218a) (Hara et al., 2002) | |||||
| North American women (285, 187) (Antoine et al., 2007) | ||||||
| Italian women (100, 100) (Orio et al., 2003a) | ||||||
| p.H447H, 1431C/T |
rs3856806 | North American women (285, 187) (Antoine et al., 2007) | Italian women—BMI (100, 100) (Orio et al., 2003a) | |||
| PPARGC1A (4p15.2, peroxisome proliferator-activated receptor gamma, coactivator 1 alpha: transcriptional coactivator for steroid receptors and nuclear receptors) | p.S482G | rs8192678 | Chinese women (201, 147) (Wang et al., 2006b) | |||
| PPP1R3A (7q31.1, protein phosphatase-1: mediates insulin resistance) | 5 bp ins/del in the 3′UTR (ARE-1/ARE-2) | rs5886683+rs5886684 | North American women—insulin sensitivity (186a) (Alcoser et al., 2004) | |||
| PTPN1 (20q13.1-q13.2, protein tyrosine phosphatase 1B: insulin signalling) | 981C/T | rs11575938 | Caucasian women (72, 42) (San Millan et al., 2004) | |||
| 1484ins/delG | Caucasian women (72, 42) (San Millan et al., 2004) | |||||
| RETN (19p13.2, resistin: mediates insulin resistance) | −179C/G (−180C/G) |
rs1862513 | Greek women (320, 180) (Xita et al., 2004) | Greek women—BMI (320, 180) (Xita et al., 2004) | ||
| SORBS1 (10q23.3-q24.1, sorbin: may play a role in insulin resistance) | p.T228A | rs2281939 | Caucasian women (72, 42) (San Millan et al., 2004) | Caucasian women—BMI (72, 42) (San Millan et al., 2004) | ||
| Mediators of vascular function or genes linked to cardiovascular risk | ||||||
| AGT (1q42-q43, angiotensin I: mediates vascular homeostasis) | p.M235T | rs699 | Italian women (95, 64) (Zulian et al., 2005) | Italian women—insulin sensitivity (95, 64) (Zulian et al., 2005) | ||
| MTHFR (1p36.3, methylenetetrahydrofolate reductase: olate metabolism, linked to cardiovascular disease) | 677C/T (p.A222V) | rs1801133 | Italian women (70, 70) (Orio et al., 2003b) | |||
| PLAT (8p12, tissue plasminogen activator: fibrinolysis system, linked to cardiovascular disease) | Alu repeat ins/del 311 bp | rs4646972 | Turkish women (91, 100) (Karadeniz et al., 2007) | Turkish women—cholesterol and LDL levels (91, 100) (Karadeniz et al., 2007) | ||
| SERPINE1 (7q21.3-q22, plasminogen-activator inhibitor-1 (PAI-1): pibrinolysis system, linked to cardiovascular disease) | −675ins/delG (4G/5G) | Several IDs: rs1799768, rs34857375, rs1799762, rs1799889 |
Greek women (98, 64) (Diamanti-Kandarakis et al., 2004) | Caucasian women (72, 42; 106, 102) (San Millan et al., 2004; Walch et al., 2005a) | North American women—rate of miscarriages (149, 234) (Glueck et al., 1999) Greek women—SERPINE1 (PAI-1) levels (98, 64) (Diamanti-Kandarakis et al., 2004) |
|
| Turkish women (91, 100) (Karadeniz et al., 2007) | ||||||
The list shows genes that have been investigated for their role in polycystic ovary syndrome. Genes are grouped according to their function (and then alphabetically). Studies showing positive or no associations of these genes with disease susceptibility and positive associations with phenotype (clinical characteristics of the condition, e.g. endocrine abnormalities) are presented. aReplicated in a family study (52 probands). NB: when only the number of cases is given, the study was not case control. BMI, body mass index; CCL5, formally know as RANTES, Regulation upon Activation Normal T cell Expressed and Secreted; LDL, low-density lipoprotein; PAI-1, plasminogen activator inhibitor-1; RFLP, restriction fragment-length polymorphism; VNTR, variable number of tandem repeats.
Adiponectin
Patients with obesity, type 2 diabetes, insulin resistance or PCOS have abnormally low levels of adiponectin, and therefore several studies have investigated the effect of two SNPs in the adiponectin gene (ADIPOQ) on PCOS susceptibility. Whereas there was an association with the 45G/T polymorphism in German and Finnish women (Haap et al., 2005; Heinonen et al., 2005), there was no association for this polymorphism or the 276G/T polymorphism in Greek or Caucasian women (Panidis et al., 2004; San Millan et al., 2004; Heinonen et al., 2005; Xita et al., 2005) (NB: 276G/T was not investigated by Haap et al.). Both polymorphisms may, however, affect insulin levels (Xita et al., 2005).
Calpain-10
The gene for the cysteine protease calpain-10 (CAPN10) is associated with type 2 diabetes, and potential associations between five different polymorphisms and PCOS have been investigated. In the CAPN10 gene, two different Spanish studies found an association between either the SNP UCSNP44 (Gonzalez et al., 2003) or UCSNP45 (Escobar-Morreale et al., 2002) and PCOS susceptibility. These associations were not observed in a German study (Haap et al., 2005). No associations were found between the UCSNP43, UCSNP19, UCSNP63 polymorphisms and PCOS (Ehrmann et al., 2002a; Gonzalez et al., 2003), although there were some links with the symptoms of PCOS (Escobar-Morreale et al., 2002; Gonzalez et al., 2003).
Plasma cell differentiation antigen
Genes encoding components of the insulin signalling system have been investigated in relation to PCOS across a number of studies. Plasma cell differentiation antigen glycoprotein (PC-1, also known as ENPP1, ectonucleotide pyrophosphatase/phosphodiesterase 1) is a mediator of insulin receptor signalling and has been implicated in insulin resistance. However, studies investigating an association between PCOS susceptibility and the p.K121Q polymorphism have reported both positive (Heinonen et al., 2004) and negative (San Millan et al., 2004; Baba et al., 2007) results. Although early case–control studies in the UK indicated a link between PCOS susceptibility and the insulin gene variable number of tandem repeats microsatellite length (Franks et al., 1999; Michelmore et al., 2001), a large subsequent study in more than 3000 women from the UK and Finland found no such association (Powell et al., 2005). In addition, studies conducted in the Czech Republic (Vankova et al., 2002) and Spain (Calvo et al., 2002) have found no association between this variant and PCOS.
Insulin receptor gene
Three polymorphisms in the insulin receptor gene (INSR) have shown a positive association with PCOS: 3364T/C (p.H1058H) in Chinese (Chen et al., 2004) and American women (Siegel et al., 2002), but not Korean women (Lee et al., 2006); 3128T/C (p.C1008C) in Chinese women (Jin et al., 2006); and 176477C/T in Korean women (Lee et al., 2007). The 2007 study by Lee et al. investigated a total of nine polymorphisms but only found an association with 176477C/T. The 3364T/C and 3128T/C polymorphisms were also associated with the phenotype of PCOS (Chen et al., 2004; Jin et al., 2006).
Insulin receptor substrates
The insulin receptor substrates IRS-1 and IRS-2 are proteins that participate in the insulin signal transduction pathway. Extensive investigations on a possible association between the p.G972R polymorphism in the IRS-1 gene and PCOS have been carried out, with both postive (Sir-Petermann et al., 2004; Dilek et al., 2005; Baba et al., 2007) and negative (El Mkadem et al., 2001; Ehrmann et al., 2002b; Haap et al., 2005; Villuendas et al., 2005) results. The polymorphism was associated with symptoms of PCOS across four different studies (El Mkadem et al., 2001; Sir-Petermann et al., 2004; Villuendas et al., 2005; Witchel et al., 2005). Metformin, an insulin-sensitizing drug that is often used in women with PCOS to induce ovulation, is believed to act via phosphorylation of IRS proteins. This could explain why a study in Turkish women with PCOS indicated that the IRS-1 genotype may influence a patient's response to metformin therapy (Ertunc et al., 2005). In contrast, investigations into the p.G1057D polymorphism in the IRS-2 gene have failed to show an association with susceptibility to PCOS (El Mkadem et al., 2001; Haap et al., 2005; Villuendas et al., 2005), but have linked this polymorphism with blood glucose levels in women with PCOS (El Mkadem et al., 2001; Ehrmann et al., 2002b).
Insulin-like growth factors
An ApaI RFLP in the insulin-like growth factor-2 (IGF-2) gene has been linked to PCOS susceptibility in Caucasian women (San Millan et al., 2004), whereas polymorphisms in genes encoding IGF-1, IGF-1 receptor and IGF-2 receptor were not associated (San Millan et al., 2004). Of three variants investigated in the paraoxonase gene (PON1) (San Millan et al., 2004), the −108C/T variant was linked to PCOS susceptibility in Caucasian women. The p.L55M polymorphisms was not associated with PCOS, but was linked with a higher BMI and greater insulin resistance in women who were homozygotic for the 55M polymorphism compared with carriers of the common 55L allele. This polymorphism is believed to contribute to impaired insulin function by increasing levels of oxidative stress in women with PCOS. The remaining polymorphism investigated (p.Q192R) was associated with neither susceptibility nor phenotype.
Peroxisome proliferator-activated receptor-γ
Peroxisome proliferator-activated receptor-γ (PPARG) regulates the expression of several anti-atherosclerotic proteins. One study of the polymorphism p.P12A showed an association with PCOS susceptibility in Finnish women (Korhonen et al., 2003a), but five other studies found no such association (Orio et al., 2003a; San Millan et al., 2004; Haap et al., 2005; Wang et al., 2006b; Antoine et al., 2007). Three studies found effects on glucose metabolism or insulin sensitivity in carriers of this polymorphism (Hara et al., 2002; Hahn et al., 2005; Tok et al., 2005) and indications that genotype also determines levels of hirsutism (Hahn et al., 2005). The 1431C/T (p.H447H) polymorphism in this gene has been linked to obesity in women with PCOS (Orio et al., 2003a) but not to susceptibility to PCOS (Antoine et al., 2007). So far, there has only been a single study of the PPARG coactivator 1 alpha (PPARGC1A) gene and no association was shown between the polymorphism studied (p.S482G) and PCOS (Wang et al., 2006b).
Glucose metabolism and insulin resistance
An insertion/deletion polymorphism in the AT-rich element within the 3′-untranslated region of the muscle-specific glycogen-targeting subunit of the protein phosphatase-1 gene (PPP1R3A), which has been associated with insulin resistance and diabetes, has been shown to influence insulin resistance and androgen levels in women with PCOS (Alcoser et al., 2004). Also related to the phenotype of PCOS is the −179C/G polymorphism in the promoter region of the resistin gene (RETN) (Xita et al., 2004) and the p.T228A polymorphism in the sorbin and SH3 domain containing 1 (SORBS1) gene (San Millan et al., 2004). Both were linked to higher BMI in carriers, but neither was linked with susceptibility (San Millan et al., 2004; Xita et al., 2004).
A number of variants of other genes involved in insulin resistance, type 2 diabetes and/or obesity have been investigated but failed to show an association with PCOS: apolipoprotein E (APOE) (Heinonen et al., 2001), forkhead box C2 (FOXC2) (Haap et al., 2005), glycogen synthetase 1 (GYS1) (Rajkhowa et al., 1996) and protein tyrosine phosphatase 1B (PTPN1) (San Millan et al., 2004).
Genes linked to cardiovascular risk
Polymorphisms in a limited number of genes linked to cardiovascular risk have been studied to assess a possible role in PCOS (Table III). In one study, the p.M235T polymorphism in the gene encoding angiotensin I (AGT), a potent vasoconstrictor, was linked to PCOS susceptibility and a genotype-dependent difference was seen in insulin sensitivity in women with PCOS (Zulian et al., 2005). Methylenetetrahydrofolate reductase (MTHFR) is an enzyme involved in the homocysteine pathway, and the p.A222V polymorphism in the MTHFR gene increases circulating homocysteine levels. As elevated levels of plasma homocysteine are associated with an increased risk of cardiovascular disease, a study investigated whether this polymorphism increases PCOS susceptibility in Italian women, but failed to show an effect (Orio et al., 2003b). As part of the fibrinolytic system, the gene encoding tissue plasminogen activator (PLAT) has been investigated in relation to PCOS. Although no association between the Alu repeat insertion/deletion and susceptibility was found, PCOS patients that were homozygous for the insertion (i/i genotype) had lower levels of low-density and total cholesterol than other genotypes (Karadeniz et al., 2007). Plasminogen activator inhibitor-1 (SERPINE1) is also involved in blood coagulation and has been implicated in cardiovascular disease. The 4G/5G polymorphism in the SERPINE1 gene has been linked with PCOS susceptibility in one study (Diamanti-Kandarakis et al., 2004), but no association was found in three other studies (San Millan et al., 2004; Walch et al., 2005a; Karadeniz et al., 2007). Women with PCOS who are carriers of the polymorphism were, however, found to have an increased risk of miscarriage (Glueck et al., 1999) and higher plasminogen activator inhibitor-1 levels (Diamanti-Kandarakis et al., 2004).
Genes involved in tissue remodelling and inflammatory processes
Tissue remodelling
Matrix metalloproteinases (MMPs) are thought to play a critical role in the remodelling of the extracellular matrix during follicular development (Goldman and Shalev, 2004). The 1607G insertion/deletion polymorphism in the MMP1 gene has been associated with an increased risk of PCOS in Caucasian women (Walch et al., 2005b) (Table IV). The association of genes of the histocompatibility family with PCOS has been studied in Japanese women. Whereas women with PCOS were more likely to be carriers of the HLA-A11 or HLA-DRB1*0403 alleles, they were less likely to be carriers of the HLA-B39 allele when compared with healthy individuals (indicating a protective effect). No association was found for the alleles tested within HLA-C (Kaibe et al., 2006). No association was found between two polymorphisms in the gene encoding haemochromatosis protein, in the MHC Class I family (HFE), and PCOS (Botella-Carretero et al., 2006).
Inflammatory cytokines
Circulating levels of several inflammatory cytokines, including tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6), correlate with insulin resistance and obesity (Milner et al., 1999; Mohlig et al., 2004). It has also been proposed that another inflammatory cytokine, IL-1, influences the processes of ovulation, fertilization and implantation. A study in Austrian women showed that the −889C/T polymorphism in the IL1A gene is associated with PCOS, which was not the case for the −511C/T and 3953G/A polymorphisms in the IL1B gene (Kolbus et al., 2007). Within the interleukin-6 (IL6) gene, the 597G/A polymorphism has been shown to be associated with PCOS susceptibility (Villuendas et al., 2002); the 572G/C polymorphism not associated (Villuendas et al., 2002) and the −174G/C was both associated with susceptibility (Villuendas et al., 2002) and not associated with (Walch et al., 2004), but related to, the PCOS phenotype (Mohlig et al., 2004; Walch et al., 2004). An investigation into polymorphisms in the genes of the IL-6 receptor complex found no association between the presence of a microsatellite CA repeat in the IL6R gene, but an association between PCOS and the p.G148R polymorphism in the IL-6 signal transducer (IL6ST) gene (Escobar-Morreale et al., 2003).
Polymorphisms in the promoter region of the TNF-α gene (TNF) do not appear to affect PCOS susceptibility (Milner et al., 1999; Mao et al., 2000; Korhonen et al., 2002), but the −308G/A polymorphism is linked with glucose tolerance in women with PCOS (Milner et al., 1999). The actions of TNF-α are mainly mediated by type 1 or type 2 TNF receptors. Of the five polymorphisms in the TNF receptor 2 gene (TNFRSF1B) investigated in relation to PCOS in Spanish and Italian women, only p.M196R was found to be associated with PCOS susceptibility and hyperandrogenism, but the other four polymorphisms investigated were not (Peral et al., 2002).
Ovarian response to gonadotrophins
Ovarian stimulation is usually performed by administering exogenous FSH following pituitary down-regulation. Ovarian response to FSH, however, varies widely among women undergoing ovarian stimulation (Georgiou et al., 1997) with wide-reaching clinical consequences. If ovarian response to stimulation is insufficient, the cycle may need to be cancelled. Similarly, if ovarian response is excessive, cycle cancellation may be necessary to avoid the risk of ovarian hyperstimulation syndrome (OHSS), a potentially life-threatening complication. Application of pharmacogenetics to the problem of predicting ovarian response utilizes information about the genetic make-up of an individual patient. This information may then be used in conjunction with existing clinical factors to predict treatment response (Fauser et al., 2008).
FSH receptor
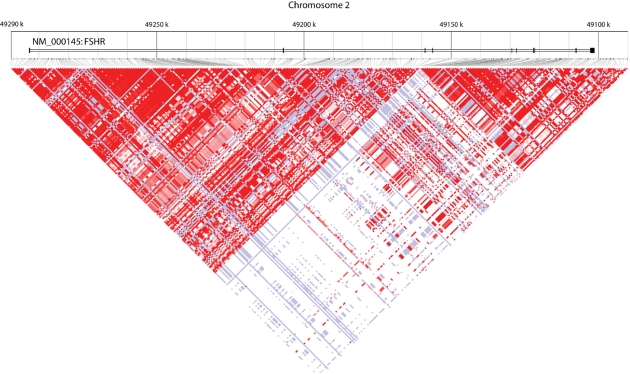
FSH induces the proliferation of granulosa cells and the synthesis of the androgen-converting enzyme aromatase, and also plays a crucial role in secondary follicle recruitment and selection of the dominant follicle (Fauser and Van Heusden, 1997). The action of FSH is mediated by FSHR, a member of the G-protein-coupled receptor family, which is expressed solely by granulosa cells. The FSHR gene harbours more than 900 SNPs, arranged in two major linkage disequilibrium blocks (Fig. 3). Two non-synonymous SNPs in strong linkage disequilibrium (p.A307T and p.N680S) have been identified in exon 10 of the FSHR gene (Simoni et al., 1999). As the interaction between FSH and its receptor plays a key role in ovarian stimulation, a number of groups have investigated the effect of polymorphisms in the FSHR gene on ovarian response (Table V).
Figure 3:
LD plot of the genomic region on chromosome 2 harbouring the FSHR gene, obtained from the HapMap website (www.hapmap.org). More than 900 SNPs are currently listed in the National Centre for Biotechnology Information SNP database. These SNPs are grouped into two major LD blocks (red triangles). FSHR, follicle-stimulating hormone receptor; LD, linkage disequilibrium.
Table V.
Polymorphisms in genes encoding sex hormones and hormone regulators, enzymes involved in metabolism and biosynthesis, or paracrine factors.
| Gene | Locus | Protein name | Protein function | Variant |
Association with ovarian response |
Phenotype (cases, controls) | ||
|---|---|---|---|---|---|---|---|---|
| Name | dbSNP ID | Positive (cases, controls) | Negative (cases, controls) | |||||
| Sex hormones and hormone regulators | ||||||||
| AMH | 19p13.3 | Anti-Müllerian hormone | Hormone | p.I49S | rs10407022 | Caucasian women—E2 levels (53, 45) (Kevenaar et al., 2007) | ||
| AMHR2 | 12q13 | Anti-Müllerian hormone type II receptor | Hormone receptor | –482A/G | rs2002555 | Caucasian women—E2 levels levels (53, 45) (Kevenaar et al., 2007) | ||
| ESR1 | 6q25.1 | Oestrogen receptor α | Hormone receptor | PvuII RFLP (−397T/C) (g.938T/C) | rs2234693 | Spanish women (170) (Mao et al., 2000; de Castro et al., 2004) | Caucasian women—follicule/oocyte ratio, pregnancy rate (100, 100) (Georgiou et al., 1997) | |
| Chinese women (200, 200) (Sundarrajan et al., 1999) | Chinese women—serum oestratdiol levels, follicule/oocyte ratio, pregnancy rate (200, 200) (Sundarrajan et al., 1999) | |||||||
| ESR2 | 14q23.2 | Oestrogen receptor β | Hormone receptor | AluI RFLP (1730A/G) (39A/G) | rs4986938 | Spanish women (170) (Mao et al., 2000; de Castro et al., 2004) | ||
| FSHR | 2p21-p16 | Follicle-stimulating hormone receptor | Hormone receptor | p.N680S (in complete LD with p.A307T, rs6165) | rs6166 | German women (93) (Perez Mayorga et al., 2000; Behre et al., 2005) | Dutch women (105) (Klinkert et al., 2006) | German women—peak oestradiol levels (93) (Behre et al., 2005) |
| Japanese women (58) (Sudo et al., 2002) | German women—circulating FSH, levels, number of follicules, lutheal phase and menstrual cycle length (23) (Perez Mayorga et al., 2000; Greb et al., 2005) | |||||||
| Spanish women (102) (de Castro et al., 2003; de Castro et al., 2004) | Japanese women (58)—basal FSH (Sudo et al., 2002) | |||||||
| Korean women (263) (Jun et al., 2006) | Dutch women (148) (Laven et al., 2003) | |||||||
| Swedish women (68) (Falconer et al., 2005) | ||||||||
| Greek women (125 (Loutradis et al., 2006) | Korean women—basal FSH, pregnancy rate (263) (Jun et al., 2006) | |||||||
| Greek women (125)—FSH levels, follicule and oocyte number (Loutradis et al., 2006) | ||||||||
| Dutch women—pregnancy rate (105) (Klinkert et al., 2006) | ||||||||
| Enzymes involved in metabolism and biosynthesis | ||||||||
| CYP19A1 | 15q21.1 | Aromatase | Steroid biosynthesis | 1672C/T | rs10046 | Spanish women (170) (de Castro et al., 2004) | ||
| Paracrine factors | ||||||||
| BMP15 | Xp11.2 | Bone morphogenic protein 15 | Oocyte and follicular development | −673C/T | No dbSNP ID | Spanish women (307) (Moron et al., 2006) | ||
| −9C/G | rs3810682 | Spanish women (307) (Moron et al., 2006) | ||||||
| 905G/A | rs3897937 | Spanish women (307) (Moron et al., 2006) | ||||||
| 308A/G (p.N103S) | rs41308602 | Spanish women (307) (Moron et al., 2006) | ||||||
| MTHFR | 1p36.3 | Methylenetetrahydrofolate reductase | Folate metabolism, linked to cardiovascular disease | 677C/T (p.A222V) | rs1801133 | German women (105) (Thaler et al., 2006) | North American women (223) (Rosen et al., 2007) | German women—E2 levels, ocyte number (105) (Thaler et al., 2006) |
| 1298A/C (p.E429A) | rs1801131 | North American women (223) (Rosen et al., 2007) | ||||||
The list shows genes that have been investigated for their role in ovarian response. Genes are grouped according to their function (and then alphabetically), and studies showing positive or no associations of these genes with ovarian response and/or phenotype (e.g. basal FSH, follicular oestradiol levels) are presented.
Studies in women with normal ovarian function demonstrate convincingly that SNPs in exon 10 modulate FSHR function and the ovarian response to FSH. This effect was first observed in a partly retrospective, non-randomized study of German women undergoing controlled ovarian hyperstimulation for assisted reproduction. The amount of FSH needed for controlled ovarian hyperstimulation to achieve similar peak estradiol levels was significantly lower in women with the N/N genotype at position 680 of the FSHR gene compared with women carrying the S/S or N/S genotypes, indicating a lower ovarian sensitivity to FSH in vivo for the S680 allele (Perez Mayorga et al., 2000). Similar results were later obtained by other investigators who studied populations from different ethnic backgrounds (Sudo et al., 2002; de Castro et al., 2003, 2004; Behre et al., 2005; Falconer et al., 2005; Jun et al., 2006; Loutradis et al., 2006). In accordance with a genetic control of FSHR p.N680S genotype status on in vitro fertilization (IVF) outcome, de Castro et al. (2003) reported that women with the S/S genotype have significantly higher rates of cycle cancellation and poor response compared with carriers of the N/S or N/N genotypes. These consistent findings in various populations indicate that the effects of the FSHR p.N680S polymorphism are independent of ethnic background and may also be present in other, previously uninvestigated, populations. Recently, it was shown that in women undergoing IVF treatment, the clinical pregnancy rate in women with the N/N genotype is significantly higher compared with those with the S/S genotype (Jun et al., 2006). However, another study using a similar study design showed opposite results, with higher pregnancy rates in women with the S/S genotype (Klinkert et al., 2006) (Table VI). These contrasting data should be interpreted with caution, and larger, well-designed and properly powered studies should be conducted before drawing conclusions about the effects of the FSHR genotype on pregnancy rates.
Table VI.
Comparison of the study designs and outcomes of two conflicting studies investigating the relation between the FSHR polymorphism at amino acid position 680 (Asn/Ser) and pregnancy rate.
| Jun et al. (2006) | Klinkert et al. (2006) | |
|---|---|---|
| Number of patients | 263 | 105 |
| Status regarding ICSI patients | Not excluded | Excluded |
| Frequency of unexplained infertility (%) | 35.6 | 25.0 |
| Genotype associated with significantly higher FSH levels | Ser/Ser | No difference |
| Genotype associated with significantly higher clinical pregnancy rate | Asn/Asn | Ser/Ser |
ICSI, intracytoplasmic sperm injection.
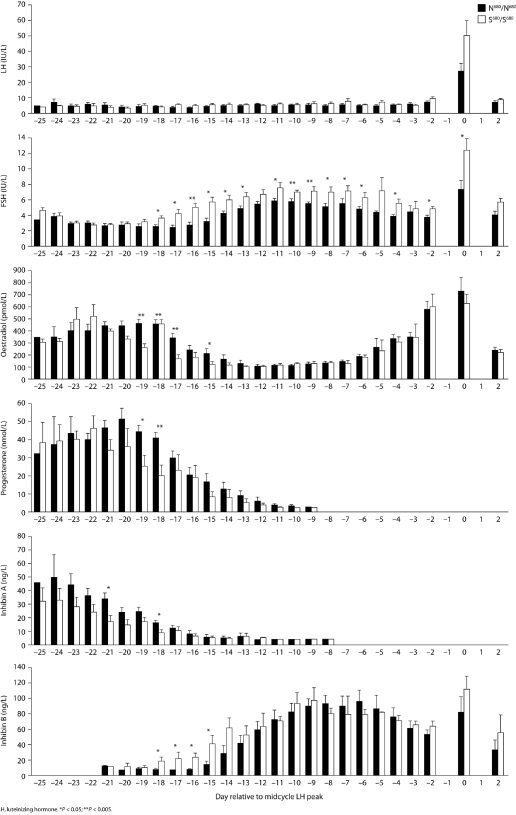
In a study involving menstrual cycle monitoring in women with normal, mono-ovulatory cycles, Greb et al. (2005) were able to show that during the luteo-follicular transition, serum levels of estradiol, progesterone and inhibin A were significantly lower and FSH started to rise earlier in women with the S/S genotype compared with women who carried the N/N genotype for the FSHR p.N680S polymorphism (Fig. 4). In addition, FSH levels were steadily and significantly higher during the follicular phase in the S/S genotype group, whereas no differences were observed between groups with regard to estradiol, inhibin B and growth velocity of the dominant follicle, showing that higher levels of endogenous FSH are necessary to achieve ovulation in carriers of the S/S genotype. Menstrual cycles were significantly longer in women with the S/S genotype, with a difference of about 2 days between these women and those with the N/N genotype. Thus, this study demonstrated that the S/S genotype results in a higher ovarian threshold for FSH, decreased negative feedback to the pituitary and a longer menstrual cycle (Greb et al., 2005). Other studies have observed that the genotype for the codon 680 polymorphism was associated with differences in cycle length (Perez Mayorga et al., 2000), basal FSH levels (Sudo et al., 2002; Laven et al., 2003; Falconer et al., 2005; Jun et al., 2006; Loutradis et al., 2006), pregnancy rates (Jun et al., 2006; Klinkert et al., 2006) and follicular/oocyte number (Loutradis et al., 2006) (Table V).
Figure 4:
Menstrual cycle-dependent serum levels of LH (A), FSH (B), estradiol (C), progesterone (D), inhibin A (E), and inhibin B (F) referenced to the day of the LH surge (0) in women with the Asn680/Asn680 (n=12) and the Ser680/Ser680 (n=9) genotype from the luteo-follicular transition phase (LH -25) until ovulation (LH +2). Means are displayed as bars, and error bars show SEM; *P<0.05; **P<0.005.
Serum hormone concentrations during the menstrual cycle in women carrying either N680/N680 or S680/S680 allele variants of the N680S polymorphism in the FSH receptor gene [Reproduced with permission from Greb et al. (2005), © 2005 The Endocrine Society].
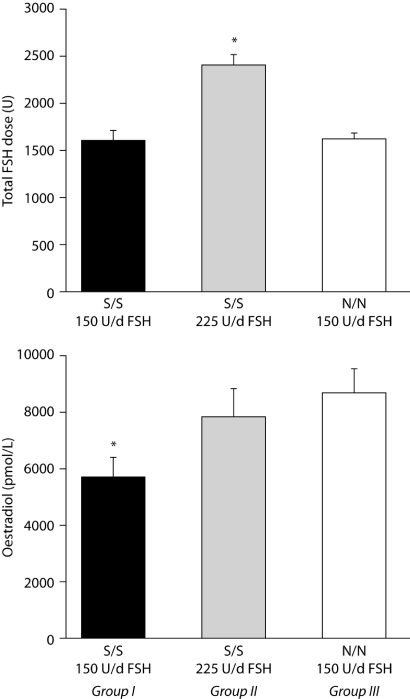
Behre et al. (2005) conducted a prospective interventional study using a randomized controlled trial design and showed that the codon 680 polymorphism of the FSHR gene caused a differential estradiol response to FSH. In this study, the same FSH dose for ovarian hyperstimulation resulted in significantly lower serum levels of estradiol in women with the S/S genotype than in women with the N/N genotype. This lower response could be overcome by increasing the FSH dose (Behre et al., 2005) (Fig. 5). Despite differences in estradiol levels, no significant differences were detected in the number of follicles or retrieved oocytes, fertilization rate, cumulative embryo score or pregnancy rate. This finding suggests that, using standard protocols, FSH might be overdosed in individual women, which may put them at risk of OHSS. Indeed, a retrospective association study demonstrated that the FSHR S680 allele was represented to a higher degree in women developing OHSS, and that the N680 allele was associated significantly with the severity of OHSS (Daelemans et al., 2004). Since OHSS is relatively rare, none of the studies performed so far in individual centres have sufficient power to demonstrate or refute convincingly any association with the FSHR gene; this is an issue that should be analysed retrospectively in a multicentre study.
Figure 5:
Serum levels of oestradiol before ovulation induction were significantly lower in women with the Ser/Ser allele variant (group I, n=24) compared to the Asn/Asn allele variant (group III, n=44) of the FSH receptor (lower panel: *significant difference between group I and III). This difference in ovarian response could be overcome by increasing the daily FSH dose from 150U/day to 225U/day (upper panel:*significant higher total FSH dose) in women with the Ser/Ser allele variant (group II, n=25); lower panel: no significant difference between group II and III.
Total FSH dose required (upper) and serum estradiol concentration (lower) in normo-ovulatory women undergoing controlled ovarian hyperstimulation, grouped according to N680S genotype for the FSH receptor gene [Reproduced with permission from Behre et al. (2005), ©2005 Lippincott Williams & Wilkins].
Taken together, these data indicate that the FSHR gene may play a significant role in the success of ovarian stimulation. Women with the FSHR S680 allele comprise 60–75% of women undergoing IVF (allelic distribution is similar to that of the general population), and are characterized by higher basal FSH serum concentrations, the need for a higher amount of exogenous FSH and a higher risk of hypo- or hyper-response. Thus, genotyping the FSHR p.N680S polymorphism may provide a means of identifying a population of poor responders before IVF procedures are initiated. Since the basis of the poor responder status of these women is a reduced response of the FSHR to FSH stimulation, a stimulation protocol designed to overcome the partial resistance to FSH response should be sufficient to improve significantly the success of IVF in these women. The clinical effectiveness of such an approach should be confirmed in randomized controlled trials.
It is important to note that studies in women with ovarian dysfunction did not find any association between FSHR polymorphisms and ovarian response to FSH; e.g. in premature ovarian failure (Conway et al., 1999; Sundblad et al., 2004). No significant difference in the FSH dose needed for monofollicular development was detected between women with different FSHR genotypes in a non-randomized trial and normogonadotrophic anovulatory women treated with low-dose FSH for ovulation induction (Laven et al., 2003). Therefore, it seems that the differential estradiol response caused by different FSHR alleles is evident so far only in women with normal ovarian function. Another study demonstrated that the S680 allele is significantly more frequent in women with a normal menstrual cycle and elevated FSH levels than in women with normal FSH levels, corroborating the idea of a higher FSH threshold (de Koning et al., 2006). Finally, an intriguing association between the A307–S680 haplotype and ovarian cancer susceptibility was recently reported (Yang et al., 2006).
Other genes
In addition to studies investigating the influence of FSHR genotype on ovarian responsiveness, other genetic factors have been analysed and reported to be involved in modulating ovarian sensitivity to FSH. Important candidates are polymorphisms in the ER1 (Georgiou et al., 1997; Sundarrajan et al., 1999; de Castro et al., 2004), and ER2 genes (de Castro et al., 2004) as well as in the anti-Müllerian hormone (AMH) and AMH type II receptor genes (Kevenaar et al., 2007) and in MTHFR (Thaler et al., 2006; Rosen et al., 2007). As ovarian responsiveness to FSH may be a polygenic trait, future studies should investigate the combined role of all these factors in large numbers of well-characterized patients undergoing ovarian hyperstimulation, using stringent genetic epidemiological criteria.
Finally, several studies have explored the effects of polymorphisms in genes for metabolic enzymes on ovarian response (Table V). De Castro et al. (2004) studied the 1672C/T polymorphism in the CYP19A1 gene, which encodes an enzyme involved in estrogen synthesis, and found that it does not influence ovarian response to exogenous FSH. Bone morphogenetic protein 15 (BMP-15), a member of the transforming growth factor-β superfamily, is a paracrine factor expressed exclusively in the ovaries and is involved in oocyte and follicular development. A recent study investigated the association between OHSS and polymorphisms in the BMP15 gene (Moron et al., 2006). Although individual polymorphisms did not show a strong association with the risk of OHSS, the TGGG haplotype was a risk factor and the CCAA haplotype was found to confer protection against OHSS (Moron et al., 2006).
Discussion
This systematic review emphasizes the inconsistency of results obtained by studies that have investigated associations between polymorphisms and PCOS or ovarian response. This may be due to methodological reasons, such as population stratification (genetic heterogeneity, population history), inappropriate disease/trait definition, subject selection issues or chance findings. One of the major problems with candidate gene studies concerns sample size, which is frequently insufficient. The sample sizes of many such studies have been calculated based on knowledge acquired while studying monogenic traits, which have a very strong effect. Unlike monogenetic traits, complex traits result from the interaction of several genetic variations and environmental factors, thus individual genetic variations have a much more modest effect (Lander and Schork, 1994). This may not have been taken into consideration, resulting in studies that are too small to reveal a genetic association in the context of a complex trait. Furthermore, it has been clearly demonstrated that studies with small populations, those that investigate a genetic variation with a small effect, or those that have a flexible design that is prone to bias are less likely to be replicated (Ioannidis, 2005). Similar conclusions were drawn by Gorroochurn et al. (2007), who showed that for commonly observed P-value thresholds (P = 0.02–0.01, when α = 0.05), replication probabilities are surprisingly low (around 60–70% chance of replication).
Inconsistent results may also be due to characteristics inherent to polymorphisms, such as incomplete penetrance, genetic heterogeneity and gene–gene or gene–environment interactions. Other shortcomings of the use of candidate genes/markers to study association include the fact that only a very tiny part of the genome is being studied, and this is done independently of any interactions that may be involved. In addition, candidate gene selection relies on prior knowledge, making it impossible to reveal an association with genes that have unknown function or that have not been known to be implicated in the disease/trait being studied.
To overcome some of these limitations, the use of genome-wide scans is an experimental tool that is becoming an increasingly realistic option. Over the past 5–10 years, refinement of technology involving polymerase chain reaction, development of microarray technology and the remarkable progress in the characterization of the human genome sequence have enabled the study of thousands of DNA variations in a single experiment. Commercial genotyping tools currently allow the study of almost a million SNPs per sample in a single assay, representing roughly 10% of the estimated total number of SNPs in the human genome. Although not all known SNPs are represented on one genotyping microarray, linkage disequilibrium allows nearly 90% of the human genome to be studied with current technology (Schork et al., 2000; Locke et al., 2006; Roeder et al., 2006). Therefore, by performing an assay for a particular SNP, it is also possible to indirectly test for the presence of other variants that are in linkage disequilibrium. Although less advanced, the same technology is also being used to study quantitative (or copy number) gene variation on a genome-wide basis; this type of variation has recently been found to impact a much larger part of the genome than originally thought (Iafrate et al., 2004). Before this technique offers clinically useful data, however, key methodological points must be addressed. These include increasing the sample size compared with single gene/marker studies and resolving statistical problems inherent to multiple testing. Although it is obvious that a number of the studies reviewed had a small sample size, it is quite difficult to give a generic sample size that will fit all studies, as this depends on the frequency and number of the markers being tested and the importance of the genetic effect of the associated marker; genome-wide studies are often carried out on more than 1000 individuals. If the association studies have been properly designed, replication of the results in an independent population is one of the best methods to confirm an association.
Regardless of whether a candidate gene or a genome-wide approach is used, we suggest that in order to improve the quality of results, genetic association studies should be carried out in two phases: an exploratory phase, followed by a validation phase. Whenever possible, exploratory studies should be carried out using a genome-wide approach to maximize the chances of identifying an association. Ideally, these exploratory studies should be replicated, and only markers that are positive in at least two exploratory studies should be considered for validation. As explained previously, the identification of an association between a certain allele or genotype and a given disease/trait does not necessarily mean that there is an aetiological link. Due to the occurrence of linkage disequilibrium, in a number of cases, the associated genetic variation will only be a marker, and not the direct cause, of the trait; for this reason, care must be taken when drawing conclusions from the results. The recommended second phase consists of validating the small set of markers that were associated with the disease/trait in the exploratory studies. Depending on the objective, validation of the markers can take several forms. In some cases, it may be enough to replicate the association with this marker in a different study population (e.g. if a genetic marker is predictive of response to treatment or of disease state). On the other hand, if the aim is to study the impact of genetic variants on molecular mechanisms, then it will be necessary to first either sequence or screen the DNA region around the associated variant with a higher density of markers in order to pinpoint the particular variation that is the causative variation (resulting in, e.g. changes in amino acid sequence or changes to the promoter region affecting gene expression). Once identified, this causative variation will only be truly validated by results of in vitro and in vivo assays, such as site-directed mutagenesis or reporter gene assays.
On the basis of the data we have summarized, there is currently only one polymorphism for which a sufficient number of studies have consistently identified a significant association. Studies in women from various ethnic backgrounds with normal ovarian function demonstrate convincingly that SNPs in exon 10 of the FSH receptor gene can be used as markers to predict differences in FSHR function and ovarian response to FSH (Perez Mayorga et al., 2000; Sudo et al., 2002; de Castro et al., 2003, 2004; Falconer et al., 2005; Jun et al., 2006; Loutradis et al., 2006), although ovarian response is a polygenic trait and the interaction with other gene polymorphisms remains to be investigated. On the contrary, no consistent association between gene polymorphism and PCOS could be identified.
Conclusions
By pursuing the most promising polymorphisms identified in this review, we hope that the key factors involved in the pathogenesis of PCOS will be identified in the not-too-distant future, and that this knowledge can be used to improve prognosis for women with this disorder. These women are also part of a wider group who would potentially benefit from further analyses to confirm the link between variants in the FSHR gene and ovarian response to gonadotrophins. Strong evidence for an association could ultimately lead to stimulation protocols that are carefully personalized to each woman's individual needs, according to the particular combination of polymorphisms inherited.
Funding
The preparation of this manuscript was sponsored by an unrestricted educational grant from Merck Serono International S.A., Geneva, Switzerland (an affiliate of Merck KGaA, Darmstadt, Germany). Funding to pay the Open Access publication charges for this article was provided by Merck Serono International S.A., an affiliate of Merck KGaA, Darmstadt, Germany.
Acknowledgements
The authors would like to thank Drs Polly Field, Imogen Horsey and Kay Elder for their assistance in drafting the manuscript.
References
- Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome—a hypothesis. J Endocrinol. 2002;174:1–5. doi: 10.1677/joe.0.1740001. [DOI] [PubMed] [Google Scholar]
- Alcoser SY, Hara M, Bell GI, Ehrmann DA. Association of the (AU)AT-rich element polymorphism in PPP1R3 with hormonal and metabolic features of polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:2973–2976. doi: 10.1210/jc.2003-031189. [DOI] [PubMed] [Google Scholar]
- Antoine HJ, Pall M, Trader BC, Chen YD, Azziz R, Goodarzi MO. Genetic variants in peroxisome proliferator-activated receptor gamma influence insulin resistance and testosterone levels in normal women, but not those with polycystic ovary syndrome. Fertil Steril. 2007;87:862–869. doi: 10.1016/j.fertnstert.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Endo T, Sata F, Honnma H, Kitajima Y, Hayashi T, Manase K, Kanaya M, Yamada H, Minakami H, et al. Polycystic ovary syndrome is associated with genetic polymorphism in the insulin signaling gene IRS-1 but not ENPP1 in a Japanese population. Life Sci. 2007;81:850–854. doi: 10.1016/j.lfs.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Babu KA, Rao KL, Kanakavalli MK, Suryanarayana VV, Deenadayal M, Singh L. CYP1A1, GSTM1 and GSTT1 genetic polymorphism is associated with susceptibility to polycystic ovaries in South Indian women. Reprod Biomed Online. 2004;9:194–200. doi: 10.1016/s1472-6483(10)62129-3. [DOI] [PubMed] [Google Scholar]
- Behre HM, Greb RR, Mempel A, Sonntag B, Kiesel L, Kaltwasser P, Seliger E, Ropke F, Gromoll J, Nieschlag E, et al. Significance of a common single nucleotide polymorphism in exon 10 of the follicle-stimulating hormone (FSH) receptor gene for the ovarian response to FSH: a pharmacogenetic approach to controlled ovarian hyperstimulation. Pharmacogenet Genomics. 2005;15:451–456. doi: 10.1097/01.fpc.0000167330.92786.5e. [DOI] [PubMed] [Google Scholar]
- Bendlova B, Zavadilova J, Vankova M, Vejrazkova D, Lukasova P, Vcelak J, Hill M, Cibula D, Vondra K, Starka L, et al. Role of D327N sex hormone-binding globulin gene polymorphism in the pathogenesis of polycystic ovary syndrome. J Steroid Biochem Mol Biol. 2007;104:68–74. doi: 10.1016/j.jsbmb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–683. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- Botella-Carretero JI, Luque-Ramirez M, varez-Blasco F, San Millan JL, Escobar-Morreale HF. Mutations in the hereditary hemochromatosis gene are not associated with the increased body iron stores observed in overweight and obese women with polycystic ovary syndrome. Diabetes Care. 2006;29:2556. doi: 10.2337/dc06-1655. [DOI] [PubMed] [Google Scholar]
- Calvo RM, Villuendas G, Sancho J, San Millan JL, Escobar-Morreale HF. Role of the follistatin gene in women with polycystic ovary syndrome. Fertil Steril. 2001;75:1020–1023. doi: 10.1016/s0015-0282(01)01697-1. [DOI] [PubMed] [Google Scholar]
- Calvo RM, Telleria D, Sancho J, San Millan JL, Escobar-Morreale HF. Insulin gene variable number of tandem repeats regulatory polymorphism is not associated with hyperandrogenism in Spanish women. Fertil Steril. 2002;77:666–668. doi: 10.1016/s0015-0282(01)03238-1. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Shi YH, Zhao YR, Li Y, Tang R, Zhao LX, Chang ZH. Correlation between single nucleotide polymorphism of insulin receptor gene with polycystic ovary syndrome. Zhonghua Fu Chan Ke Za Zhi. 2004;39:582–585. [PubMed] [Google Scholar]
- Conway GS, Honour JW, Jacobs HS. Heterogeneity of the polycystic ovary syndrome: clinical, endocrine and ultrasound features in 556 patients. Clin Endocrinol (Oxf) 1989;30:459–470. doi: 10.1111/j.1365-2265.1989.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Conway GS, Conway E, Walker C, Hoppner W, Gromoll J, Simoni M. Mutation screening and isoform prevalence of the follicle stimulating hormone receptor gene in women with premature ovarian failure, resistant ovary syndrome and polycystic ovary syndrome. Clin Endocrinol (Oxf) 1999;51:97–99. doi: 10.1046/j.1365-2265.1999.00745.x. [DOI] [PubMed] [Google Scholar]
- Daelemans C, Smits G, de M V, Costagliola S, Englert Y, Vassart G, Delbaere A. Prediction of severity of symptoms in iatrogenic ovarian hyperstimulation syndrome by follicle-stimulating hormone receptor Ser680Asn polymorphism. J Clin Endocrinol Metab. 2004;89:6310–6315. doi: 10.1210/jc.2004-1044. [DOI] [PubMed] [Google Scholar]
- de Castro F, Ruiz R, Montoro L, Perez-Hernandez D, Sanchez-Casas PE, Real LM, Ruiz A. Role of follicle-stimulating hormone receptor Ser680Asn polymorphism in the efficacy of follicle-stimulating hormone. Fertil Steril. 2003;80:571–576. doi: 10.1016/s0015-0282(03)00795-7. [DOI] [PubMed] [Google Scholar]
- de Castro F, Moron FJ, Montoro L, Galan JJ, Hernandez DP, Padilla ES, Ramirez-Lorca R, Real LM, Ruiz A. Human controlled ovarian hyperstimulation outcome is a polygenic trait. Pharmacogenetics. 2004;14:285–293. doi: 10.1097/00008571-200405000-00003. [DOI] [PubMed] [Google Scholar]
- de Koning CH, Benjamins T, Harms P, Homburg R, van Montfrans JM, Gromoll J, Simoni M, Lambalk CB. The distribution of FSH receptor isoforms is related to basal FSH levels in subfertile women with normal menstrual cycles. Hum Reprod. 2006;21:443–446. doi: 10.1093/humrep/dei317. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bartzis MI, Zapanti ED, Spina GG, Filandra FA, Tsianateli TC, Bergiele AT, Kouli CR. Polymorphism T–>C (−34 bp) of gene CYP17 promoter in Greek patients with polycystic ovary syndrome. Fertil Steril. 1999;71:431–435. doi: 10.1016/s0015-0282(98)00512-3. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bartzis MI, Bergiele AT, Tsianateli TC, Kouli CR. Microsatellite polymorphism (tttta)(n) at -528 base pairs of gene CYP11alpha influences hyperandrogenemia in patients with polycystic ovary syndrome. Fertil Steril. 2000;73:735–741. doi: 10.1016/s0015-0282(99)00628-7. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Palioniko G, Alexandraki K, Bergiele A, Koutsouba T, Bartzis M. The prevalence of 4G5G polymorphism of plasminogen activator ihibitor-1 (PAI-1) gene in polycystic ovarian syndrome and its association with plasma PAI-1 levels. Eur J Endocrinol. 2004;150:793–798. doi: 10.1530/eje.0.1500793. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Piperi C. Genetics of polycystic ovary syndrome: searching for the way out of the labyrinth. Hum Reprod Update. 2005;11:631–643. doi: 10.1093/humupd/dmi025. [DOI] [PubMed] [Google Scholar]
- Dilek S, Ertunc D, Tok EC, Erdal EM, Aktas A. Association of Gly972Arg variant of insulin receptor substrate-1 with metabolic features in women with polycystic ovary syndrome. Fertil Steril. 2005;84:407–412. doi: 10.1016/j.fertnstert.2005.01.133. [DOI] [PubMed] [Google Scholar]
- Draper N, Powell BL, Franks S, Conway GS, Stewart PM, McCarthy MI. Variants implicated in cortisone reductase deficiency do not contribute to susceptibility to common forms of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2006;65:64–70. doi: 10.1111/j.1365-2265.2006.02547.x. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Schwarz PE, Hara M, Tang X, Horikawa Y, Imperial J, Bell GI, Cox NJ. Relationship of calpain-10 genotype to phenotypic features of polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;a 87:1669–1673. doi: 10.1210/jcem.87.4.8385. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Tang X, Yoshiuchi I, Cox NJ, Bell GI. Relationship of insulin receptor substrate-1 and -2 genotypes to phenotypic features of polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;b87:4297–4300. doi: 10.1210/jc.2002-020216. [DOI] [PubMed] [Google Scholar]
- El Mkadem SA, Lautier C, Macari F, Molinari N, Lefebvre P, Renard E, Gris JC, Cros G, Daures JP, Bringer J, et al. Role of allelic variants Gly972Arg of IRS-1 and Gly1057Asp of IRS-2 in moderate-to-severe insulin resistance of women with polycystic ovary syndrome. Diabetes. 2001;50:2164–2168. doi: 10.2337/diabetes.50.9.2164. [DOI] [PubMed] [Google Scholar]
- Elter K, Erel CT, Cine N, Ozbek U, Hacihanefioglu B, Ertungealp E. Role of the mutations Trp8=> Arg and Ile15=> Thr of the human luteinizing hormone beta-subunit in women with polycystic ovary syndrome. Fertil Steril. 1999;71:425–430. doi: 10.1016/s0015-0282(98)00491-9. [DOI] [PubMed] [Google Scholar]
- Ertunc D, Tok EC, Aktas A, Erdal EM, Dilek S. The importance of IRS-1 Gly972Arg polymorphism in evaluating the response to metformin treatment in polycystic ovary syndrome. Hum Reprod. 2005;20:1207–1212. doi: 10.1093/humrep/deh747. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Peral B, Villuendas G, Calvo RM, Sancho J, San Millan JL. Common single nucleotide polymorphisms in intron 3 of the calpain-10 gene influence hirsutism. Fertil Steril. 2002;77:581–587. doi: 10.1016/s0015-0282(01)03206-x. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Calvo RM, Villuendas G, Sancho J, San Millan JL. Association of polymorphisms in the interleukin 6 receptor complex with obesity and hyperandrogenism. Obes Res. 2003;11:987–996. doi: 10.1038/oby.2003.136. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Luque-Ramirez M, San Millan JL. The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev. 2005;26:251–282. doi: 10.1210/er.2004-0004. [DOI] [PubMed] [Google Scholar]
- Falconer H, Andersson E, Aanesen A, Fried G. Follicle-stimulating hormone receptor polymorphisms in a population of infertile women. Acta Obstet Gynecol Scand. 2005;84:806–811. doi: 10.1111/j.0001-6349.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- Fauser BC, Van Heusden AM. Manipulation of human ovarian function: physiological concepts and clinical consequences. Endocr Rev. 1997;18:71–106. doi: 10.1210/edrv.18.1.0290. [DOI] [PubMed] [Google Scholar]
- Fauser BC, Diedrich K, Devroey P. Predictors of ovarian response: progress towards individualized treatment in ovulation induction and ovarian stimulation. Hum Reprod Update. 2008;14:1–14. doi: 10.1093/humupd/dmm034. [DOI] [PubMed] [Google Scholar]
- Ferk P, Teran N, Gersak K. The (TAAAA)n microsatellite polymorphism in the SHBG gene influences serum SHBG levels in women with polycystic ovary syndrome. Hum Reprod. 2007;22:1031–1036. doi: 10.1093/humrep/del457. [DOI] [PubMed] [Google Scholar]
- Franks S, Gharani N, McCarthy M. Genetic abnormalities in polycystic ovary syndrome. Ann Endocrinol (Paris) 1999;60:131–133. [PubMed] [Google Scholar]
- Fratantonio E, Vicari E, Pafumi C, Calogero AE. Genetics of polycystic ovarian syndrome. Reprod Biomed Online. 2005;10:713–720. doi: 10.1016/s1472-6483(10)61114-5. [DOI] [PubMed] [Google Scholar]
- Gaasenbeek M, Powell BL, Sovio U, Haddad L, Gharani N, Bennett A, Groves CJ, Rush K, Goh MJ, Conway GS, et al. Large-scale analysis of the relationship between CYP11A promoter variation, polycystic ovarian syndrome, and serum testosterone. J Clin Endocrinol Metab. 2004;89:2408–2413. doi: 10.1210/jc.2003-031640. [DOI] [PubMed] [Google Scholar]
- Georgiou I, Konstantelli M, Syrrou M, Messinis IE, Lolis DE. Oestrogen receptor gene polymorphisms and ovarian stimulation for in-vitro fertilization. Hum Reprod. 1997;12:1430–1433. doi: 10.1093/humrep/12.7.1430. [DOI] [PubMed] [Google Scholar]
- Gharani N, Waterworth DM, Batty S, White D, Gilling-Smith C, Conway GS, McCarthy M, Franks S, Williamson R. Association of the steroid synthesis gene CYP11a with polycystic ovary syndrome and hyperandrogenism. Hum Mol Genet. 1997;6:397–402. doi: 10.1093/hmg/6.3.397. [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Wang P, Fontaine RN, Sieve-Smith L, Tracy T, Moore SK. Plasminogen activator inhibitor activity: an independent risk factor for the high miscarriage rate during pregnancy in women with polycystic ovary syndrome. Metabolism. 1999;48:1589–1595. doi: 10.1016/s0026-0495(99)90250-0. [DOI] [PubMed] [Google Scholar]
- Goldman S, Shalev E. MMPS and TIMPS in ovarian physiology and pathophysiology. Front Biosci. 2004;9:2474–2483. doi: 10.2741/1409. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Abril E, Roca A, Aragon MJ, Figueroa MJ, Velarde P, Ruiz R, Fayez O, Galan JJ, Herreros JA, et al. Specific CAPN10 gene haplotypes influence the clinical profile of polycystic ovary patients. J Clin Endocrinol Metab. 2003;88:5529–5536. doi: 10.1210/jc.2003-030322. [DOI] [PubMed] [Google Scholar]
- Goodarzi MO, Shah NA, Antoine HJ, Pall M, Guo X, Azziz R. Variants in the 5alpha-reductase type 1 and type 2 genes are associated with polycystic ovary syndrome and the severity of hirsutism in affected women. J Clin Endocrinol Metab. 2006;91:4085–4091. doi: 10.1210/jc.2006-0227. [DOI] [PubMed] [Google Scholar]
- Gorroochurn P, Hodge SE, Heiman GA, Durner M, Greenberg DA. Non-replication of association studies: ‘pseudo-failures’ to replicate? Genet Med. 2007;9:325–331. doi: 10.1097/gim.0b013e3180676d79. [DOI] [PubMed] [Google Scholar]
- Greb RR, Grieshaber K, Gromoll J, Sonntag B, Nieschlag E, Kiesel L, Simoni M. A common single nucleotide polymorphism in exon 10 of the human follicle stimulating hormone receptor is a major determinant of length and hormonal dynamics of the menstrual cycle. J Clin Endocrinol Metab. 2005;90:4866–4872. doi: 10.1210/jc.2004-2268. [DOI] [PubMed] [Google Scholar]
- Haap M, Machicao F, Stefan N, Thamer C, Tschritter O, Schnuck F, Wallwiener D, Stumvoll M, Haring HU, Fritsche A. Genetic determinants of insulin action in polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2005;113:275–281. doi: 10.1055/s-2005-837665. [DOI] [PubMed] [Google Scholar]
- Hahn S, Fingerhut A, Khomtsiv U, Khomtsiv L, Tan S, Quadbeck B, Herrmann BL, Knebel B, Muller-Wieland D, Mann K, et al. The peroxisome proliferator activated receptor gamma Pro12Ala polymorphism is associated with a lower hirsutism score and increased insulin sensitivity in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2005;62:573–579. doi: 10.1111/j.1365-2265.2005.02261.x. [DOI] [PubMed] [Google Scholar]
- Hahn S, Frey UH, Siffert W, Tan S, Mann K, Janssen OE. The CC genotype of the GNAS T393C polymorphism is associated with obesity and insulin resistance in women with polycystic ovary syndrome. Eur J Endocrinol. 2006;155:763–770. doi: 10.1530/eje.1.02275. [DOI] [PubMed] [Google Scholar]
- Hara M, Alcoser SY, Qaadir A, Beiswenger KK, Cox NJ, Ehrmann DA. Insulin resistance is attenuated in women with polycystic ovary syndrome with the Pro(12)Ala polymorphism in the PPARgamma gene. J Clin Endocrinol Metab. 2002;87:772–775. doi: 10.1210/jcem.87.2.8255. [DOI] [PubMed] [Google Scholar]
- Heinonen S, Korhonen S, Hippelainen M, Hiltunen M, Mannermaa A, Saarikoski S. Apolipoprotein E alleles in women with polycystic ovary syndrome. Fertil Steril. 2001;75:878–880. doi: 10.1016/s0015-0282(01)01691-0. [DOI] [PubMed] [Google Scholar]
- Heinonen S, Korhonen S, Helisalmi S, Koivunen R, Tapanainen JS, Laakso M. The 121Q allele of the plasma cell membrane glycoprotein 1 gene predisposes to polycystic ovary syndrome. Fertil Steril. 2004;82:743–745. doi: 10.1016/j.fertnstert.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Heinonen S, Korhonen S, Helisalmi S, Koivunen R, Tapanainen J, Hippelainen M, Laakso M. Associations between two single nucleotide polymorphisms in the adiponectin gene and polycystic ovary syndrome. Gynecol Endocrinol. 2005;21:165–169. doi: 10.1080/09513590500238796. [DOI] [PubMed] [Google Scholar]
- Hickey T, Chandy A, Norman RJ. The androgen receptor CAG repeat polymorphism and X-chromosome inactivation in Australian Caucasian women with infertility related to polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:161–165. doi: 10.1210/jcem.87.1.8137. [DOI] [PubMed] [Google Scholar]
- Iafrate AJ, Feuk L, Rivera MN, Listewnik ML, Donahoe PK, Qi Y, Scherer SW, Lee C. Detection of large-scale variation in the human genome. Nat Genet. 2004;36:949–951. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaskelainen J, Korhonen S, Voutilainen R, Hippelainen M, Heinonen S. Androgen receptor gene CAG length polymorphism in women with polycystic ovary syndrome. Fertil Steril. 2005;83:1724–1728. doi: 10.1016/j.fertnstert.2004.11.080. [DOI] [PubMed] [Google Scholar]
- Jahanfar S, Eden JA. Genetic and non-genetic theories on the etiology of polycystic ovary syndrome. Gynecol Endocrinol. 1996;10:357–364. doi: 10.3109/09513599609012823. [DOI] [PubMed] [Google Scholar]
- Jin L, Zhu XM, Luo Q, Qian Y, Jin F, Huang HF. A novel SNP at exon 17 of INSR is associated with decreased insulin sensitivity in Chinese women with PCOS. Mol Hum Reprod. 2006;12:151–155. doi: 10.1093/molehr/gal022. [DOI] [PubMed] [Google Scholar]
- Jones MR, Italiano L, Wilson SG, Mullin BH, Mead R, Dudbridge F, Watts GF, Stuckey BG. Polymorphism in HSD17B6 is associated with key features of polycystic ovary syndrome. Fertil Steril. 2006;86:1438–1446. doi: 10.1016/j.fertnstert.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Jones MR, Wilson SG, Mullin BH, Mead R, Watts GF, Stuckey BG. Polymorphism of the follistatin gene in polycystic ovary syndrome. Mol Hum Reprod. 2007;13:237–241. doi: 10.1093/molehr/gal120. [DOI] [PubMed] [Google Scholar]
- Jun JK, Yoon JS, Ku SY, Choi YM, Hwang KR, Park SY, Lee GH, Lee WD, Kim SH, Kim JG, et al. Follicle-stimulating hormone receptor gene polymorphism and ovarian responses to controlled ovarian hyperstimulation for IVF-ET. J Hum Genet. 2006;51:665–670. doi: 10.1007/s10038-006-0005-5. [DOI] [PubMed] [Google Scholar]
- Kahsar-Miller M, Boots LR, Azziz R. Dopamine D3 receptor polymorphism is not associated with the polycystic ovary syndrome. Fertil Steril. 1999;71:436–438. doi: 10.1016/s0015-0282(98)00485-3. [DOI] [PubMed] [Google Scholar]
- Kaibe M, Takakuwa K, Murakawa H, Ishii K, Tamura M, Tanaka K. Studies on the human leukocyte antigens in patients with polycystic ovary syndrome in a Japanese population–possible susceptibility of HLA-A11 and -DRB1*0403 to patient population with polycystic ovary syndrome. Am J Reprod Immunol. 2006;55:301–306. doi: 10.1111/j.1600-0897.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- Karadeniz M, Erdogan M, Berdeli A, Saygili F, Yilmaz C. 4G/5G Polymorphism of PAI-1 gene and Alu-repeat I/D polymorphism of TPA gene in Turkish patients with polycystic ovary syndrome. J Assist Reprod Genet. 2007;24:412–418. doi: 10.1007/s10815-007-9160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevenaar ME, Themmen AP, Laven JS, Sonntag B, Fong SL, Uitterlinden AG, de Jong FH, Pols HA, Simoni M, Visser JA. Anti-Mullerian hormone and anti-Mullerian hormone type II receptor polymorphisms are associated with follicular phase estradiol levels in normo-ovulatory women. Hum Reprod. 2007;22:1547–1554. doi: 10.1093/humrep/dem036. [DOI] [PubMed] [Google Scholar]
- Kim JY, Moon SM, Ryu HJ, Kim JJ, Kim HT, Park C, Kimm K, Oh B, Lee JK. Identification of regulatory polymorphisms in the TNF-TNF receptor superfamily. Immunogenetics. 2005;57:297–303. doi: 10.1007/s00251-005-0800-8. [DOI] [PubMed] [Google Scholar]
- Klinkert ER, te Velde ER, Weima S, van Zandvoort PM, Hanssen RG, Nilsson PR, de Jong FH, Looman CW, Broekmans FJ. FSH receptor genotype is associated with pregnancy but not with ovarian response in IVF. Reprod Biomed Online. 2006;13:687–695. doi: 10.1016/s1472-6483(10)60660-8. [DOI] [PubMed] [Google Scholar]
- Kolbus A, Walch K, Nagele F, Wenzl R, Unfried G, Huber JC. Interleukin-1 alpha but not interleukin-1 beta gene polymorphism is associated with polycystic ovary syndrome. J Reprod Immunol. 2007;73:188–193. doi: 10.1016/j.jri.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Korhonen S, Romppanen EL, Hiltunen M, Mannermaa A, Punnonen K, Hippelainen M, Heinonen S. Lack of association between C-850T polymorphism of the gene encoding tumor necrosis factor-alpha and polycystic ovary syndrome. Gynecol Endocrinol. 2002;16:271–274. doi: 10.1080/gye.16.4.271.274. [DOI] [PubMed] [Google Scholar]
- Korhonen S, Heinonen S, Hiltunen M, Helisalmi S, Hippelainen M, Koivunen R, Tapanainen JS, Laakso M. Polymorphism in the peroxisome proliferator-activated receptor-gamma gene in women with polycystic ovary syndrome. Hum Reprod. 2003;a 18:540–543. doi: 10.1093/humrep/deg128. [DOI] [PubMed] [Google Scholar]
- Korhonen S, Romppanen EL, Hiltunen M, Helisalmi S, Punnonen K, Hippelainen M, Heinonen S. Two exonic single nucleotide polymorphisms in the microsomal epoxide hydrolase gene are associated with polycystic ovary syndrome. Fertil Steril. 2003;b 79:1353–1357. doi: 10.1016/s0015-0282(03)00385-6. [DOI] [PubMed] [Google Scholar]
- Kurabayashi T, Yahata T, Quan J, Tanaka K. Association of polymorphisms in the beta2 and beta3 adrenoceptor gene with polycystic ovary syndrome. J Reprod Med. 2006;51:389–393. [PubMed] [Google Scholar]
- Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- Laven JS, Imani B, Eijkemans MJ, Fauser BC. New approach to polycystic ovary syndrome and other forms of anovulatory infertility. Obstet Gynecol Surv. 2002;57:755–767. doi: 10.1097/00006254-200211000-00022. [DOI] [PubMed] [Google Scholar]
- Laven JS, Mulders AG, Suryandari DA, Gromoll J, Nieschlag E, Fauser BC, Simoni M. Follicle-stimulating hormone receptor polymorphisms in women with normogonadotropic anovulatory infertility. Fertil Steril. 2003;80:986–992. doi: 10.1016/s0015-0282(03)01115-4. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Oh B, Lee JY, Kimm K, Lee SH, Baek KH. A novel single nucleotide polymorphism of INSR gene for polycystic ovary syndrome. Fertil Steril. 2007 doi: 10.1016/j.fertnstert.2007.05.026. in press. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Yoo KJ, Kim SJ, Lee SH, Cha KY, Baek KH. Single nucleotide polymorphism in exon 17 of the insulin receptor gene is not associated with polycystic ovary syndrome in a Korean population. Fertil Steril. 2006;86:380–384. doi: 10.1016/j.fertnstert.2005.12.073. [DOI] [PubMed] [Google Scholar]
- Locke DP, Sharp AJ, McCarroll SA, McGrath SD, Newman TL, Cheng Z, Schwartz S, Albertson DG, Pinkel D, Altshuler DM, et al. Linkage disequilibrium and heritability of copy-number polymorphisms within duplicated regions of the human genome. Am J Hum Genet. 2006;79:275–290. doi: 10.1086/505653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutradis D, Patsoula E, Minas V, Koussidis GA, Antsaklis A, Michalas S, Makrigiannakis A. FSH receptor gene polymorphisms have a role for different ovarian response to stimulation in patients entering IVF/ICSI-ET programs. J Assist Reprod Genet. 2006;23:177–184. doi: 10.1007/s10815-005-9015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao W, Yu L, Chen Y. Study on the relationship between a polymorphism of tumor necrosis factor-alpha gene and the pathogenesis of polycystic ovary syndrome. Zhonghua Fu Chan Ke Za Zhi. 2000;35:536–539. [PubMed] [Google Scholar]
- Marszalek B, Lacinski M, Babych N, Capla E, Biernacka-Lukanty J, Warenik-Szymankiewicz A, Trzeciak WH. Investigations on the genetic polymorphism in the region of CYP17 gene encoding 5′-UTR in patients with polycystic ovarian syndrome. Gynecol Endocrinol. 2001;15:123–128. doi: 10.1080/gye.15.2.123.128. [DOI] [PubMed] [Google Scholar]
- Michelmore K, Ong K, Mason S, Bennett S, Perry L, Vessey M, Balen A, Dunger D. Clinical features in women with polycystic ovaries: relationships to insulin sensitivity, insulin gene VNTR and birth weight. Clin Endocrinol (Oxf) 2001;55:439–446. doi: 10.1046/j.1365-2265.2001.01375.x. [DOI] [PubMed] [Google Scholar]
- Mifsud A, Ramirez S, Yong EL. Androgen receptor gene CAG trinucleotide repeats in anovulatory infertility and polycystic ovaries. J Clin Endocrinol Metab. 2000;85:3484–3488. doi: 10.1210/jcem.85.9.6832. [DOI] [PubMed] [Google Scholar]
- Milner CR, Craig JE, Hussey ND, Norman RJ. No association between the -308 polymorphism in the tumour necrosis factor alpha (TNFalpha) promoter region and polycystic ovaries. Mol Hum Reprod. 1999;5:5–9. doi: 10.1093/molehr/5.1.5. [DOI] [PubMed] [Google Scholar]
- Mohlig M, Spranger J, Osterhoff M, Ristow M, Pfeiffer AF, Schill T, Schlosser HW, Brabant G, Schofl C. The polycystic ovary syndrome per se is not associated with increased chronic inflammation. Eur J Endocrinol. 2004;150:525–532. doi: 10.1530/eje.0.1500525. [DOI] [PubMed] [Google Scholar]
- Moron FJ, de CF, Royo JL, Montoro L, Mira E, Saez ME, Real LM, Gonzalez A, Manes S, Ruiz A. Bone morphogenetic protein 15 (BMP15) alleles predict over-response to recombinant follicle stimulation hormone and iatrogenic ovarian hyperstimulation syndrome (OHSS) Pharmacogenet Genomics. 2006;16:485–495. doi: 10.1097/01.fpc.0000215073.44589.96. [DOI] [PubMed] [Google Scholar]
- Oksanen L, Tiitinen A, Kaprio J, Koistinen HA, Karonen S, Kontula K. No evidence for mutations of the leptin or leptin receptor genes in women with polycystic ovary syndrome. Mol Hum Reprod. 2000;6:873–876. doi: 10.1093/molehr/6.10.873. [DOI] [PubMed] [Google Scholar]
- Orio F, Jr, Matarese G, Di BS, Palomba S, Labella D, Sanna V, Savastano S, Zullo F, Colao A, Lombardi G. Exon 6 and 2 peroxisome proliferator-activated receptor-gamma polymorphisms in polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;a 88:5887–5892. doi: 10.1210/jc.2002-021816. [DOI] [PubMed] [Google Scholar]
- Orio F, Jr, Palomba S, Di BS, Colao A, Tauchmanova L, Savastano S, Labella D, Russo T, Zullo F, Lombardi G. Homocysteine levels and C677T polymorphism of methylenetetrahydrofolate reductase in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;b 88:673–679. doi: 10.1210/jc.2002-021142. [DOI] [PubMed] [Google Scholar]
- Panidis D, Kourtis A, Kukuvitis A, Farmakiotis D, Xita N, Georgiou I, Tsatsoulis A. Association of the T45G polymorphism in exon 2 of the adiponectin gene with polycystic ovary syndrome: role of Delta4-androstenedione. Hum Reprod. 2004;19:1728–1733. doi: 10.1093/humrep/deh336. [DOI] [PubMed] [Google Scholar]
- Peral B, San Millan JL, Castello R, Moghetti P, Escobar-Morreale HF. Comment: the methionine 196 arginine polymorphism in exon 6 of the TNF receptor 2 gene (TNFRSF1B) is associated with the polycystic ovary syndrome and hyperandrogenism. J Clin Endocrinol Metab. 2002;87:3977–3983. doi: 10.1210/jcem.87.8.8715. [DOI] [PubMed] [Google Scholar]
- Perez Mayorga M, Gromoll J, Behre HM, Gassner C, Nieschlag E, Simoni M. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptor genotype. J Clin Endocrinol Metab. 2000;85:3365–3369. doi: 10.1210/jcem.85.9.6789. [DOI] [PubMed] [Google Scholar]
- Perez-Bravo F, Echiburu B, Maliqueo M, Santos JL, Sir-Petermann T. Tryptophan 64 –> arginine polymorphism of beta-3-adrenergic receptor in Chilean women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2005;62:126–131. doi: 10.1111/j.1365-2265.2004.02183.x. [DOI] [PubMed] [Google Scholar]
- Petry CJ, Ong KK, Michelmore KF, Artigas S, Wingate DL, Balen AH, de ZF, Ibanez L, Dunger DB. Association of aromatase (CYP 19) gene variation with features of hyperandrogenism in two populations of young women. Hum Reprod. 2005;20:1837–1843. doi: 10.1093/humrep/deh900. [DOI] [PubMed] [Google Scholar]
- Powell BL, Haddad L, Bennett A, Gharani N, Sovio U, Groves CJ, Rush K, Goh MJ, Conway GS, Ruokonen A, et al. Analysis of multiple data sets reveals no association between the insulin gene variable number tandem repeat element and polycystic ovary syndrome or related traits. J Clin Endocrinol Metab. 2005;90:2988–2993. doi: 10.1210/jc.2004-2485. [DOI] [PubMed] [Google Scholar]
- Qin K, Ehrmann DA, Cox N, Refetoff S, Rosenfield RL. Identification of a functional polymorphism of the human type 5 17beta-hydroxysteroid dehydrogenase gene associated with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:270–276. doi: 10.1210/jc.2005-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkhowa M, Talbot JA, Jones PW, Pettersson K, Haavisto AM, Huhtaniemi I, Clayton RN. Prevalence of an immunological LH beta-subunit variant in a UK population of healthy women and women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 1995;43:297–303. doi: 10.1111/j.1365-2265.1995.tb02035.x. [DOI] [PubMed] [Google Scholar]
- Rajkhowa M, Talbot JA, Jones PW, Clayton RN. Polymorphism of glycogen synthetase gene in polycystic ovary syndrome. Clin Endocrinol (Oxf) 1996;44:85–90. doi: 10.1046/j.1365-2265.1996.658474.x. [DOI] [PubMed] [Google Scholar]
- Roeder K, Bacanu SA, Wasserman L, Devlin B. Using linkage genome scans to improve power of association in genome scans. Am J Hum Genet. 2006;78:243–252. doi: 10.1086/500026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MP, Shen S, McCulloch CE, Rinaudo PF, Cedars MI, Dobson AT. Methylenetetrahydrofolate reductase (MTHFR) is associated with ovarian follicular activity. Fertil Steril. 2007;88:632–638. doi: 10.1016/j.fertnstert.2006.11.165. [DOI] [PubMed] [Google Scholar]
- San Millan JL, Corton M, Villuendas G, Sancho J, Peral B, Escobar-Morreale HF. Association of the polycystic ovary syndrome with genomic variants related to insulin resistance, type 2 diabetes mellitus, and obesity. J Clin Endocrinol Metab. 2004;89:2640–2646. doi: 10.1210/jc.2003-031252. [DOI] [PubMed] [Google Scholar]
- San Millan JL, Botella-Carretero JI, varez-Blasco F, Luque-Ramirez M, Sancho J, Moghetti P, Escobar-Morreale HF. A study of the hexose-6-phosphate dehydrogenase gene R453Q and 11beta-hydroxysteroid dehydrogenase type 1 gene 83557insA polymorphisms in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4157–4162. doi: 10.1210/jc.2004-1523. [DOI] [PubMed] [Google Scholar]
- Schork NJ, Nath SK, Fallin D, Chakravarti A. Linkage disequilibrium analysis of biallelic DNA markers, human quantitative trait loci, and threshold-defined case and control subjects. Am J Hum Genet. 2000;67:1208–1218. doi: 10.1086/321201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S, Futterweit W, Davies TF, Concepcion ES, Greenberg DA, Villanueva R, Tomer Y. A C/T single nucleotide polymorphism at the tyrosine kinase domain of the insulin receptor gene is associated with polycystic ovary syndrome. Fertil Steril. 2002;78:1240–1243. doi: 10.1016/s0015-0282(02)04241-3. [DOI] [PubMed] [Google Scholar]
- Simoni M, Gromoll J, Höppner W, Kamischke A, Krafft T, Stähle D, Nieschlag E. Mutational analysis of the follicle-stimulating hormone (FSH) receptor in normal and infertile men: identification and characterization of two discrete FSH receptor isoforms. J Clin Endocrinol Metab. 1999;84:751–755. doi: 10.1210/jcem.84.2.5500. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Angel B, Maliqueo M, Santos JL, Riesco MV, Toloza H, Perez-Bravo F. Insulin secretion in women who have polycystic ovary syndrome and carry the Gly972Arg variant of insulin receptor substrate-1 in response to a high-glycemic or low-glycemic carbohydrate load. Nutrition. 2004;20:905–910. doi: 10.1016/j.nut.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Stewart DR, Dombroski BA, Urbanek M, Ankener W, Ewens KG, Wood JR, Legro RS, Strauss JF, III, Dunaif A, Spielman RS. Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab. 2006;91:4112–4117. doi: 10.1210/jc.2006-0951. [DOI] [PubMed] [Google Scholar]
- Sudo S, Kudo M, Wada S, Sato O, Hsueh AJ, Fujimoto S. Genetic and functional analyses of polymorphisms in the human FSH receptor gene. Mol Hum Reprod. 2002;8:893–899. doi: 10.1093/molehr/8.10.893. [DOI] [PubMed] [Google Scholar]
- Sundarrajan C, Liao W, Roy AC, Ng SC. Association of oestrogen receptor gene polymorphisms with outcome of ovarian stimulation in patients undergoing IVF. Mol Hum Reprod. 1999;5:797–802. doi: 10.1093/molehr/5.9.797. [DOI] [PubMed] [Google Scholar]
- Sundblad V, Chiauzzi VA, Escobar ME, Dain L, Charreau EH. Screening of FSH receptor gene in Argentine women with premature ovarian failure (POF) Mol Cell Endocrinol. 2004;222:53–59. doi: 10.1016/j.mce.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Techatraisak K, Conway GS, Rumsby G. Frequency of a polymorphism in the regulatory region of the 17 alpha-hydroxylase-17,20-lyase (CYP17) gene in hyperandrogenic states. Clin Endocrinol (Oxf) 1997;46:131–134. doi: 10.1046/j.1365-2265.1997.8700880.x. [DOI] [PubMed] [Google Scholar]
- Thaler CJB, Budiman H, Ruebsamen H, Nagel D, Lohse P. Effects of the common 677C>T mutation of the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene on ovarian responsiveness to recombinant follicle-stimulating hormone. Am J Reprod Immunol. 2006;55:251–258. doi: 10.1111/j.1600-0897.2005.00357.x. [DOI] [PubMed] [Google Scholar]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;a 81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;b 19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- Tok EC, Aktas A, Ertunc D, Erdal EM, Dilek S. Evaluation of glucose metabolism and reproductive hormones in polycystic ovary syndrome on the basis of peroxisome proliferator-activated receptor (PPAR)-gamma2 Pro12Ala genotype. Hum Reprod. 2005;20:1590–1595. doi: 10.1093/humrep/deh769. [DOI] [PubMed] [Google Scholar]
- Tong Y, Liao WX, Roy AC, Ng SC. Association of AccI polymorphism in the follicle-stimulating hormone beta gene with polycystic ovary syndrome. Fertil Steril. 2000;74:1233–1236. doi: 10.1016/s0015-0282(00)01616-2. [DOI] [PubMed] [Google Scholar]
- Tong Y, Liao WX, Roy AC, Ng SC. Absence of mutations in the coding regions of follicle-stimulating hormone receptor gene in Singapore Chinese women with premature ovarian failure and polycystic ovary syndrome. Horm Metab Res. 2001;33:221–226. doi: 10.1055/s-2001-14941. [DOI] [PubMed] [Google Scholar]
- Urbanek M, Legro RS, Driscoll D, Strauss JF, III, Dunaif A, Spielman RS. Searching for the polycystic ovary syndrome genes. J Pediatr Endocrinol Metab. 2000;a 13(Suppl 5):1311–1313. [PubMed] [Google Scholar]
- Urbanek M, Wu X, Vickery KR, Kao LC, Christenson LK, Schneyer A, Legro RS, Driscoll DA, Strauss JF, III, Dunaif A, et al. Allelic variants of the follistatin gene in polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;b 85:4455–4461. doi: 10.1210/jcem.85.12.7026. [DOI] [PubMed] [Google Scholar]
- Urbanek M, Woodroffe A, Ewens KG, amanti-Kandarakis E, Legro RS, Strauss JF, III, Dunaif A, Spielman RS. Candidate gene region for polycystic ovary syndrome on chromosome 19p13.2. J Clin Endocrinol Metab. 2005;90:6623–6629. doi: 10.1210/jc.2005-0622. [DOI] [PubMed] [Google Scholar]
- Urbanek M, Du Y, Silander K, Collins FS, Steppan CM, Strauss JF, III, Dunaif A, Spielman RS, Legro RS. Variation in resistin gene promoter not associated with polycystic ovary syndrome. Diabetes. 2003;52:214–217. doi: 10.2337/diabetes.52.1.214. [DOI] [PubMed] [Google Scholar]
- Vankova M, Vrbikova J, Hill M, Cinek O, Bendlova B. Association of insulin gene VNTR polymorphism with polycystic ovary syndrome. Ann N Y Acad Sci. 2002;967:558–565. doi: 10.1111/j.1749-6632.2002.tb04317.x. [DOI] [PubMed] [Google Scholar]
- Villuendas G, San Millan JL, Sancho J, Escobar-Morreale HF. The -597 G–>A and -174 G–>C polymorphisms in the promoter of the IL-6 gene are associated with hyperandrogenism. J Clin Endocrinol Metab. 2002;87:1134–1141. doi: 10.1210/jcem.87.3.8309. [DOI] [PubMed] [Google Scholar]
- Villuendas G, Botella-Carretero JI, Roldan B, Sancho J, Escobar-Morreale HF, San Millan JL. Polymorphisms in the insulin receptor substrate-1 (IRS-1) gene and the insulin receptor substrate-2 (IRS-2) gene influence glucose homeostasis and body mass index in women with polycystic ovary syndrome and non-hyperandrogenic controls. Hum Reprod. 2005;20:3184–3191. doi: 10.1093/humrep/dei205. [DOI] [PubMed] [Google Scholar]
- Walch K, Grimm C, Zeillinger R, Huber JC, Nagele F, Hefler LA. A common interleukin-6 gene promoter polymorphism influences the clinical characteristics of women with polycystic ovary syndrome. Fertil Steril. 2004;81:1638–1641. doi: 10.1016/j.fertnstert.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Walch K, Grimm C, Huber JC, Nagele F, Kolbus A, Hefler LA. A polymorphism of the plasminogen activator inhibitor-1 gene promoter and the polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2005;a 123:77–81. doi: 10.1016/j.ejogrb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Walch K, Nagele F, Zeillinger R, Vytiska-Binstorfer E, Huber JC, Hefler LA. A polymorphism in the matrix metalloproteinase-1 gene promoter is associated with the presence of polycystic ovary syndrome in Caucasian women. Fertil Steril. 2005;b 83:1565–1567. doi: 10.1016/j.fertnstert.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Wang D, Johnson AD, Papp AC, Kroetz DL, Sadee W. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C>T affects mRNA stability. Pharmacogenet Genomics. 2005;15:693–704. [PubMed] [Google Scholar]
- Wang Y, Wu X, Cao Y, Yi L, Chen J. A microsatellite polymorphism (tttta)n in the promoter of the CYP11a gene in Chinese women with polycystic ovary syndrome. Fertil Steril. 2006;a 86:223–226. doi: 10.1016/j.fertnstert.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu X, Cao Y, Yi L, Fan H, Chen J. Polymorphisms of the peroxisome proliferator-activated receptor-gamma and its coactivator-1alpha genes in Chinese women with polycystic ovary syndrome. Fertil Steril. 2006;b 85:1536–1540. doi: 10.1016/j.fertnstert.2005.10.047. [DOI] [PubMed] [Google Scholar]
- Witchel SF, Kahsar-Miller M, Aston CE, White C, Azziz R. Prevalence of CYP21 mutations and IRS1 variant among women with polycystic ovary syndrome and adrenal androgen excess. Fertil Steril. 2005;83:371–375. doi: 10.1016/j.fertnstert.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Xita N, Tsatsoulis A, Chatzikyriakidou A, Georgiou I. Association of the (TAAAA)n repeat polymorphism in the sex hormone-binding globulin (SHBG) gene with polycystic ovary syndrome and relation to SHBG serum levels. J Clin Endocrinol Metab. 2003;88:5976–5980. doi: 10.1210/jc.2003-030197. [DOI] [PubMed] [Google Scholar]
- Xita N, Georgiou I, Tsatsoulis A, Kourtis A, Kukuvitis A, Panidis D. A polymorphism in the resistin gene promoter is associated with body mass index in women with polycystic ovary syndrome. Fertil Steril. 2004;82:1466–1467. doi: 10.1016/j.fertnstert.2004.04.050. [DOI] [PubMed] [Google Scholar]
- Xita N, Georgiou I, Chatzikyriakidou A, Vounatsou M, Papassotiriou GP, Papassotiriou I, Tsatsoulis A. Effect of adiponectin gene polymorphisms on circulating adiponectin and insulin resistance indexes in women with polycystic ovary syndrome. Clin Chem. 2005;51:416–423. doi: 10.1373/clinchem.2004.043109. [DOI] [PubMed] [Google Scholar]
- Yang CQ, Chan KY, Ngan HY, Khoo US, Chiu PM, Chan QK, Xue WC, Cheung AN. Single nucleotide polymorphisms of follicle-stimulating hormone receptor are associated with ovarian cancer susceptibility. Carcinogenesis. 2006;27:1502–1506. doi: 10.1093/carcin/bgl014. [DOI] [PubMed] [Google Scholar]
- Zhao SP, Tang XM, Shao DH, Dai HY, Dai SZ. Association study between a polymorphism of aldosterone synthetase gene and the pathogenesis of polycystic ovary syndrome. Zhonghua Fu Chan Ke Za Zhi. 2003;38:94–97. [PubMed] [Google Scholar]
- Zulian E, Sartorato P, Schiavi F, Moghetti P, Castello R, Mantero F, Opocher G, Scaroni C. The M235T polymorphism of the angiotensinogen gene in women with polycystic ovary syndrome. Fertil Steril. 2005;84:1520–1521. doi: 10.1016/j.fertnstert.2005.05.043. [DOI] [PubMed] [Google Scholar]