Abstract
Acute graft-versus-host disease (GVHD) remains a significant cause of mortality after hematopoietic cell transplantation (HCT). Tumor necrosis factor–alpha (TNF-α) mediates GVHD by amplifying donor immune responses to host tissues and by direct toxicity to target organs. We measured TNF receptor 1 (TNFR1) as a surrogate marker for TNF-α in 438 recipients of myeloablative HCT before transplantation and at day 7 after transplantation. Increases in TNFR1 levels more than or equal to 2.5 baseline correlated with eventual development of GVHD grade 2 to 4 (58% vs 32%, P < .001) and with treatment-related mortality (39% vs 17%, P < .001). In a multivariate analysis including age, degree of HLA match, donor type, recipient and donor sex, disease, and status at HCT, the increase in TNFR1 level at day 7 remained a significant predictor for outcome. Measurement of TNFR1 levels early after transplantation provides independent information in advance of important clinical outcomes, such as GVHD and death.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is an important therapeutic option for a variety of malignant and nonmalignant disorders. Acute graft-versus-host disease (GVHD) remains a significant complication of allogeneic HCT and limits the broader application of this therapy.1 The importance of inflammatory cytokines in the pathogenesis of acute GVHD is now well accepted,2–4 particularly tumor necrosis factor–alpha (TNF-α).5–8 In the absence of complications, TNF-α levels rise after conditioning-induced tissue damage and return to baseline levels within 1 week.9 In small numbers of patients with GVHD, persistent elevation of TNF-α levels has been observed before and during the onset of disease.10,11 Furthermore, clinical trials have demonstrated that the inhibition of TNF-α can be effective as initial or salvage therapy for acute GVHD.12–14 TNF-α binds to its receptors, TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2), on the surface of multiple cell types15; after ligation, the receptor-ligand complexes are shed into the plasma, where they are easily measured. Prior work has shown that TNFR1 levels are elevated in patients with GVHD14 and correlate with TNF-α levels.16,17 We therefore hypothesized that significant increases in TNF-α, as measured bound to its receptor, TNFR1, would occur before clinical manifestations of GVHD and would be elevated by day 7 in patients who eventually develop the disease.
Methods
Patient blood samples were obtained under informed consent in patients undergoing allogeneic HCT after myeloablative conditioning at the University of Michigan between 2000 and 2005 under an Institutional Review Board–approved protocol. Informed consent was obtained in accordance with the Declaration of Helsinki.
Samples from 438 patients were collected both before transplantation (baseline) and 7 days after allogeneic HCT, separated into cellular and plasma components on the day of collection, and frozen for later analysis. TNFR1 levels were measured from each sample in duplicate using an enzyme-linked immunosorbent assay according to the manufacturer's protocol (R&D Systems, Minneapolis, MN). Enzyme-linked immunosorbent assay plates were read by a microplate reader (Bio-Rad, Hercules, CA). The day 7 TNFR1 value is presented as a ratio over baseline because of the wide variability (up to 50-fold differences) in baseline TNFR1 levels. All ratios are expressed as the mean plus or minus SEM.
Statistical comparisons between patient groups were performed using Wilcoxon rank sum test with continuous variables and Fisher exact test with categorical variables. Patients and recipients with single HLA-antigen disparities by DNA techniques were considered mismatched for purposes of analysis. The Kaplan-Meier method was used for estimating overall survival (OS), the log-rank test was used to assess differences in OS among patient groups, and a Cox proportional hazards model was used to assess the impact of multiple variables on OS. Unadjusted and adjusted cumulative incidence estimates of treatment-related mortality (TRM) and GVHD and comparisons between patient groups were computed using the proportional subdistribution hazard methods of Fine and Gray.18 In the models of GVHD and TRM, relapse was treated as a competing risk for both outcomes.
Results and discussion
Patient characteristics, including age, donor match, degree of match, transplant conditioning regimens, recipient and donor gender, diagnosis, and status at HCT, are shown in Table 1. All patients received the same GVHD prophylaxis, consisting of tacrolimus and mini-methotrexate (5 mg/m2 on days 1, 3, 6, and 11 after transplantation) and either norfloxacin or levofloxacin for gut decontamination. The mean day 7 TNFR1 ratio in patients who never developed clinically significant GVHD (grade 0-1, n = 269, 61%) was 1.91 plus or minus 0.09. This near doubling in TNFR1 level at day 7 over baseline presumably reflects conditioning-induced tissue damage.19 The day 7 TNFR1 ratio in patients with GVHD grade 2 (n = 83, 19%) was 2.32 plus or minus 0.20 and for patients with GVHD grades 3 or 4 (n = 86, 20%) was 2.92 plus or minus 0.26. The additional elevation in the day 7 TNFR1 ratio thus strongly correlated with GVHD severity (P < .001). The median day of onset was 30 in BMT recipients from related donors and 20 in BMT recipients from unrelated donors. The day 7 TNFR1 ratios were therefore elevated 2 to 3 weeks earlier than the median onset of clinical symptoms.
Table 1.
Patient characteristics (n = 438) for patients who underwent myeloablative allogeneic transplantation at the University of Michigan from January 2000 to December 2005
| Characteristic | Value |
|---|---|
| Median age, y (range) | 42 (0-65) |
| Conditioning, no. (%) | |
| Busulfan-based | 298 (68) |
| BCNU-based | 88 (20) |
| TBI-based | 52 (12) |
| Donor source/match, no. (%) | |
| 6/6 related | 247 (56) |
| 5/6 related | 20 (5) |
| 6/6 unrelated | 124 (28) |
| 5/6 unrelated | 47 (11) |
| Sex (donor → recipient), no. (%) | |
| Male→ male | 164 (37) |
| Female → male | 101 (23) |
| Male→ female | 107 (24) |
| Female → female | 66 (15) |
| Diagnosis, no. (%) | |
| Malignant | 415 (95) |
| Nonmalignant | 23 (5) |
| Status at transplantation, no. (%) | |
| Standard risk | 264 (61) |
| Advanced risk | 151 (34) |
| Nonmalignant | 23 (5) |
GVHD is a major contributor to increased TRM after allogeneic HCT.20–22 We thus analyzed TRM and overall survival. We found that multiple ratios between 1.5 and 3.0 were statistically significant discriminators for likelihood of grades 2 to 4 GVHD, TRM, and OS (data not shown). We next used Classification and Regression Trees (CART)23 to identify the day 7 TNFR1 ratios that best discriminated between those developing and not developing GVHD. CART identified a day 7 TNFR1 ratio of 2.7 as the best fitting threshold. We then resampled the data 5000 times with replacement and fit CART to each of these resampled datasets to derive a bootstrap distribution of plausible thresholds. The interquartile range of this distribution was 2.4 to 2.9. We then selected a day 7 TNFR1 ratio of 2.5 for data presentation because this ratio was statistically valid and clinically optimal given that it divided patients such that the smaller group (those with ratios ≥ 2.5) still comprised a considerable portion of the study population and roughly corresponded to the mean TNFR1 ratio in patients with grades 2 to 4 GVHD.
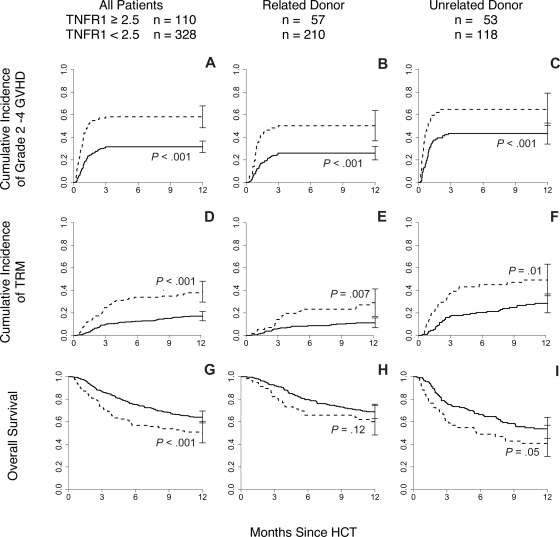
The 110 patients (25%) with a TNFR1 ratio more than or equal to 2.5 were almost twice as likely to develop grades 2 to 4 GVHD (58% vs 32%, P < .001, Figure 1A) and were more than twice as likely to die of transplant-related causes within 1 year (39% vs 17%, P < .001, Figure 1D). This increase in 1-year TRM translated into inferior 1-year survival (50% vs 64%, P < .001, Figure 1G).
Figure 1.
Day 7 TNFR1 ratio correlates with cumulative incidence of GVHD and OS. (A) Patients (n = 438) with TNFR1 ratio more than or equal to 2.5 ( ) experienced increased incidence of GVHD grades 2 to 4 compared with those with a ratio less than 2.5 (
) experienced increased incidence of GVHD grades 2 to 4 compared with those with a ratio less than 2.5 ( P < .001). Similar differences were observed when the patients were further subdivided by donor type: (B) related donor (RD; n = 267, P < .001) and (C) unrelated donor (URD; n = 171, P < .001). (D) Patients with TNFR1 ratio more than 2.5 experienced increased incidence of treatment-related mortality compared with those with a ratio of less than 2.5 (P < .001). Similar differences were observed when the patients were further subdivided by donor type: (E) RD (P = .007) and (F) URD (P = .01). (G) Patients with TNFR1 ratio more than 2.5 were more likely to die within the first year after transplantation compared with those with ratio less than 2.5 (P < .001). Similar differences were observed when the patients were further subdivided by donor type: (H) RD (P = .12) and (I) URD (P = .05).
P < .001). Similar differences were observed when the patients were further subdivided by donor type: (B) related donor (RD; n = 267, P < .001) and (C) unrelated donor (URD; n = 171, P < .001). (D) Patients with TNFR1 ratio more than 2.5 experienced increased incidence of treatment-related mortality compared with those with a ratio of less than 2.5 (P < .001). Similar differences were observed when the patients were further subdivided by donor type: (E) RD (P = .007) and (F) URD (P = .01). (G) Patients with TNFR1 ratio more than 2.5 were more likely to die within the first year after transplantation compared with those with ratio less than 2.5 (P < .001). Similar differences were observed when the patients were further subdivided by donor type: (H) RD (P = .12) and (I) URD (P = .05).
Donor type (related vs unrelated) is a major predictor for GVHD risk,24,25 and we therefore tested whether the relationship between the day 7 TNFR1 ratio correlated with GVHD rates, TRM, and OS within specific donor groups. Mean day 7 TNFR1 ratios were 2.4 plus or minus 0.2 for recipients of unrelated HCT and 2.0 plus or minus 0.1 in recipients of related donor HCT (P = .04). Recipients of related donor HCT (n = 267) with a TNFR1 ratio more than or equal to 2.5 were more likely to develop GVHD (50% vs 26%, P < .001, Figure 1B) and more likely to die of TRM within one year (29% vs 11%, P = .007, Figure 1E) than patients with a ratio less than 2.5 (n = 210). Although OS trended downward in these patients (60% vs 69% at 1 year, P = .12, Figure 1H), this difference was not statistically significant. Results were virtually identical for recipients of unrelated donor transplants (n = 171; Figure 1C,F,I). In a multivariate analysis, including age (treated as a continuous variable), degree of HLA-match, donor type, recipient and donor sex, disease, and status at HCT, a day 7 TNFR1 ratio more than 2.5 strongly correlated with significant GVHD (P < .001), TRM (P < .001), and OS (P = .03), confirming that the day 7 TNFR1 ratio is an independent predictor for these outcomes (Table 2). As expected,24,25 donor type remained a strong independent predictor for GVHD (P < .001), TRM (P < .001), and OS (P < .003) with the risk associated with a day 7 TNFR1 ratio more than 2.5 comparable with the risk associated with receiving an HCT from an unrelated donor. Other findings from the multivariate analysis were also consistent with expectations.26 A single antigen mismatch predicted for increased risk of GVHD (P = .005), whereas younger age at transplantation conferred protection against TRM (P = .001) and death (P = .04). The presence of advanced disease status at transplantation had the greatest impact on survival of all variables tested (HR 7.78, P = .005). For further verification of our findings, we repeated the multivariate analysis with the day 7 TNFR1 ratios treated as a continuous variable and found similar results (Table 3).
Table 2.
Results of multivariate models of time to GVHD, time to treatment-related mortality, and overall survival using threshold day 7 TNFR1 ratio
| Variable | Category | GVHD |
TRM |
OS |
|||
|---|---|---|---|---|---|---|---|
| HR | P | HR | P | HR | P | ||
| Day 7 TNFR1 ratio | ≤ 2.5 | Ref | — | Ref | — | Ref | — |
| > 2.5 | 2.44 | <.001 | 2.40 | <.001 | 1.39 | .03 | |
| Donor type | MRD | Ref | — | Ref | — | Ref | — |
| MUD | 1.87 | <.001 | 3.25 | <.001 | 1.49 | .006 | |
| Match | Yes | Ref | — | Ref | — | Ref | — |
| No | 1.93 | .01 | 1.09 | .79 | 1.25 | .28 | |
| Recipient age* | 1.05 | .35 | 1.28 | .002 | 1.10 | .03 | |
| Donor → recipient | Male → male | Ref | — | Ref | — | Ref | — |
| Female → male | 1.29 | .19 | 1.26 | .39 | 1.02 | .90 | |
| Sex | Male → female | .63 | .04 | .69 | .19 | .88 | .46 |
| Female → female | .80 | .54 | .75 | .57 | 1.01 | .98 | |
| Diagnosis and disease status | Nonmalignant | Ref | — | Ref | — | Ref | — |
| Standard risk | 1.65 | .36 | .96 | .96 | 4.26 | .05 | |
| Advanced risk | 1.74 | .31 | 1.31 | .70 | 7.67 | .005 | |
| Preparative regimen | Non-TBI | Ref | — | Ref | — | Ref | — |
| TBI | 1.14 | .63 | .61 | .20 | 1.18 | .45 | |
GVHD indicates graft-versus-host disease; TRM, treatment-related mortality; OS, overall survival; HR, hazard ratio; TNFR1; TNF receptor 1; Ref, reference group; —, not applicable; MRD, matched related donor; MUD, matched unrelated donor; Standard risk, lymphoma with chemosensitive disease, leukemia in complete remission (ie, CR1, CR2, or CR3), or untreated myelodysplastic syndrome at the time of transplantation; Advanced risk, leukemia not in remission, or lymphoma with stable or progressive disease at the time of transplantation; and TBI, total body irradiation.
For recipient age, the HR reflects the change in hazard for every 10 years.
Table 3.
Results of multivariate models of time to GVHD, time to treatment-related mortality, and overall survival using continuous day 7 TNFR1 ratio
| Variable | Category | GVHD |
TRM |
OS |
|||
|---|---|---|---|---|---|---|---|
| HR | P | HR | P | HR | P | ||
| Day 7 TNFR1 ratio | 1.14 | <0.001 | 1.17 | <0.001 | 1.08 | .02 | |
| Donor type | MRD | Ref | — | Ref | — | Ref | — |
| MUD | 1.90 | <0.001 | 3.37 | <0.001 | 1.50 | .006 | |
| Match | Yes | Ref | — | Ref | — | Ref | — |
| No | 1.99 | 0.005 | 1.07 | 0.83 | 1.24 | .29 | |
| Recipient age* | 1.05 | 0.39 | 1.29 | <0.001 | 1.11 | .03 | |
| Donor → recipient | Male → male | Ref | — | Ref | — | Ref | — |
| Female → male | 1.20 | 0.34 | 1.21 | 0.48 | 1.03 | .87 | |
| Sex | Male → female | 0.62 | 0.03 | 0.71 | 0.23 | 0.89 | .54 |
| Female → female | 0.85 | 0.67 | 0.79 | 0.62 | 1.00 | >.99 | |
| Diagnosis and disease status | Nonmalignant | Ref | — | Ref | — | Ref | — |
| Standard risk | 1.70 | 0.32 | 0.94 | 0.93 | 4.17 | .05 | |
| Advanced risk | 1.66 | 0.35 | 1.25 | 0.74 | 7.42 | .006 | |
| Preparative regimen | Non-TBI | Ref | — | Ref | — | Ref | — |
| TBI | 1.08 | 0.80 | 0.63 | 0.23 | 1.19 | .42 | |
GVHD indicates graft-versus-host disease; TRM, treatment-related mortality; OS, overall survival; HR, hazard ratio; TNFR1; TNF receptor 1; Ref, reference group; —, not applicable; MRD, matched related donor; MUD, matched unrelated donor; Standard risk, lymphoma with chemosensitive disease, leukemia in complete remission (ie, CR1, CR2, or CR3), or untreated myelodysplastic syndrome at the time of transplantation; Advanced risk, leukemia not in remission, or lymphoma with stable or progressive disease at the time of transplantation; and TBI, total body irradiation.
For recipient age, the HR reflects the change in hazard for every 10 years.
Our data indicate that the magnitude of rise in TNFR1 levels 2 to 3 weeks in advance of clinical symptoms strongly correlates with the severity and incidence of GVHD, 1-year TRM, and 1-year OS even within risk groups stratified by donor source. The specificity of a day 7 TNFR1 ratio more than or equal to 2.5 for predicting GVHD was 83%. However, the sensitivity of a TNFR1 ratio more than or equal to 2.5 to predict GVHD is only 38% because the majority of patients who develop grades 2 to 4 GVHD have a TNFR1 ratio less than 2.5. Nevertheless, in patients with a TNFR1 ratio more than or equal to 2.5, the likelihood of developing significant GVHD is sufficiently high (58%) to justify the study of preemptive treatment strategies. A recent study has shown that patients with GVHD who are treated with the TNF-α inhibitor etanercept in addition to standard, high-dose steroids experience higher complete response rates than patients treated with steroids alone.14 One logical strategy to treat patients at high risk for GVHD would therefore be to initiate preemptive TNF-α blockade in patients with high day 7 TNFR1 ratios in an attempt to prevent the occurrence of GVHD in this high-risk group. The identification of additional biomarkers that predict GVHD may further refine risk groups and thereby aid in the development of preemptive strategies that could ultimately reduce transplantation-related toxicity.
Acknowledgments
The authors thank Pam James and Charlotte Zinkus for their technical assistance, the BMT Program Team at the University of Michigan Clinical Trials Office for outstanding data collection and management, and the patients and their families whose participation made this research possible.
This work was supported by grants from the National Institutes of Health (2P01CA039542-18 and 5P30CA046592) and the Doris Duke Charitable Foundation. J.L.M.F. is the recipient of a Doris Duke Distinguished Clinical Scientist Award. S.W.C. is a recipient of the Alex's Lemonade Stand Foundation Young Investigator Award.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.W.C., J.E.L., T.B., and J.L.M.F. designed and planned the study; T.B. was the study statistician; S.P. and J.W. performed the cytokine assays; S.W.C., D.J., C.L.K., and S.P. were in charge of data collection and quality assurance; and all authors participated in writing the report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John E. Levine, University of Michigan Comprehensive Cancer Center, 1500 East Medical Center Drive, 5303 Comprehensive Cancer Center, Ann Arbor, MI 48109-5942; e-mail: jelevine@med.umich.edu.
References
- 1.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 2.Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–2968. [PubMed] [Google Scholar]
- 3.Krenger W, Hill GR, Ferrara JL. Cytokine cascades in acute graft-versus-host disease. Transplantation. 1997;64:553–558. doi: 10.1097/00007890-199708270-00001. [DOI] [PubMed] [Google Scholar]
- 4.Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001;29:259–277. doi: 10.1016/s0301-472x(00)00677-9. [DOI] [PubMed] [Google Scholar]
- 5.Hattori K, Hirano T, Miyajima H, et al. Differential effects of anti-Fas ligand and anti-tumor necrosis factor alpha antibodies on acute graft-versus-host disease pathologies. Blood. 1998;91:4051–4055. [PubMed] [Google Scholar]
- 6.Cooke KR, Hill GR, Crawford JM, et al. Tumor necrosis factor- alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998;102:1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara JL, Levy R, Chao NJ. Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transplant. 1999;5:347–356. doi: 10.1016/s1083-8791(99)70011-x. [DOI] [PubMed] [Google Scholar]
- 8.Korngold R, Marini JC, De Baca ME, Murphy GF, Giles-Komar J. Role of tumor necrosis factor-alpha in graft-versus-host disease and graft-versus-leukemia responses. Biol Blood Marrow Transplant. 2003;9:292–303. doi: 10.1016/s1083-8791(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 9.Xun CQ, Thompson JS, Jennings CD, Brown SA, Widmer MB. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83:2360–2367. [PubMed] [Google Scholar]
- 10.Holler E, Kolb HJ, Moller A, et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990;75:1011–1016. [PubMed] [Google Scholar]
- 11.Holler E, Kolb HJ, Hintermeier-Knabe R, et al. Role of tumor necrosis factor alpha in acute graft-versus-host disease and complications following allogeneic bone marrow transplantation. Transplant Proc. 1993;25:1234–1236. [PubMed] [Google Scholar]
- 12.Jacobsohn DA, Hallick J, Anders V, McMillan S, Morris L, Vogelsang GB. Infliximab for steroid-refractory acute GVHD: A case series. Am J Hematol. 2003;74:119–124. doi: 10.1002/ajh.10392. [DOI] [PubMed] [Google Scholar]
- 13.Couriel D, Saliba R, Hicks K, et al. Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood. 2004;104:649–654. doi: 10.1182/blood-2003-12-4241. [DOI] [PubMed] [Google Scholar]
- 14.Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute GVHD. Blood. 2008;111:2470–2475. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumor necrosis factor receptors: structure and function. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 16.Hintermeier-Knabe R, Brockhaus M, Holler E, et al. Sequential release of tumor necrosis factor alpha and tumor necrosis factor receptors in complications of human bone marrow transplantation. In: Freund M, Link H, Schmidt RE, editors. Cytokines in Hematopoiesis, Oncology, and AIDS II. Berlin: Springer-Verlag; 1992. pp. 443–448. [Google Scholar]
- 17.Or R, Kalinkovich A, Nagler A, et al. Soluble tumor necrosis factor (sTNF) receptors: a possible prognostic marker for bone marrow transplantation-related complications. Cytokines Mol Ther. 1996:243–250. [PubMed] [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 20.Thomas ED, Storb R, Clift CD, et al. Bone-marrow transplantation, second of two parts. N Engl J Med. 1975;292:895–902. doi: 10.1056/NEJM197504242921706. [DOI] [PubMed] [Google Scholar]
- 21.Armitage JO. Bone marrow transplantation. N Engl J Med. 1994;330:827–838. doi: 10.1056/NEJM199403243301206. [DOI] [PubMed] [Google Scholar]
- 22.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: secondary treatment. Blood. 1991;77:1821–1828. [PubMed] [Google Scholar]
- 23.Breiman L, Friedman JH, Olshen RA, Stone CJ. Monterey, CA: Wadsworth; 1984. Classification and Regression Trees. [Google Scholar]
- 24.de Lima M, Champlin R. Unrelated donor hematopoietic transplantation. Rev Clin Exp Hematol. 2001;5:100–134. doi: 10.1046/j.1468-0734.2001.00035.x. [DOI] [PubMed] [Google Scholar]
- 25.Yakoub-Agha I, Mesnil F, Kuentz M, et al. Allogeneic marrow stem cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24:5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan K M. Graft versus host disease. In: Blume KG, Foreman SJ, Appelbaum FR, editors. Thomas' Hematopoietic Cell Transplantation. Oxford, United Kingdom: Blackwell; 2003. pp. 635–664. [Google Scholar]