Abstract
Several lines of evidence support the presence of dosage-sensitive genes on chromosome 21 that regulate leukemogenesis and hematopoiesis. We report a detailed clinical and molecular characterization of 3 patients with chronic thrombocytopenia caused by distinct constitutional microdeletions involving chromosomal region 21q22.12. The patients exhibited growth restriction, dysmorphic features, and developmental delays. One patient developed acute myelogenous leukemia (AML) at 6 years of age. All 3 deletions included the RUNX1, CLIC6, DSCR, and KCNE1 genes. Our data provide additional support for the role of RUNX1 haploinsufficiency in megakaryopoiesis and predisposition to AML. The leukemic clone had trisomy 21 resulting from duplication of chromosome 21 containing the RUNX1 deletion. This shows that genes other than RUNX1 must also play a role in AML associated with trisomy 21. We recommend that children with syndromic thrombocytopenia have clinical array-comparative genomic hybridization analysis and appropriate cytogenetic studies to facilitate our ability to provide a definitive diagnosis.
Introduction
Acquired and constitutional structural genomic aberrations leading to the activation of oncogenes or haploinsufficiency of tumor suppressor genes are well-known pathogenic mechanisms in cancer. The predisposition for leukemia and myelodysplasia1,2 and the common hematologic abnormalities3 seen in children with trisomy 21 suggest that dosage-sensitive genes on chromosome 21 are involved in leukemogenesis and hematopoiesis. The “leukemogenic” role of an additional chromosome 21 was further supported by showing the selective involvement of trisomic cells with leukemia in patients with trisomy 21 mosaicism.4 In addition, trisomy/polysomy 21and translocations that disrupt the RUNX1 gene on chromosome 21q22 are nonrandom and are among the most frequently acquired chromosomal abnormalities in acute lymphoblastic leukemia (ALL) and acute myelocytic leukemia (AML).5–7 This dosage effect is also seen in patients with a subtype of ALL with intrachromosomal amplification of chromosome 21 encompassing the RUNX1 gene (iAMP21) who have poor outcome.8
The significant role of RUNX1 in megakaryopoiesis and leukemogenesis was further supported by the finding that haploinsufficiency of the RUNX1 gene is the genetic basis of the autosomal dominant familial platelet disorder with predisposition to acute myelogenous leukaemia (FPD/AML; MIM 601399).9 Similar to patients with the FPD/AML syndrome, there was an approximately 15% reduction in the number of platelets in the Runx1+/− mice,10 but the development of AML could not be recapitulated in these mice.10,11 In addition, somatic mutations in RUNX1 and its cofactor CBFB are frequently found in acute leukemias and myelodysplastic syndrome (MDS).12,13
Here, we present the molecular analysis and fine mapping of constitutional microdeletions on 21q encompassing the RUNX1 gene in 3 patients with chronic thrombocytopenia. One of the 3 patients developed AML at the age of 6 years. In addition, the patients had growth restriction, dysmorphic features, and variable degrees of developmental delay.
Methods
The parents of all 3 subjects gave informed consent to participate in human subject protocols approved by the Institutional Review Board (IRB) of Baylor College of Medicine. Patient 1 was enrolled on IRB-approved protocol entitled “The Molecular Basis of Familial Cancer Predisposition Syndromes.” Patients 2 and 3 were enrolled on IRB-approved protocol entitled “Use of microarrays (DNA chips) for global detection of cytogenetic abnormalities by comparative genomic hybridization.” Both studies include forms that specifically allow parents to provide consent for the use of photographs for publication. The informed consents were obtained in accordance with the Declaration of Helsinki.
Cytogenetic and fluorescence in situ hybridization analyses
Chromosome analysis of phytohemagglutinin (PHA)–stimulated peripheral blood lymphocytes (for all patients) and of bone marrow (for patient 1) was performed by GTG-banding analysis using standard cytogenetic laboratory procedures. Fluorescence in situ hybridization (FISH) analysis using probes specific for ETO and RUNX1 genes and other specific probes for chromosome 21 were performed in a PHA-stimulated peripheral blood sample obtained from the patients. DNA for home-brewed FISH probes was extracted and directly labeled according to the manufacturers' instructions (Abbott Molecular, Abbott Park, IL).
Clinical chromosome microarray analysis
The Baylor College of Medicine Chromosome Microarray Analysis (CMA) (http://www.bcm.edu/geneticlabs/cma/tables/detectionratesoligo.pdf) Version 6.3 (CMA V6.3) and CMA V5 were used to study patient 2 and patient 3, respectively.14,15
Deletion mapping by high-resolution oligonucleotide array
Further characterization of the deletions was performed using the Agilent 244K Whole Human Genome CGH arrays (Agilent Technologies, Palo Alto, CA) containing 236 000 probes. The procedures for DNA digestion, labeling, hybridization, and data analyses were performed according to the manufacturer's protocol (Agilent Technologies).
Results
Clinical reports
In this report, we describe the clinical and molecular characterization of 3 patients with chronic thrombocytopenia, dysmorphic features (see Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), congenital anomalies, and variable developmental delay. A summary of the clinical features of all 3 patients is provided in Table 1. The average platelets counts were similar in all 3 patients: 78.0 × 109/L (78 000 platelets/mm3), 60.0 × 109/L (60 000 platelets/mm3), and 74.0 × 109/L (74 000 platelets/mm3) in patients 1, 2, and 3, respectively. Before the cytogenetic analyses described below, all 3 patients had extensive clinical work-ups, eg, diepoxy butane (DEB) breakage, which failed to show the cause of their thrombocytopenia. In patient 3, the platelet count was only requested after the karyotype and microarray analyses showed that the deletion encompassed the RUNX1 gene. More detailed description of the clinical features and diagnostic work-up of each patient are provided in Document S1.
Table 1.
Clinical features of the 3 patients described with syndromic thrombocytopenia
| Feature | Patient 1 (male) | Patient 2 (female) | Patient 3 (female) |
|---|---|---|---|
| Race-ethnicity | White-European | White-European/Filipino | White-Hispanic |
| Age thrombocytopenia first detected, mo | 18 | 19 | 9 |
| Average platelet count, × 109/L (platelets/mm3) | 78.0 (78 000) | 60.0 (60 000) | 74.0 (74 000) |
| Other hematologic findings | MDS/AML at age 6 y | Macrocytosis | — |
| Birth weight, g (percentile) | 2920 (25th) | 2041 (<3rd) | 1955 (<3rd) |
| Growth delay | + | + (gastric tube) | + |
| Developmental delay | Fine motor | Global | Global |
| FOC* | 5th-10th percentile | <3rd percentile | <3rd percentile |
| Weight* | 5th percentile | <3rd percentile | <3rd percentile |
| Height* | <3rd percentile | <3rd percentile | <3rd percentile |
| Eyes | Hypertelorism, almond-shaped | Hypertelorism, epicanthal folds, sparse eyebrows | Hypertelorism, epicanthal folds, small/deep-set, strabismus |
| Palate/mouth | High-arched/abnormal enamel | Intact/smooth philtrum | Intact |
| Nose | Anteverted, broad nasal root | Short, anteverted | Flat nasal bridge |
| Chest | Inverted nipples | Inverted nipples | Inverted nipples |
| Skull | Prominent forehead | Normocephalic | High, broad forehead; flat occiput |
| Other anomalies | Transposition of great arteries, absence of left testis | Toenail hypoplasia, delayed myelination and dysgenic corpus callosum | Neonatal seizures, alopecia |
FOC indicates fronto-occipital circumference; and MDS/AML, myelodysplastic syndrome and acute myelogenous leukemia.
Measurements at last clinical evaluation.
Clinical cytogenetic, FISH, and array–comparative genome hybridization analyses
When patient 1 was 6 years old, he presented to our center with chronic thrombocytopenia and newly diagnosed MDS/AML. Cytogenetic evaluation of unstimulated bone marrow showed an abnormal cell line with duplication of chromosome 7p14-p15 along with 3 copies of chromosome 21 in 13 of 20 cells examined (Figure 1A). Cytogenetic evaluation of PHA-stimulated peripheral blood showed the same abnormal cell line in 7 of 20 cells examined. Given chronic thrombocytopenia, AML, multiple congenital anomalies, and dysmorphic features the clinical diagnosis of a constitutional deletion involving the RUNX1 locus was made. The clinically available array comparative genomic hybridization (array-CGH) analysis at that time did not adequately cover the RUNX1 locus; therefore, direct FISH analysis of the RUNX1 locus was requested. FISH evaluation of PHA-stimulated peripheral blood that used probes specific for ETO and RUNX1 genes showed 1 strong RUNX1 signal with 1 “dim” RUNX1 signal in 20% interphase cells examined and 1 strong RUNX1 signal and 2 dim RUNX1 signals in the remaining 80% of cells (Figure 1B). A bone marrow aspirate at the same time showed similar findings with the same RUNX1 patterns described above (data not shown). FISH analysis of peripheral blood from both parents showed 2 strong RUNX1 signals (data not shown), consistent with a de novo deletion in patient 1. The patient successfully received a bone marrow transplant, and follow-up studies showed engraftment with female 46 XX donor cells.
Figure 1.
Karyotype and FISH analysis in patient 1. (A). Cytogenetic analysis of peripheral blood abnormal cell showing duplication of 7p14-p15 (black arrow) and 3 copies of chromosome 21 (red arrow). (B). FISH on peripheral blood using probes specific for ETO (orange) and RUNX1 (green) genes. Note the presence of one strong and one dim green signal in the metaphase and lower left interphase nucleus confirming the constitutional RUNX1 deletion. In the lower right interphase nucleus there are 1 strong and 2 “dim” RUNX1 signals, representing a trisomy 21 cell line with duplication of the deleted chromosome 21.
The peripheral blood karyotype of patient 2 at age 19 months showed normal 46 XX. Clinical array-CGH with CMA V6.3 showed a loss in copy number on 21q, detected with oligonucleotides emulating a single BAC clone (Figure 2A), and confirmed by FISH analysis with BAC clone [RP11-17O20] that includes the RUNX1 gene (Figure 2B). Parental FISH analysis with the same BAC showed a normal pattern in the mother. In the father, the BAC hybridized to one chromosome 21 and a second hybridization signal on the long arm of one chromosome 11 (Figure 2B). The same insertional rearrangement was found in 1 of 4 siblings of patient 2.
Figure 2.
Array-CGH and FISH analyses of patient 2. (A). An output of the clinical array-CGH (CMA V6.3) of patient 2. This profile represents the averaged combined data of hybridizations performed using reference DNA. Oligonucleotides emulating the clone RP11-17O20 (arrow) shows down displacement, indicating a loss of chromosome 21 material in the patient versus the reference DNA. (B). Representative data from metaphase FISH analysis in patient 2 and her father. The RP11-17O20 clone was used as probe (red). The green signal is a subtelomeric probe used as a control. The patient has a single red signal consistent with a deletion on 1 of the 2 copies of chromosome 21. The FISH analysis in the father shows one red signal on chromosome 21 and the second signal inserted in the long arm of chromosome 11. Reverse banding (inset) confirmed the insertion to chromosome 11.
Karyotype analysis in patient 3 at birth showed a deletion at 21q22.1. The mother's karyotype was normal, but the father was not available for analysis. On the basis of the molecular results described below, FISH analysis was performed using 2 BAC clones from 21q: RP11-17O20 more distal and RP11-92D3 more proximal. In 15% of interphase nuclei there was 1 signal for both BACs, and in 85% there were 2 signals for the proximal BAC and a single signal for the distal (more telomeric) BAC (data not shown).
The clinical CMA result for patient 3 also showed a loss of copy number with 11 clones (from RP11-17O20 to clone RP4-639D23) that encompass at least 11 megabases on 21q22.1 (data not shown). The T statistic values for centromeric clones were higher than for the more telomeric clones, consistent with the finding from FISH analysis that there is a mixed population of cells with different size microdeletions.
High-resolution genomic analyses by 244K array-CGH
Patient 1 had high-resolution analysis using the Affymetrix 50K SNPchip (data not shown), which was consistent with the Agilent array data described below. Subsequently, all 3 patients had research analysis using the Agilent 244K Whole Human Genome CGH Microarray to better define the extent of deletion. The results are compared with genome sequence data (hg18; NCBI Build 36.1) (Figure 3). The analysis was done using genomic DNA isolated from a blood sample for all 3 patients. This was performed at the time of diagnosis of leukemia in patient 1, and the deletion was found to be approximately 0.7 Mb (megabases) in size on band 21q22.12 (position 34 796-35 507 Kb). This small microdeletion is embedded on a microarray profile that shows gain of all the other oligonucleotides from chromosome 21, again reflecting trisomy 21 in the leukemic clone. In addition, the microarray analysis showed gain of approximately 24.1 Mb on the short arm of chromosome 7 between positions 19 767 and 44 578 Kb (data not shown).
Figure 3.
Agilent 244K Human Genome CGH Microarray hybridization profile of chromosome 21 for the 3 patients. The size of the deletions is indicated by the blue bars. The small microdeletion in patient 1 is embedded within a profile that shows copy number gain with all the other oligonucleotides from chromosome 21 (red bar) reflecting the trisomy 21. The profile of patient 3 shows that the deflection of the log2 ratio (the green deflection from 0) is not uniform, indicating the presence of mosaicism in the cell population. On the basis of the FISH data, only 15% of the cells in patient 3 have the large (approximately 19.7 Mb) deletion and are deleted for the RUNX1 gene.
The size of the deletion in patient 2 was approximately 1.81 Mb, extending from 33 833 to 35 647 Kb on 21q22.12. The deletion in patient 3 is much larger and is approximately 19.8 Mb, encompassing the region between 27 198 and 46 915 Kb (terminal part of 21q). Consistent with the CMA and FISH analyses, there was evidence for a mixed population (mosaicism) with nonuniform deflection of the log2 ratio; approximately half with a 19.7-Mb deletion and approximately half with deletion of only the distal 9.1 Mb of the same region.
Discussion
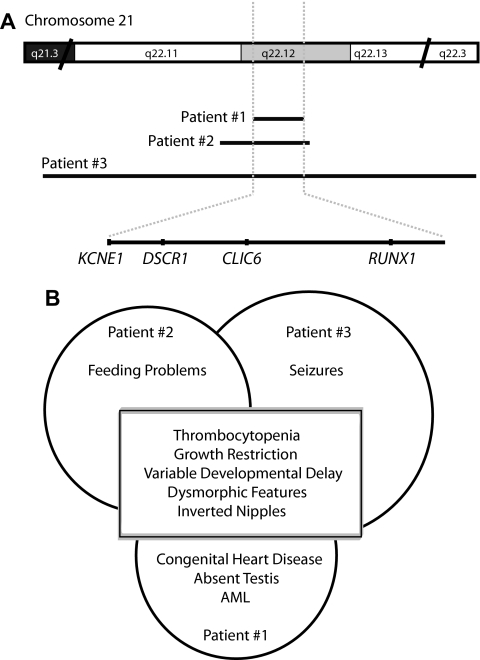
We report 3 patients with chronic thrombocytopenia associated with multiple congenital anomalies, dysmorphic features, growth restriction, and developmental delay. One of the 3 patients developed AML at the age of 6 years. All 3 patients showed constitutional microdeletions encompassing band 21q22.12. The deletions were variable in size and therefore were detected clinically by different techniques, including routine karyotype for the largest deletion, clinical array-CGH, and targeted FISH for the RUNX1 locus. Research studies using the Agilent 244K oligonucleotide array more finely delineated the deletions. There is a common deleted region of approximately 0.7 Mb among the 3 patients which encompasses 4 known genes: RUNX1, CLIC6, DSCR1, and a portion of KCNE1 (Figure 4A).
Figure 4.
Summary of the genetic and clinical findings in our patients. (A) Schematic representation of the deleted regions on 21q22.12 (not drawn to scale). The deletion map was constructed using the USCS human genome browser (http://genome.ucsc.edu/). The black lines show the extents of the deletions. The shaded area is the overlap between the deletions in the 3 patients. The genes located within this interval are at the bottom of the figure. (B) Diagram of the shared and unique clinical features of the 3 patients described in this report.
The FISH analysis with a RUNX1 probe on cells from patient 1 showed that the microdeletion was constitutional and not secondary to leukemia development. Both FISH and array-CGH show that the trisomic chromosome in the leukemia cells contains the RUNX1 deletion. This finding provides strong evidence that, although deletion of the RUNX1 locus is responsible for the thrombocytopenia and predisposition to AML, other dosage-sensitive genes on chromosome 21 also play a role in the development of leukemia. In addition, the leukemic clone contains duplication of 7p that has not been previously reported in AML (http://www.cancerindex.org). Interestingly, the duplicated region contains the cluster of HOX genes that have been implicated in AML pathogenesis.16,17
In all 3 cases, the subjects had undergone extensive evaluations, including a variety of different molecular and biochemical analyses, chromosome fragility testing, and muscle biopsy (see Document S1). Clinical use of array-CGH analysis earlier in the diagnostic work-up for syndromic thrombocytopenia will allow for rapid identification of the causative deletion.
The molecular basis of the deletion was different in all 3 patients with patient 1 resulting from a de novo deletion, patient 2 from a paternal insertional translocation, and patient 3 with likely postzygotic deletions resulting in mosaicism for 2 different-sized deletions. Patient 3 had thrombocytopenia, although only 15% of the cells were deleted for the RUNX1 gene. Of note, the father and sibling of patient 2 are cytogenetically balanced and would appear normal on array-CGH analyses because of the approximately 1.8-Mb insertional rearrangement. This illustrates the need for the appropriate cytogenetic analyses of the parents of affected patients to correctly define the recurrence risk and to offer prenatal testing to the family if so desired.
Comparing these findings with those from published cases is challenging because hematologic variables are not always reported; deletions are heterogeneous in nature and can occur with additional chromosomal rearrangements. Descriptions from the literature show that patients with partial monosomy 21 exhibit variable phenotype dominated by prenatal and postnatal growth restriction, severe developmental delay, abnormal muscle tone, a variety of dysmorphic facial features, and heart defects.18,19 Hematologic findings and thrombocytopenia were reported in 4 cases with isolated 21q deletion only but may have been more commonly present if assayed.20,21All 3 patients described here share the following features: thrombocytopenia, variable developmental delay, hypertelorism, and inverted nipples (Figure 4B). Phenotypic findings that were found in 2 of our patients included broad forehead, epicanthal folds, and abnormally shaped eyes and nose. Our data indicate that one or more of the genes within the minimal overlap region (RUNX1, CLIC6, DSCR1, and KCNE1) are involved in the dysmorphic phenotype and possibly the developmental delay in patients with 21q microdeletion.
The KCNE1 gene encodes the potassium voltage-gated channel involved in the long QT syndrome (LQTS). Heterozygous loss-of-function mutations of KCNE1 cause the LQTS (MIM 176261),22 and homozygous or compound heterozygous mutations of KCNE are responsible for prolonged QT and sensorineural hearing loss (Jervell and Lange-Nielsen syndrome; MIM 220400).23 Interestingly, although these abnormalities (including measurement of the QT interval) were not found in our patients, a cardiology evaluation is indicated in this microdeletion syndrome, given that not all patients with LQTS show an increased QT interval at rest.
The molecular data on our patients provide additional support for the significant role of RUNX1 haploinsufficiency in the development of thrombocytopenia because it results from a complete gene deletion as opposed to any potential dominant-negative effect from point mutations as previously described in FPD/AML.9
The hematologic abnormalities, clinical history, physical findings, and molecular defects in our patients add to our understanding of the heterogeneous group of hereditary thrombocytopenias.24 In particular, we show the clinical utility of array-CGH technology in the assessment of patients with thrombocytopenia and congenital anomalies, developmental delay, or both.
Conclusions
Deletions encompassing the RUNX1 gene on 21q22.12 are responsible for syndromic thrombocytopenia and predisposition to AML. Patients with this contiguous gene syndrome also exhibit dysmorphic features, developmental delay, and growth restriction (Figure 4B). Molecular analysis of leukemia samples from one patient deleted for RUNX1 confirm that haploinsufficiency of RUNX1 is associated with AML development but that other genes on chromosome 21 also play a role in trisomy 21 associated with AML. We predict that a wider clinical application of array-CGH will significantly improve our ability to provide a definitive diagnosis for patients with syndromic chronic thrombocytopenia or AML and will limit the extensive diagnostic work-up that is usually performed for these patients.
Supplementary Material
Acknowledgments
We thank the patients and families described here for their willingness to participate in our research study.
This work was supported by a grant from Alex's Lemonade Stand Foundation (S.E.P.), by the National Institutes of Health/National Institute of General Medical Sciences T32 (GM07526) (A.E.), by a Hematology Training Grant (T32 DK60445; D.L.S.), and by the Molecular Medicine Scholars Program (T32HL066991; D.L.S.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.S. wrote the paper and performed the clinical evaluation of patient 3; A.E. performed the 244K microarray experiments; D.L.S. participated in the molecular analysis of patient 1; B.L. participated in the clinical evaluation of patient 2; R.N. performed the FISH analysis on patient 1; G.W. participated in the 244K microarray experiments; A.C.C. participated in the 244K microarray experiments and data analysis; S.W.C. reviewed and provided the cytogenetic and clinical microarray data; and S.E.P. (principal investigator of this study) performed the clinical evaluation of patient 1 and edited the paper.
Conflict-of-interest disclosure: The Department of Molecular and Human Genetics at Baylor College of Medicine offers extensive genetic laboratory testing and S.W.C., A.C.C., and G.W. derive revenue from this activity. The remaining authors declare no competing financial interests.
Correspondence: Sharon E. Plon, MC3-3320, 6621 Fannin Street, Houston, TX 77030; e-mail: splon@bcm.edu.
References
- 1.Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down's syndrome. Lancet. 2000;355:165–169. doi: 10.1016/S0140-6736(99)05264-2. [DOI] [PubMed] [Google Scholar]
- 2.Izraeli S, Rainis L, Hertzberg L, Smooha G, Birger Y. Trisomy of chromosome 21 in leukemogenesis. Blood Cells Mol Dis. 2007;39:156–159. doi: 10.1016/j.bcmd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Henry E, Walker D, Wiedmeier SE, Christensen RD. Hematological abnormalities during the first week of life among neonates with Down syndrome: data from a multihospital healthcare system. Am J Med Genet A. 2007;143:42–50. doi: 10.1002/ajmg.a.31442. [DOI] [PubMed] [Google Scholar]
- 4.Ferster A, Verhest A, Vamos E, De Maertelaere E, Otten J. Leukemia in a trisomy 21 mosaic: specific involvement of the trisomic cells. Cancer Genet Cytogenet. 1986;20:109–113. doi: 10.1016/0165-4608(86)90113-5. [DOI] [PubMed] [Google Scholar]
- 5.Berger R. Acute lymphoblastic leukemia and chromosome 21. Cancer Genet Cytogenet. 1997;94:8–12. doi: 10.1016/s0165-4608(96)00351-2. [DOI] [PubMed] [Google Scholar]
- 6.Rubnitz JE, Look AT. Molecular genetics of childhood leukemias. J Pediatr Hematol Oncol. 1998;20:1–11. doi: 10.1097/00043426-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Look AT. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278:1059–1064. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- 8.Strefford JC, van Delft FW, Robinson HM, et al. Complex genomic alterations and gene expression in acute lymphoblastic leukemia with intra-chromosomal amplification of chromosome 21. Proc Natl Acad Sci U S A. 2006;103:8167–8172. doi: 10.1073/pnas.0602360103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song WJ, Sullivan MG, Legare RD, et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet. 1999;23:166–175. doi: 10.1038/13793. [DOI] [PubMed] [Google Scholar]
- 10.Sun C, Downing JR. Haploinsufficiency of AML1 results in a decrease in the number of LTR-HSCs while simultaneously inducing an increase in more mature progenitors. Blood. 2004;104:3565–3572. doi: 10.1182/blood-2003-12-4349. [DOI] [PubMed] [Google Scholar]
- 11.Matheny CJ, Speck ME, Cushing PR, et al. Disease mutations in RUNX1 and RUNX2 create nonfunctional, dominant-negative, or hypomorphic alleles. EMBO J. 2007;26:1163–1175. doi: 10.1038/sj.emboj.7601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada H, Harada Y, Niimi H, Kyo T, Kimura A, Inaba T. High incidence of somatic mutations in the AML1/RUNX1 gene in myelodysplastic syndrome and low blast percentage myeloid leukemia with myelodysplasia. Blood. 2004;103:2316–2324. doi: 10.1182/blood-2003-09-3074. [DOI] [PubMed] [Google Scholar]
- 13.Osato M. Point mutations in the RUNX1/AML1 gene: another actor in RUNX leukemia. Oncogene. 2004;23:4284–4296. doi: 10.1038/sj.onc.1207779. [DOI] [PubMed] [Google Scholar]
- 14.Cheung SW, Shaw CA, Yu W, et al. Development and validation of a CGH microarray for clinical cytogenetic diagnosis. Genet Med. 2005;7:422–432. doi: 10.1097/01.gim.0000170992.63691.32. [DOI] [PubMed] [Google Scholar]
- 15.Ou Z, Kang S-HL, Shaw CA, et al. BAC-emulation oligonucleotide arrays for targeted clinical array-CGH analyses. Genet Med. 2008;10:278–289. doi: 10.1097/GIM.0b013e31816b4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischbach NA, Rozenfeld S, Shen W, et al. HOXB6 overexpression in murine bone marrow immortalizes a myelomonocytic precursor in vitro and causes hematopoietic stem cell expansion and acute myeloid leukemia in vivo. Blood. 2005;105:1456–1466. doi: 10.1182/blood-2004-04-1583. [DOI] [PubMed] [Google Scholar]
- 17.Fröhling S, Scholl C, Bansal D, Huntly BJ. HOX gene regulation in acute myeloid leukemia: CDX marks the spot? Cell Cycle. 2007;6:2241–2245. doi: 10.4161/cc.6.18.4656. [DOI] [PubMed] [Google Scholar]
- 18.Chettouh Z, Croquette MF, Delobel B, et al. Molecular mapping of 21 features associated with partial monosomy 21: involvement of the APP-SOD1 region. Am J Hum Genet. 1995;57:62–71. [PMC free article] [PubMed] [Google Scholar]
- 19.Yao G, Chen XN, Flores-Sarnat L, et al. Deletion of chromosome 21 disturbs human brain morphogenesis. Genet Med. 2006;8:1–7. doi: 10.1097/01.gim.0000195892.60506.3f. [DOI] [PubMed] [Google Scholar]
- 20.Warren RJ, Rimoin DL. The G deletion syndromes. J Pediatr. 1970;77:658–663. doi: 10.1016/s0022-3476(70)80209-8. [DOI] [PubMed] [Google Scholar]
- 21.Huret JL, Léonard C, Chery M, et al. Monosomy 21q: two cases of del(21q) and review of the literature. Clin Genet. 1995;48:140–147. doi: 10.1111/j.1399-0004.1995.tb04074.x. [DOI] [PubMed] [Google Scholar]
- 22.Splawski I, Tristani-Firouzi M, Lehmann MH, et al. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet. 1997;17:338–340. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 23.Schulze-Bahr E, Wang Q, Wedekind H, et al. KCNE1 mutations cause Jervell and Lange-Nielsen syndrome. Nature Genet. 1997;17:267–268. doi: 10.1038/ng1197-267. [DOI] [PubMed] [Google Scholar]
- 24.Balduini CL, Iolascon A, Savoia A. Inherited thrombocytopenias: from genes to therapy. Haematologica. 2002;87:860–880. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.