Abstract
The potent bioactive sphingolipid mediator, sphingosine-1-phosphate (S1P), is produced by 2 sphingosine kinase isoenzymes, SphK1 and SphK2. Expression of SphK1 is up-regulated in cancers, including leukemia, and associated with cancer progression. A screen of sphingosine analogs identified (2R,3S,4E)-N-methyl-5-(4′-pentylphenyl)-2-aminopent-4-ene-1,3-diol, designated SK1-I (BML-258), as a potent, water-soluble, isoenzyme-specific inhibitor of SphK1. In contrast to pan-SphK inhibitors, SK1-I did not inhibit SphK2, PKC, or numerous other protein kinases. SK1-I decreased growth and survival of human leukemia U937 and Jurkat cells, and enhanced apoptosis and cleavage of Bcl-2. Lethality of SK1-I was reversed by caspase inhibitors and by expression of Bcl-2. SK1-I not only decreased S1P levels but concomitantly increased levels of its proapoptotic precursor ceramide. Conversely, S1P protected against SK1-I–induced apoptosis. SK1-I also induced multiple perturbations in activation of signaling and survival-related proteins, including diminished phosphorylation of ERK1/2 and Akt. Expression of constitutively active Akt protected against SK1-I–induced apoptosis. Notably, SK1-I potently induced apoptosis in leukemic blasts isolated from patients with acute myelogenous leukemia but was relatively sparing of normal peripheral blood mononuclear leukocytes. Moreover, SK1-I markedly reduced growth of AML xenograft tumors. Our results suggest that specific inhibitors of SphK1 warrant attention as potential additions to the therapeutic armamentarium in leukemia.
Introduction
Sphingosine-1-phosphate (S1P), a potent lipid mediator produced from sphingosine by sphingosine kinases (SphKs), regulates many processes important for cancer progression, including cell growth and survival.1 In contrast to S1P, its precursors, sphingosine and ceramide, are associated with growth arrest and induction of apoptosis.2 Thus, the balance between these interconvertible sphingolipid metabolites has been viewed as a cellular rheostat determining cell fate.3 Numerous studies have shown that perturbations in the S1P/ceramide rheostat are involved in the regulation of resistance to chemotherapy and radiation therapy of neoplastic cells, including those of hematopoietic origin.2,4,5
Two SphK isoenzymes, SphK1 and SphK2, have been described that, although sharing many features,6,7 exhibit distinct functions. SphK1 promotes cell growth and survival,8–11 whereas SphK2, when overexpressed, has opposite effects.12,13 SphK1 is a key enzyme that regulates the S1P/ceramide rheostat.12,14,15 Indeed, S1P and SphK1 have long been implicated in resistance of both primary leukemic cells and leukemia cell lines to apoptosis induced by commonly used cytotoxic agents.3,16–18 Non–isoenzyme-specific inhibitors of SphKs, such as l-threo-dihydrosphingosine (safingol) and N,N-dimethylsphingosine (DMS), are cytotoxic to leukemia cells.18,19 Interestingly, multidrug-resistant HL-60 myelogenous leukemia cells were more sensitive to DMS than the parental cells.18 Moreover, SphK1 activity was lower in HL-60 cells sensitive to doxorubicin or etoposide than in MDR (multidrug resistance protein)–1- or MRP1 (multidrug resistance protein 1)–positive HL-60 cells. Enforced expression of SphK1 in sensitive HL-60 cells blocked apoptosis, whereas down-regulation of SphK1 overcame chemoresistance by inducing mitochondria-dependent apoptosis.10 These observations take on added significance in light of evidence that MDR expression is a strong prognostic indicator in acute myelogenous leukemia (AML)20 and that the MDR phenotype, which commonly arises after treatment of AML with anthracyclines or plant-based alkaloids, is thought to represent an obstacle to successful chemotherapy. In addition, resistance of K562 human chronic myeloid leukemia cells to imatinib, an inhibitor of Bcr-Abl tyrosine kinase, correlated with expression of SphK1 and generation of S1P, whereas down-regulation of SphK1 increased sensitivity to imatinib-induced apoptosis in resistant cells.21 Thus, the development of effective and specific inhibitors of SphK1 might prove useful not only in diminishing levels of prosurvival S1P, but also in potentiating ceramide generation, a process that mediates, at least in part, the proapoptotic actions of certain cytotoxic agents.22–24 Here we describe a potent, water-soluble inhibitor of SphK1, SK1-I, that triggers multiple perturbations in activation of various signaling and survival-related proteins. SK1-I markedly induced apoptosis in human leukemic cell lines as well as blasts obtained from patients with AML and inhibited growth of AML xenograft tumors.
Methods
Materials
SK1-I, (2R,3S,4E)-N-methyl-5-(4′-pentylphenyl)-2-aminopent-4-ene-1,3-diol (BML-258), was synthesized as the HCl salt by BIOMOL International (Plymouth Meeting, PA) and will be described elsewhere. Sphingosine and N,N-dimethylsphingosine were obtained from BIOMOL International. [γ-32P]ATP (3000 Ci/mmol [111 × 1012 bq/mmol]) was purchased from Perkin Elmer (Boston, MA). Boc-D-FMK (BOC), Z-VAD-FMK (ZVAD), and etoposide were from EMD Biosciences (San Diego, CA). Terminal deoxynucleotidyl transferase Br-dUTP nick end labeling (TUNEL) kit for flow cytometry was from Sigma-Aldrich (St Louis, MO). TUNEL kit for immunohistochemistry was from Roche Applied Science (Indianapolis, IN). FITC-labeled annexin V/propidium iodide staining kit for apoptosis was from BD Biosciences (San Jose, CA).
Cells and cell culture
U937 human histiocytic leukemia and Jurkat acute T-cell leukemia cells were obtained from ATCC (Manassas, VA). Cells were cultured and maintained in logarithmic growth phase in RPMI 1640 medium supplemented with l-glutamate, penicillin, streptomycin, and 10% fetal bovine serum (Invitrogen, Carlsbad, CA),25 unless indicated otherwise. U937 cells stably overexpressing Bcl-2, Bcl-xL, constitutively active Akt (Myc-tagged myristoylated Akt), and their empty vector counterparts were obtained and cultured in the presence of the appropriate selection antibiotics exactly as described.23
Leukemic blasts were obtained with informed consent from 2 acute myelogenous leukemia (AML) patients undergoing routine diagnostic aspirations with approval from the Institutional Review Board of Virginia Commonwealth University. Informed consent was obtained in accordance with the Declaration of Helsinki. The characterization of the 2 patient samples was as follows: patient no. 1, FAB (French-American-British) subtype M2, no known fusion or mutant proteins, no known chromosome abnormalities; patient no. 2, FAB subtype M4, no known fusion or mutant proteins, inversion of chromosome 16.
Samples, which contained 85% blasts in each case, were separated by centrifugation over Ficoll/Hypaque (specific gravity: 1.077-1.081; Sigma-Aldrich) at 400g at room temperature. The interface layer, containing primarily blasts, was removed using a sterile Pasteur pipette, and resuspended in medium containing 10% FBS. Cells exhibited more than8 95% viability by trypan blue exclusion and were cultured as described.25 Peripheral blood mononuclear leukocytes were isolated similarly from healthy donors.
RNA interference
U937 cells were transfected with 100 pmol RNAi oligonucleotides targeted to SphK1 (sequence targeted: GGGCAAGGCCTTGCAGCTC) and nontargeting control siRNA (nonspecific random sequence) obtained from Qiagen (Valencia, CA). Transfections were performed with the Amaxa Nucleofector (program V-001) with Cell Line Nucleofector Kit V (Amaxa, Cologne, Germany) according to the manufacturer's instructions.
Expression and activity of sphingosine kinases
HEK 293 cells were cultured in DMEM containing 10% fetal bovine serum and transfected with V5-His-pcDNA3.1 vector (Invitrogen), V5-His-tagged human SphK1, or V5-His-tagged human SphK2 using Lipofectamine PLUS (Invitrogen) as previously described.26 Cells were then cultured for 2 days and lysed by freeze-thawing, and SphK1 activity was determined with [γ-32P]ATP (10 μCi [0.37 MBq], 1 mM, containing 10 mM MgCl2) and sphingosine in 0.25% Triton X-100, which inhibits SphK2.27 SphK2 activity was determined with sphingosine added as a complex with 4 mg/mL BSA in the presence of 1 M KCl, conditions in which SphK2 activity is optimal and SphK1 is strongly inhibited.27 Labeled S1P was extracted and separated by TLC on silica gel G60 with chloroform/acetone/methanol/acetic acid/H2O (10:4:3:2:1, vol/vol) as solvent. Radioactive bands corresponding to S1P were quantified with a FX Molecular Imager (Bio-Rad, Hercules, CA). SphK-specific activity is expressed as picomole of S1P formed per minute per milligram of protein.
Western blot analysis
Cells were resuspended in cell lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% Nonidet P40, 0.5% sodium deoxycholate, 1 mM PMSF, 5 μg/mL leupeptin, 5 μg/mL aprotinin, 1 mM DTT). Equal amounts of protein (60 μg) were separated by 10% sodium dodecyl sulfide–polyacrylamide gel electrophoresis (SDS-PAGE) and then transblotted to nitrocellulose. Blots were incubated with primary antibodies (1:1000) overnight in Tris-buffered saline (TBS) containing 5% nonfat dry milk and 0.1% Tween 20 followed by anti–rabbit HRP-conjugated IgG (1:10 000; Jackson Immunoresearch Laboratories, West Grove, PA). Immunocomplexes were visualized by enhanced chemiluminescence (Pierce, Rockford, IL) with Kodak (Rochester, NY) or Phenix Research Products (Candler, NC) X-ray film. Western blots were quantitated using AlphaEaseFC 4.0.0 software from Alpha Innotech (San Leandro, CA).
The following were used as primary antibodies: phospho-p44/42 MAP kinase (Thr202/Tyr204) antibody, phospho-p38 MAP kinase (Thr180/Tyr182) antibody (Cell Signaling, Beverly, MA), phospho-JNK (Thr183/Tyr185) antibody, Bcl-xS/L antibody (S-18; Santa Cruz Biotechnology, Santa Cruz, CA), antihuman Bcl-2 (Dako, Carpinteria, CA), Mcl-1 antibody, anti–caspase-3, and anti–caspase-9 (BD PharMingen, San Diego, CA), and anti-PARP (BIOMOL International).
Protein kinase profiling
Effects of SK1-I on the activity of various protein kinases was assessed by SelectScreen Kinase Profiling (Invitrogen Drug Discovery Solutions, Madison, WI). Briefly, assays were performed in 384-well plates using a fluorescence resonance energy transfer (FRET)–based kinase assay system with peptide substrates containing 2 fluorophores that make up a FRET pair, in the absence or presence of 5 μM SK1-I and at an ATP concentration of Kmapp for each protein kinase. The development reagent contains a protease that specifically digests nonphosphorylated peptide and produces a fluorescent signal. Coumarin fluorescence and the fluorescein FRET signal were monitored at 445 nm and 520 nm, respectively. The coumarin emission excites fluorescein by FRET in the uncleaved (phosphorylated) substrate peptide only. Reactions containing unphosphorylated peptide and kinase in the absence of ATP and stoichiometrically phosphorylated peptide served as 0% and 100% phosphorylation controls, respectively. Raw fluorescence values were corrected for background. Reaction end points were calculated as emission ratios of coumarin fluorescence divided by the fluorescein FRET signal. These ratios were then normalized to the ratio obtained with the 100% phosphorylation control.
Annexin V/PI assays for apoptosis
Cells were stained with annexin V–fluorescein isothiocyanate and propidium iodide (PI) and then evaluated for apoptosis by flow cytometry according to the manufacturer's protocol (BD PharMingen). Briefly, 106 cells were washed twice with phosphate-buffered saline (PBS) and stained with 5 μL annexin V–fluorescein isothiocyanate and 5 μL PI (50 μg/mL) in buffer containing 10 mM HEPES, pH 7.4, 140 mM NaOH, and 2.5 mM CaCl2 for 15 minutes at room temperature in the dark. The apoptotic cells were determined using a Coulter Epics-XL-MCL cytofluorometer with the EXPO32 Flow Cytometry analytic program (Beckman Coulter, Fullerton, CA). The percentages in the lower right quadrant correspond to early apoptotic cells (annexin V–positive), whereas percentages in the upper right quadrant correspond to late apoptotic cells (annexin V– and PI-positive; see Figure 2).
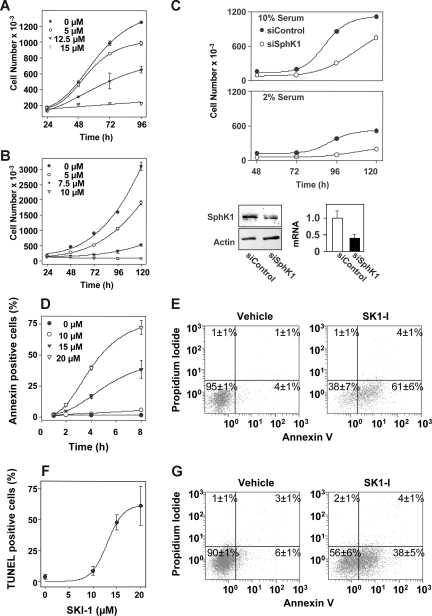
Figure 2.
SK1-I and siSphK1 decrease cellular proliferation and viability. (A) U937 cells or (B) Jurkat cells (105 cells/mL) were cultured in medium containing 10% serum in the absence or presence of the indicated concentrations of SK1-I. Cell numbers were determined with a Coulter counter model Z1 (Beckman Coulter). (C) Knockdown of SphK1 by siRNA reduces cell growth. U937 cells were transiently transfected siRNA targeted to SphK1 (○) or siRNA control (●). Cells (105 cells/mL) were then cultured in medium containing 10% (top panel) or 2% (middle panel) serum for the indicated times and cell numbers determined with a Coulter counter. (Bottom panel) Equal amounts of cell lysate proteins (60 μg) from duplicate cultures were separated by SDS-PAGE and immunoblotted with anti-SphK1 antibody. Blots were stripped and probed with actin antibody to ensure equal loading and transfer. SphK1 mRNA levels were normalized to actin and the ratio relative to siControl from 3 independent experiments is shown. *P < .05. (D-G) SK1-I promotes apoptosis. U937 cells cultured in 10% serum were treated without or with 10, 15, or 20 μM SK1-I for the indicated times (D) or with 20 μM SK1-I for 24 hours (E) and stained with annexin V/PI, and apoptosis was determined by flow cytometry. Early apoptotic cells are annexin V positive, late apoptotic cells are both annexin V and PI positive, whereas necrotic cells are PI positive only. (F) Duplicate cultures were treated with the indicated concentration of SK1-I for 24 hours, and the fraction of TUNEL-positive cells was determined. (G) U937 cells were cultured in medium containing 2% serum in the absence or presence of 10 μM SK1-I for 24 hours and then stained with annexin V/PI, and apoptosis was determined by flow cytometry. The percentages in the lower right quadrant correspond to early apoptotic cells (annexin V positive), whereas percentages in the upper right quadrant correspond to late apoptotic cells (annexin V/PI-positive).
DNA strand break detection by TUNEL assay
Cells (106) were fixed with 1% (wt/vol) paraformaldehyde on ice for 15 minutes, washed twice with PBS, and permeabilized in 70% ethanol on ice for 30 minutes. Cells were washed and resuspended in a DNA labeling solution containing terminal deoxyribonucleotide transferase and bromodeoxyuridine (BrdU) and incubated at 37°C for 1 hour according to the manufacturer's instructions (Sigma-Aldrich). Cells were then incubated with anti–BrdU-fluorescein antibody in the dark for 30 minutes at room temperature and analyzed using a Coulter Epics-XL-MCL cytofluorometer with the EXPO32 Flow Cytometry analytic program (Beckman Coulter).
Mass spectrometric analysis of sphingolipids and metabolites
Cells were washed extensively with cold PBS and pelleted by centrifugation at 2000g for 10 minutes. An aliquot of cells was taken for DNA and protein measurements. To the rest, internal standards were added (0.5 nmol each C12-SM, C12-Cer, C12-GlcCer, C12-LacCer, C17-sphingosine, C17-sphinganine, C17-sphingosine 1-phosphate, C17-sphinganine-1-phosphate, and C12-Cer-phosphate; Avanti Polar Lipids, Alabaster, AL), lipids extracted, and individual ceramide acyl chain species quantified by liquid chromatography, electrospray ionization-tandem mass spectrometry (ESI-MS/MS, 4000 QTRAP; Applied Biosystems, Foster City, CA) as described previously.28
Xenograft tumor model
All experiments involving animals were approved by the Virginia Commonwealth University (VCU) Institutional Animal Care and Use Committee (IACUC). U937 cells (2 × 106, suspended in 100 μL sterile PBS) were injected into 2 sites on both flanks of 6-week-old CB17 severe combined immunodeficient (SCID)/beige mice (Taconic Farms, Germantown, NY) and allowed to grow to palpable tumors for 7 days. When tumors reached a volume of 50 to 100 mm3, animals were randomly assigned to 2 groups that were injected intraperitoneally with 200 μL saline or SK1-I (20 mg/kg) on 7 consecutive days. Tumor measurements were made daily with calipers, and tumor volume was calculated using the formula: (π × [length in millimeters] × [width in millimeters2]/6. At the end of the experiment, the animals were killed and the tumors removed, fixed in formalin, and embedded in paraffin or frozen in liquid nitrogen. Formalin-fixed sections were stained with hematoxylin-eosin, or with antibodies against Ki-67 (Novocastra, Newcastle, United Kingdom). Antibody binding was detected by immunohistochemistry and peroxidase-conjugated species-specific secondary antibodies and visualized with 3,3-diaminobenzidine. Paraffin sections were dewaxed, rehydrated, and proteinase K–treated before permeabilization. Frozen sections were stained with a fluorescein TUNEL labeling kit followed by counterstaining with DAPI. Slides were analyzed by fluorescence microscopy.
Statistical analysis
Experiments were repeated at least 3 times with consistent results. For each experiment, data from triplicate samples were calculated and expressed as the mean plus or minus standard deviation (SD). The significance of differences between experimental conditions was determined using the Student t test for unpaired observations.
Results
SK1-I is a potent and selective inhibitor of SphK1 but not SphK2
Currently, no structural information is available for SphKs to allow use of computational docking methods for the rational design of inhibitors. Therefore, an alternative approach is to use information obtained from inhibitor studies to design more potent and selective inhibitors. Various chemically synthesized short-chain sphingosine and dihydrosphingosine analogs have previously been investigated as inhibitors of SphK.29–32 It was found that replacement of the alkyl chain with a phenyl ring or substituting fluorine for the 3-hydroxyl group yielded potent SphK inhibitors. Moreover, analogs with a 4,5-trans double bond were generally superior inhibitors.30 Based on these earlier observations, we synthesized (2R,3S,4E)-N-methyl-5-(4′-pentylphenyl)-2-aminopent-4-ene-1,3-diol (Figure 1A) and examined its effects on recombinant SphK1 and SphK2 (Figure 1B-E). This water-soluble sphingosine analog potently inhibited SphK1 activity in a dose-dependent manner (Figure 1C) with 60% to 70% inhibition at 5 μM. As previously reported,6,29 N,N-dimethylsphingosine (DMS) also inhibited SphK1 activity, albeit with less potency. Importantly, in contrast to DMS, which also inhibits SphK27 and ceramide kinase,33 our compound did not inhibit recombinant SphK2 (Figure 1C,E) or ceramide kinase (data not shown). Because of its specific inhibitory effect on SphK1, this compound is hereafter referred to as SK1-I.
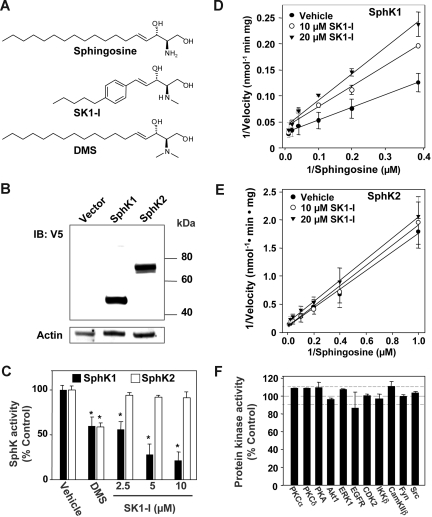
Figure 1.
SK1-I is a specific SphK1 inhibitor. (A) Chemical structures of sphingosine, SK1-I (2-amino-3[2-(4-pentylphenyl)ethyl-1-ene]1,3-propanediol), and DMS (N,N-dimethylsphingosine). (B) HEK 293 cells were transiently transfected with vector, V5-hSphK1 or V5-hSphK2. Cell lysates were prepared and equal amounts of proteins were resolved by SDS-PAGE and analyzed by Western blotting with anti-V5 antibody. Blots were stripped and reprobed with antiactin as a loading control. (C) SphK1 and SphK2 activities in cell lysates were measured with 10 μM sphingosine in the absence or presence of the indicated concentrations of either SK1-I or 10 μM DMS. SphK1 activity was determined in the presence of Triton X-100 and SphK2 activity was measured in the presence of high salt concentrations, conditions that favor SphK1 and SphK2, respectively. Data are expressed as percentage SphK activity measured in the absence of inhibitor. *P ≤ .01. (D,E) Lineweaver-Burk plots. SphK1 and SphK2 activity was measured with increasing concentrations of sphingosine and the indicated concentrations of SK1-I. Regression analysis revealed a Km of 10 plus or minus 2 μM, Vmax of 38 000 plus or minus 2000 pmol/min per milligram of protein, and Ki of 10 plus or minus 5 μM for SphK1 (D) and Km of 12 plus or minus 1 μM and Vmax 8100 plus or minus 300 pmol/min per milligram of protein for hSphK2 (E), without significant inhibition by SK1-I. (F) Effects of SK1-I on activity of the indicated protein kinases were assessed by SelectScreen Kinase Profiling as described in “Methods.” Data are expressed as percentage of control activity (in the absence of SK1-I) and are averages of 2 independent measurements.
Lineweaver-Burk analysis revealed that SphK1 activity was inhibited competitively by SK1-I with a Ki value of approximately 10 μM (Figure 1D), nearly identical to the Km for sphingosine. SK1-I was not phosphorylated by either SphK1 or SphK2 (data not shown). Because DMS and several other pan-SphK inhibitors also inhibit protein kinase C34 and potentially other kinases,35,36 it was important to examine the effects of SK1-I on protein kinases. A protein kinase activity screen was used that contained several different recombinant protein kinases, a fluorescently labeled polypeptide substrate, and ATP at the Kmapp for each kinase. SK1-I did not significantly inhibit any of the protein kinases including 2 different members of the PKC family, PKCα and PKCδ, PKA, Akt1, ERK1, EGFR, CDK2, IKKβ, or CamKIIβ (Figure 1F). Hence, SK1-I is unique among known SphK inhibitors in view of its isoenzyme selectivity, water solubility, and lack of effect on protein kinases.
SK1-I potently inhibits growth of human leukemia cells
Previous studies have shown that the pan-SphK inhibitor DMS markedly induces apoptosis of U937 and Jurkat T cells.3,17,29,37,38 As shown in Figure 2A, a concentration of SK1-I as low as 5 μM significantly decreased growth of U937 cells cultured in the presence of 10% serum, which was evident after 72 hours of culture. T-lymphoblastic Jurkat cells were even more sensitive to SK1-I, as a concentration of 5 μM inhibited growth by 50% and 10 μM completely prevented cell growth (Figure 2B). Similarly, 10 μM SKI-1 decreased growth of other leukemia cell lines, including promyelocytic HL-60, Molt-4 T-cell leukemia, and K-562 CML cells by 50%, 70%, and 90%, respectively, within 48 hours of treatment.
Similar to the effects of treatment with SK1-I and in agreement with studies in other leukemic cell lines,10,21 down-regulation of SphK1 expression with siRNA targeted to a unique sequence, which reduced SphK1 protein and mRNA levels by more than 60% (Figure 2C), markedly reduced the rate of growth of U937 cells cultured in the presence of either 2% or 10% serum (Figure 2C). Together, these findings are consistent with the notion that specific inhibition of SphK1 by pharmacologic or genetic means significantly inhibits the growth of human myeloid and lymphoid leukemia cells.
SK1-I induces apoptosis in human leukemia cells
Inhibition of SphK with DMS or down-regulation of SphK1 expression has been associated with induction of apoptosis in many cell types, including human leukemia cells.3,10,18,19 Thus, we next examined the effects of SK1-I on apoptosis of U937 cells using flow cytometry to monitor cells expressing phosphatidylserine on the outer plasma membrane by annexin V staining and PI as a measure of membrane permeability. There was a time- and concentration-dependent increase in apoptosis of U937 cells upon exposure to SK1-I (Figure 2D). As shown in Figure 2E, the majority of the cells were early apoptotic and a very small percentage were necrotic (PI-positive only). These results correlated closely with the occurrence of DNA strand breaks, as determined by TUNEL assays (Figure 2F). Moreover, similar to down-regulation of SphK1, which inhibits cell growth more effectively when cells are cultured in the presence of lower concentrations of serum (Figure 2C), U937 cells were more susceptible to SK1-I–induced apoptosis when the serum concentration was reduced (Figure 2G).
Functional roles of caspase activation and Bcl-2 cleavage in SK1-I–induced cell death
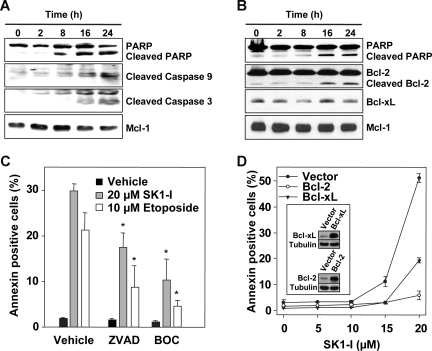
It has previously been demonstrated that down-regulation of SphK1 in HL-60 cells with siRNA10 or inhibition of SphK with DMS in Jurkat cells39 is sufficient to trigger activation of executioner caspase-3 as well as cleavage of PARP, hallmarks of apoptosis. Similarly, concomitant with induction of apoptosis, treatment of U937 cells with SK1-I increased activation of caspases-3 and -9 and induced cleavage of poly-ADP ribose polymerase (PARP; Figure 3A,B). Furthermore, exposure to SK1-I for 16 to 24 hours resulted in cleavage of Bcl-2 (Figure 3B), an antiapoptotic protein that prevents mitochondrial dysfunction. On the other hand, levels of Mcl-1, an antiapoptotic protein that plays a key role in the survival of malignant hematopoietic cells,40 were not significantly altered (Figure 3A,B). Next, we investigated the functional roles of caspase activation and Bcl-2 cleavage in SK1-I–induced lethality. Pretreatment of U937 cells with the pan-caspase inhibitors ZVAD and BOC significantly attenuated SK1-I–induced apoptosis as well as that induced by the DNA-damaging agent etoposide (Figure 3C). Furthermore, ectopic expression of Bcl-2 completely protected against SK1-I–induced lethality and expression of Bcl-xL reduced cell death by 60% (Figure 3D). Together, these findings indicate that the lethality of SK1-I is primarily mediated via the intrinsic mitochondrial pathway, an event opposed by Bcl-2.
Figure 3.
Caspase inhibitors and overexpression of Bcl-2 protect against apoptosis induced by SK1-I. U937 cells were treated without or with 10 μM SK1-I (A) or 20 μM SK1-I (B) for the indicated times. Cell lysates were prepared and equal amounts of proteins (20 μg) analyzed by Western blotting with the indicated antibodies. Blots were stripped and reprobed with anti–Mcl-1 to ensure equivalent loading and transfer. (C) U937 cells were pretreated for 30 minutes without or with 20 μM Boc-D-FMK (BOC) or Z-VAD-FMK (ZVAD) and then treated with 20 μM SK1-I or 10 μM etoposide. After 4 hours, apoptosis was determined by flow cytometric analysis of annexin V/PI-stained cells. *P ≤ .01. (D) U937 cells stably expressing vector, Bcl-2, or Bcl-xL were treated with the indicated concentrations of SK1-I for 8 hours. Apoptosis was determined by flow cytometric analysis of annexin V/PI-stained cells. Insets show overexpression of Bcl-2 and Bcl-xL by immunoblotting.
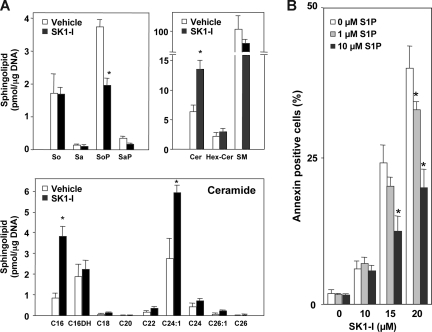
Because SphK1 is a critical regulator of the balance between proapoptotic ceramide and antiapoptotic S1P,8,10 the effect of SK1-I on levels of these sphingolipid metabolites was determined by high-performance liquid chromatography ESI-MS/MS.28 SK1-I treatment caused a 50% decrease in total cellular S1P (Figure 4A), without altering levels of sphingosine or dihydrosphingosine (sphinganine), with a concomitant increase in total cellular ceramide and a decrease in sphingomyelin (Figure 4A). The most abundant ceramide species in U937 cells was 24:1 (Figure 4A). SK1-I treatment increased levels of C16:0 and C24:1 ceramide species by 3- and 2-fold, respectively, but had no significant effects on other ceramide species (Figure 4A).
Figure 4.
SK1-I decreases S1P and increases ceramide levels and S1P protects against SK1-I–induced apoptosis. (A) U937 cells were treated without or with 20 μM SK1-I for 6 hours. Lipids were extracted and sphingosine (So), sphinganine (Sa), sphingosine-1-phosphate (SoP), sphinganine-1-phosphate (SaP), and ceramide species were determined by ESI-MS/MS. Data are means of triplicate determinations and are expressed as picomole of lipid per microgram of DNA. Numbers indicate fatty acid chain length followed by the number of double bonds. C16DH indicates C16-dihydroceramide. (B) U937 cells were treated without or with SK1-I (10, 15, 20 μM) in the absence and presence of S1P (1, 10 μM) for 4 hours. Cells were stained with annexin V/PI and apoptosis was determined by flow cytometry. *P ≤ .01.
To confirm that the apoptotic effects of SK1-I were due to its ability to inhibit SphK1, S1P add-back experiments were carried out. Consistent with the reduction in levels of S1P by SK1-I, apoptosis induced by SK1-I was diminished by addition of exogenous S1P in a dose-dependent manner (Figure 4B). Collectively, these findings indicate that SK1-I induces apoptosis in human leukemia cells by inhibiting SphK1 and production of S1P with a concomitant increase in ceramide.
Apoptosis induced by SK1-I is associated with inactivation of ERK1/2 and Akt survival signals
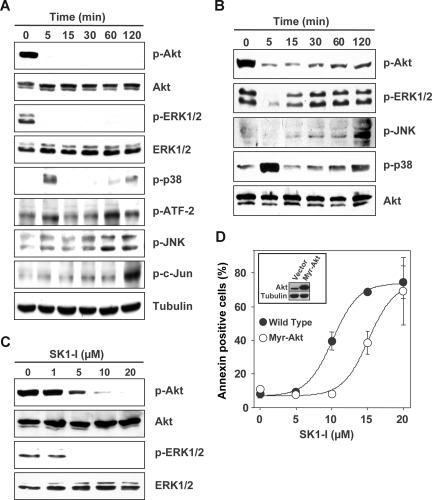
Abundant evidence indicates that the mitogen-activated protein kinases (ERK1/2, JNK, and p38 MAPK) and Akt play a critical role in leukemia cell fate.41 Treatment of U937 cells with SK1-I caused a rapid and marked decrease in phosphorylation of ERK1/2 and Akt (Figure 5A-C). These deactivations of survival signaling were sustained up to 2 hours in the presence of low concentrations of serum (Figure 5A), whereas in the presence of 10% serum, the attenuation of p-ERK1/2 and p-Akt levels was gradually overcome (Figure 5B) and was dependent on the concentration of SK1-I (Figure 5C). Furthermore, a transient increase in p38 phosphorylation was observed at 5 minutes followed later by a less robust activation (Figure 5A,B). In addition, JNK activation and c-Jun phosphorylation were also detected at later times (Figure 5A,B). Because SK1-I profoundly deactivates Akt, it was of interest to determine its role in the lethal effects of SK1-I. Overexpression of constitutively active myristoylated Akt in U937 cells significantly attenuated apoptosis induced by SK1-I at a concentration less than 15 μM (Figure 5D), suggesting that deactivation of Akt might be one of the factors contributing to the apoptotic effects of SK1-I.
Figure 5.
SK1-I alters survival signaling. (A-C) U937 cells were cultured in medium containing 2% serum in the presence of 10 μM SK1-I for the indicated times (A); in medium containing 10% serum in the presence of 20 μM SK1-I for the indicated times (B); or with the indicated concentrations of SK1-I for 5 minutes (C). Equal amounts of lysate proteins were resolved by SDS-PAGE and analyzed by Western blotting with antibodies against pAkt, Akt, p-ERK1/2, ERK1/2, p-p38, p-ATF-2, p-JNK, and p-cJun. Blots were stripped and reprobed with antitubulin (A), anti-Akt (B), or anti-ERK1/2 (C) to demonstrate equal loading. (D) Enforced expression of myristoylated Akt protects cells from SK1-I–mediated lethality. U937 cells stably expressing empty vector or constitutively active Akt (myristoylated Akt) were treated with the indicated concentrations of SK1-I for 24 hours. Cells were stained with annexin V/PI and apoptosis was determined by flow cytometry. (Inset) Immunoblot of lysate proteins from duplicate cultures with anti-Akt and antitubulin antibodies.
Primary human AML blasts are highly sensitive to apoptosis induced by SK1-I
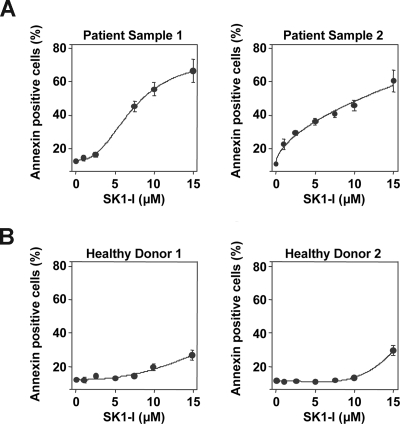
To examine the effectiveness of SK1-I on primary AML specimens, parallel studies were performed in leukemic blasts obtained from bone marrow aspirates of 2 patients with AML (FAB subtype M2). Treatment of blasts with increasing concentrations of SK1-I revealed enhanced sensitivity to apoptosis induction compared with U937 and Jurkat cell lines. Both patient samples exhibited a marked increase in apoptosis when exposed to SK1-I for 24 hours and 40% to 50% apoptosis was observed with 7.5 μM SK1-I as revealed by annexin V/PI analysis (Figure 6A). In agreement with previous results,42 less than 10% of blasts exhibited apoptosis in the absence of treatment, which is very similar to apoptosis of normal peripheral blood mononuclear cells. Notably, SK1-I had a much less pronounced effect on the survival of normal peripheral blood mononuclear cells (Figure 6B). These results suggest that primary human AML cells, reported to overexpress SphK1,43 are more susceptible to SK1-I than continuously cultured leukemia cell lines, whereas SK1-I is relatively sparing of normal peripheral blood mononuclear leukocytes.
Figure 6.
SK1-I induces apoptosis in primary AML blasts. (A) Primary blasts from 2 patients with AML (FAB classification F2; > 70% blasts) and peripheral blood mononuclear leukocytes from 2 healthy donors (B) were obtained as described in “Cells and cell culture,” cultured in medium containing 10% serum for 24 hours in the absence or presence of the indicated concentrations of SK1-I. Cells were stained with annexin V/PI and apoptosis determined by flow cytometry.
Antileukemic activity of SK1-I in vivo
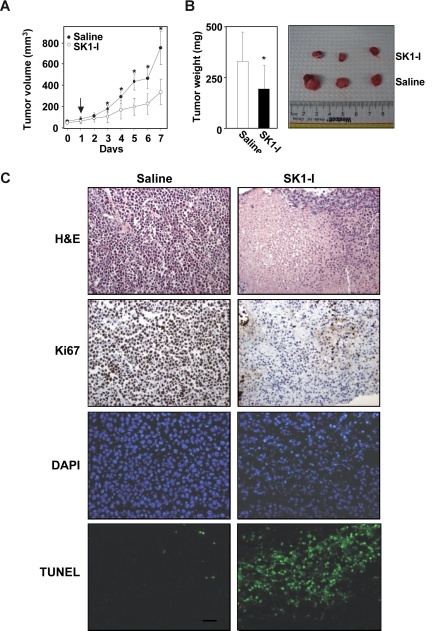
We next evaluated the ability of SK1-I to inhibit tumor growth of leukemia cells in xenografts in immunodeficient mice, a model that has been extensively used to facilitate development of several new treatment modalities.44 U937 cells subcutaneously injected into the flanks of SCID/beige mice rapidly gave rise to exponentially growing tumors. When tumors reached a volume of 50 to 100 mm3, mice were injected intraperitoneally with saline or SK1-I (20 mg/kg) daily. As can be seen in Figure 7A, SK1-I significantly decreased tumor growth. After 7 days, the mean volume of the U937 tumors in mice treated with SK1-I was more than 50% smaller than the tumors in the saline-treated mice (control group mean = 747 mm3, SK1-I group mean = 332 mm3; P < .001). Tumor weights at autopsy of SK1-1–treated mice were also significantly lower (Figure 7B). Mice treated with SK1-I did not show signs of wasting and the body weights after 7 days were not significantly different from controls.
Figure 7.
SK1-I suppresses U937 xenograft tumor growth. CB17 SCID/beige mice (5 mice per group) with palpable U937 cell tumors (4 tumors per mouse) were injected intraperitoneally with saline or SK1-I (20 mg/kg) for 7 days. (A) Tumor volumes were measured daily. Data are expressed as mean volumes (± SD). (B) After 7 days, animals were killed and tumors excised and weighed. Average and representative results are shown. *P ≤ .01. (C) Tumor histology. Paraffin-embedded tumor sections were stained with either H&E or immunostained with Ki67. Apoptotic cells were visualized by TUNEL staining and counterstained with DAPI. Slides were examined with an Olympus BX-40 microscope (Olympus, Melville, NY) equipped with a UPlan F1 20×/0.5 NA objective and WH10x eyepiece, and analyzed with RS Image version 1.7.3 (Alpha Innotech). Representative sections from n = 4 individual treated tumors. Scale bar represents 30 μm.
As expected, tumors from saline-treated mice stained strongly with Ki67, indicating a tumor composition of highly proliferating cells with very few apoptotic cells stained by TUNEL (Figure 7C). In contrast, immunohistochemical analysis of tumors from SK1-I–treated mice revealed many apoptotic cells as determined by nuclear fragmentation (TUNEL staining) and condensed nuclei (Figure 7C). SK1-I also drastically reduced mitotic cells in the tumors (Figure 7C). These results indicate that SK1-I has potent antileukemic activity in vivo.
Discussion
Ample evidence indicates that in many types of cancers, S1P production is dysregulated leading to abnormal cell growth and survival signaling.5,45 SphK1 is overexpressed in a variety of solid tumors46 and also in AML.43 Moreover, bcr/abl, a common genetic aberration in chronic myelogenous leukemia and a poor prognostic indicator for at least 20% of patients with acute lymphocytic leukemia, has been shown to up-regulate SphK1 expression.47 It was recently shown that SphK1 expression can predict sensitivity of leukemia cells to daunorubicin.48 Hence, SphK1 is now considered a potential target for pharmacologic intervention, particularly in leukemic cells where its level correlates with chemoresistance and radioresistance.10,21,48 Previous studies of the role of SphK1 in leukemic cells have focused on its down-regulation by specific siRNA or the use of pharmacologic agents that inhibited both SphK1 and SphK2, and potentially protein kinases. This study describes the development of the first potent and water-soluble SphK1 isoenzyme-specific inhibitor, SK1-I. Moreover, SK1-I does not inhibit PKC or a large number of other protein kinases. In contrast to most small-molecule protein kinase inhibitors that are competitive with ATP at the well-conserved ATP-binding pocket and potentially cross-react, SK1-I is competitive with the lipid substrate.
SK1-I potently induced apoptosis in several leukemic cells lines and AML leukemic blasts, reflected by externalization of phosphatidylserine, increased DNA strand breaks, activation of caspases-3 and -9, and cleavage of PARP and Bcl-2. By what mechanisms does SK1-I so profoundly induce these lethal effects? This could be due to several non–mutually exclusive interrelated actions. SK1-I inhibits production of prosurvival S1P that can act intracellularly to enhance growth and survival, although its intracellular targets have not yet been elucidated.49 It is also well accepted that intracellularly produced S1P can be released from cells50 and act through its cell surface receptors that are linked to survival pathways including ERK1/2 and Akt.1 In this regard, the ability of SK1-I to decrease activated ERK1/2 and Akt in leukemic cells might be relevant, as the Raf/MEK/ERK and PI3K/Akt pathways are frequently constitutively activated in AML.41,51 Because ERK1/2 phosphorylates and activates SphK1,52 leading to increased S1P, which in turn can stimulate ERK1/2, SK1-I can interrupt this positive feedback loop by inhibiting SphK1, decreasing progrowth and survival S1P while simultaneously increasing its precursor, the proapoptotic ceramide. Hence, SK1-I integrates multiple molecular therapeutic targets in leukemia.
Ceramide generation has long been implicated in apoptosis induction in human leukemia cells53 and recently, the synergistic actions of several different signal transduction inhibitors on apoptosis have been linked to dramatic increases in ceramide generation. For example, coadministration of histone deaceylase inhibitors with perifosine in human leukemia cells leads to Akt and ERK disruption, a marked increase in ceramide and reactive oxygen species production, and a striking increase in mitochondrial injury and apoptosis.23,24 Ceramide can transduce its apoptotic actions via multiple pathways.2 Important identified ceramide targets include the serine/threonine protein phosphatases PP1 and PP2A that dephosphorylate Akt as well as SR proteins, regulators of alternative splicing of Bcl-2.2 We found that exposure to SK1-I resulted in cleavage of Bcl-2, a response that has been associated with mitochondria-dependent apoptosis.54 Moreover, it is well established that overexpression of Bcl-2 prevents ceramide formation and protects against ceramide-induced apoptosis in many cell types including acute lymphoblastic leukemia and AML.2,55,56 In agreement, we found that overexpression of Bcl-2 also prevented SK1-I–induced lethality, emphasizing the importance of the intrinsic mitochondrial death pathway. Consistent with this notion, it has recently been demonstrated that S1P exerts its cytoprotective effect on mitochondrial events during apoptosis of Jurkat cells by blocking translocation of Bax to the mitochondria in a MEK/ERK1/2-dependent manner.57 A recent study suggested that sustained elevation of ceramide at the endoplasmic reticulum coordinately activates the ER stress response and inactivates antiapoptotic Akt leading to apoptosis.58 It is thus possible that SK1-I–induced Akt deactivation is mediated not only by decreased formation of S1P, but also by increased ceramide. The immunosuppressant drug FTY720, which structurally resembles SKI-1, but is a competitive substrate of SphKs rather than a competitive inhibitor, has also been shown to reduce Akt phosphorylation.59 FTY720, which had relatively little toxicity in clinical trials for multiple sclerosis,60 has recently been proposed to be an alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome–positive acute lymphocytic leukemia.61
Our finding that SK1-I potently induced apoptosis in leukemic blasts isolated from patients with AML but was relatively noncytotoxic to normal peripheral blood mononuclear leukocytes highlights its selectivity for leukemia cells. Moreover, in a xenograft AML model, SK1-I had clear single-agent activity that suppressed tumor growth, induced apoptosis in the tumor, and decreased proliferation, analogous to its actions in vitro. Preliminary analysis of toxicity of liver, kidney, and spleen did not reveal any noticeable effects. Thus, specific SphK1 inhibitors deserve consideration for potential pharmacologic intervention in leukemia, used either alone or as adjuncts to conventional or other known targeted agents.
Acknowledgments
We thank Dr Elaine Wang, Dr Samuel Kelley, Jeremy Allegood, and Dr Alfred H. Merrill for assistance with the mass spectrometry and for helpful discussions. We thank Dr Yun Dai for help with tumor pathology analysis. We thank the Developmental Therapeutics Program, National Cancer Institute (NCI)/National Institutes of Health (NIH; Bethesda, MD) for additional cell line screening (http://dtp.nci.nih.gov/index.html).
This work was supported by NIH grants R01CA61774 and R37 GM043880 (S.S.), National Institute of Allergy and Infectious Diseases (NIAID, Bethesda, MD) Training Grant T32AI007407 (S.W.P.), and the Intramural Research Program of the National Institute of Mental Health (Bethesda, MD; S.M.). Confocal microscopy and flow cytometry were supported in part by NIH Grant P30 CA16059 to the Massey Cancer Center (Richmond, VA).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.W.P. made the initial discovery, performed research, analyzed data, and wrote the first draft; B.S.P., M.R., D.K., and J.A.A. performed research; T.K. analyzed data and contributed vital reagents; J.K.A. and R.E.Z. synthesized compounds and analyzed data; S.M. and S.G. analyzed data and wrote the paper; and S.S. directed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sarah Spiegel, Department of Biochemistry and Molecular Biology, Virginia Commonwealth University School of Medicine, 1101 E Marshall St, Richmond, VA 23298-0614; e-mail: sspiegel@vcu.edu.
References
- 1.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nature Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 2.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nature Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 3.Cuvillier O, Pirianov G, Kleuser B, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 4.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Milstien S, Spiegel S. Targeting sphingosine-1-phosphate: a novel avenue for cancer therapeutics. Cancer Cell. 2006;9:148–150. doi: 10.1016/j.ccr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273:23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Sugiura M, Nava VE, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 8.Olivera A, Kohama T, Edsall LC, et al. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia P, Wang L, Moretti PA, et al. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J Biol Chem. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 10.Bonhoure E, Pchejetski D, Aouali N, et al. Overcoming MDR-associated chemoresistance in HL-60 acute myeloid leukemia cells by targeting sphingosine kinase-1. Leukemia. 2006;20:95–102. doi: 10.1038/sj.leu.2404023. [DOI] [PubMed] [Google Scholar]
- 11.Sukocheva O, Wadham C, Holmes A, et al. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol. 2006;173:301–310. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maceyka M, Sankala H, Hait NC, et al. Sphk1 and Sphk2: sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 13.Okada T, Ding G, Sonoda H, et al. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J Biol Chem. 2005;280:36318–36325. doi: 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- 14.Berdyshev EV, Gorshkova IA, Usatyuk P, et al. De novo biosynthesis of dihydrosphingosine-1-phosphate by sphingosine kinase 1 in mammalian cells. Cell Signal. 2006;18:1779–1792. doi: 10.1016/j.cellsig.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Taha TA, Kitatani K, El-Alwani M, Bielawski J, Hannun YA, Obeid LM. Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: modulation of sphingolipid levels and the induction of apoptosis. FASEB J. 2006;20:482–484. doi: 10.1096/fj.05-4412fje. [DOI] [PubMed] [Google Scholar]
- 16.Cuvillier O, Rosenthal DS, Smulson ME, Spiegel S. Sphingosine 1-phosphate inhibits activation of caspases that cleave poly(ADP-ribose) polymerase and lamins during Fas- and ceramide-mediated apoptosis in Jurkat T lymphocytes. J Biol Chem. 1998;273:2910–2916. doi: 10.1074/jbc.273.5.2910. [DOI] [PubMed] [Google Scholar]
- 17.Cuvillier O, Levade T. Sphingosine 1-phosphate antagonizes apoptosis of human leukemia cells by inhibiting release of cytochrome c and Smac/DIABLO from mitochondria. Blood. 2001;98:2828–2836. doi: 10.1182/blood.v98.9.2828. [DOI] [PubMed] [Google Scholar]
- 18.Jendiroba DB, Klostergaard J, Keyhani A, Pagliaro L, Freireich EJ. Effective cytoto-xicity against human leukemias and chemotherapy-resistant leukemia cell lines by N-N-dimethylsphingosine. Leuk Res. 2002;26:301–310. doi: 10.1016/s0145-2126(01)00129-1. [DOI] [PubMed] [Google Scholar]
- 19.Jarvis WD, Fornari FA, Jr, Tombes RM, et al. Evidence for involvement of mitogen-activated protein kinase, rather than stress-activated protein kinase, in potentiation of 1-beta-D-arabinofuranosylcytosine-induced apoptosis by interruption of protein kinase C signaling. Mol Pharmacol. 1998;54:844–856. doi: 10.1124/mol.54.5.844. [DOI] [PubMed] [Google Scholar]
- 20.Filipits M, Stranzl T, Pohl G, et al. Drug resistance factors in acute myeloid leukemia: a comparative analysis. Leukemia. 2000;14:68–76. doi: 10.1038/sj.leu.2401634. [DOI] [PubMed] [Google Scholar]
- 21.Baran Y, Salas A, Senkal CE, et al. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J Biol Chem. 2007;282:10922–10934. doi: 10.1074/jbc.M610157200. [DOI] [PubMed] [Google Scholar]
- 22.Maggio SC, Rosato RR, Kramer LB, et al. The histone deacetylase inhibitor MS-275 interacts synergistically with fludarabine to induce apoptosis in human leukemia cells. Cancer Res. 2004;64:2590–2600. doi: 10.1158/0008-5472.can-03-2631. [DOI] [PubMed] [Google Scholar]
- 23.Rahmani M, Reese E, Dai Y, et al. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res. 2005;65:2422–2432. doi: 10.1158/0008-5472.CAN-04-2440. [DOI] [PubMed] [Google Scholar]
- 24.Rosato RR, Maggio SC, Almenara JA, et al. The histone deacetylase inhibitor LAQ-824 induces human leukemia cell death through a process involving XIAP down-regulation, oxidative injury, and the acid sphingomyelinase-dependent generation of ceramide. Mol Pharmacol. 2006;69:216–225. doi: 10.1124/mol.105.017145. [DOI] [PubMed] [Google Scholar]
- 25.Dai Y, Yu C, Singh V, et al. Pharmacological inhibitors of the mitogen-activated protein kinase (MAPK) kinase/MAPK cascade interact synergistically with UCN-01 to induce mitochondrial dysfunction and apoptosis in human leukemia cells. Cancer Res. 2001;61:5106–5115. [PubMed] [Google Scholar]
- 26.Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett. 2003;554:189–193. doi: 10.1016/s0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- 27.Hait NC, Sarkar S, Le Stunff H, et al. Role of sphingosine kinase 2 in cell migration towards epidermal growth factor. J Biol Chem. 2005;280:29462–29469. doi: 10.1074/jbc.M502922200. [DOI] [PubMed] [Google Scholar]
- 28.Sullards MC, Merrill AH., Jr Analysis of sphingosine 1-phosphate, ceramides, and other bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Science STKE. 2001;2001 doi: 10.1126/stke.2001.67.pl1. L1; PMID: 11752637. [DOI] [PubMed] [Google Scholar]
- 29.Edsall LC, Van Brocklyn JR, Cuvillier O, Kleuser B, Spiegel S. N. N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry. 1998;37:12892–12898. doi: 10.1021/bi980744d. [DOI] [PubMed] [Google Scholar]
- 30.De Jonghe S, Van Overmeire I, Poulton S, et al. Structure-activity relationship of short-chain sphingoid bases as inhibitors of sphingosine kinase. Bioorg Med Chem Lett. 1999;9:3175–4180. doi: 10.1016/s0960-894x(99)00554-5. [DOI] [PubMed] [Google Scholar]
- 31.Johnson CR, Chun J, Bittman R, Jarvis WD. Intrinsic cytotoxicity and chemomodulatory actions of novel phenethylisothiocyanate sphingoid base derivatives in HL-60 human promyelocytic leukemia cells. J Pharmacol Exp Ther. 2004;309:452–461. doi: 10.1124/jpet.103.060665. [DOI] [PubMed] [Google Scholar]
- 32.Niiro H, Azuma H, Tanago S, et al. (3Z)-2-Acetylamino-3-octadecen-1-ol as a potent apoptotic agent against HL-60 cells. Bioorg Med Chem. 2004;12:45–51. doi: 10.1016/j.bmc.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 33.Sugiura M, Kono K, Liu H, et al. Ceramide kinase, a novel lipid kinase: molecular cloning and functional characterization. J Biol Chem. 2002;277:23294–23300. doi: 10.1074/jbc.M201535200. [DOI] [PubMed] [Google Scholar]
- 34.Igarashi Y, Hakomori S, Toyokuni T, et al. Effect of chemically well-defined sphingosine and its N-methyl derivatives on protein kinase C and src kinase activities. Biochemistry. 1989;28:6796–6800. doi: 10.1021/bi00443a002. [DOI] [PubMed] [Google Scholar]
- 35.De Luca T, Morre DM, Zhao H, Morre DJ. NAD+/NADH and/or CoQ/CoQH2 ratios from plasma membrane electron transport may determine ceramide and sphingosine-1-phosphate levels accompanying G1 arrest and apoptosis. Biofactors. 2005;25:43–60. doi: 10.1002/biof.5520250106. [DOI] [PubMed] [Google Scholar]
- 36.Gamble JR, Xia P, Hahn CN, et al. Phenoxodiol, an experimental anticancer drug, shows potent antiangiogenic properties in addition to its antitumour effects. Int J Cancer. 2006;118:2412–2420. doi: 10.1002/ijc.21682. [DOI] [PubMed] [Google Scholar]
- 37.Jarvis WD, Fornari FA, Jr, Auer KL, et al. Coordinate regulation of stress- and mitogen-activated protein kinases in the apoptotic actions of ceramide and sphingosine. Mol Pharmacol. 1997;52:935–947. doi: 10.1124/mol.52.6.935. [DOI] [PubMed] [Google Scholar]
- 38.Hamada K, Nakamura H, Oda T, Hirano T, Shimizu N, Utiyama H. Involvement of Mac-1-mediated adherence and sphingosine 1-phosphate in survival of phorbol ester-treated U937 cells. Biochem Biophys Res Commun. 1998;244:745–750. doi: 10.1006/bbrc.1998.8328. [DOI] [PubMed] [Google Scholar]
- 39.Cuvillier O, Edsall L, Spiegel S. Involvement of sphingosine in mitochondria-dependent fas-induced apoptosis of type II jurkat T cells. J Biol Chem. 2000;275:15691–15700. doi: 10.1074/jbc.M000280200. [DOI] [PubMed] [Google Scholar]
- 40.Moulding DA, Giles RV, Spiller DG, White MR, Tidd DM, Edwards SW. Apoptosis is rapidly triggered by antisense depletion of MCL-1 in differentiating U937 cells. Blood. 2000;96:1756–1763. [PubMed] [Google Scholar]
- 41.Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- 42.Rosato RR, Almenara JA, Kolla SS, et al. Mechanism and functional role of XIAP and Mcl-1 down-regulation in flavopiridol/vorinostat antileukemic interactions. Mol Cancer Ther. 2007;6:692–702. doi: 10.1158/1535-7163.MCT-06-0562. [DOI] [PubMed] [Google Scholar]
- 43.Sobue S, Iwasaki T, Sugisaki C, et al. Quantitative RT-PCR analysis of sphingolipid metabolic enzymes in acute leukemia and myelodysplastic syndromes. Leukemia. 2006;20:2042–2046. doi: 10.1038/sj.leu.2404386. [DOI] [PubMed] [Google Scholar]
- 44.McCormack E, Bruserud O, Gjertsen BT. Animal models of acute myelogenous leukaemia: development, application and future perspectives. Leukemia. 2005;19:687–706. doi: 10.1038/sj.leu.2403670. [DOI] [PubMed] [Google Scholar]
- 45.Sabbadini RA. Targeting sphingosine-1-phosphate for cancer therapy. Br J Cancer. 2006;95:1131–1135. doi: 10.1038/sj.bjc.6603400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.French KJ, Schrecengost RS, Lee BD, et al. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- 47.Li QF, Huang WR, Duan HF, Wang H, Wu CT, Wang LS. Sphingosine kinase-1 mediates BCR/ABL-induced up-regulation of Mcl-1 in chronic myeloid leukemia cells. Oncogene. 2007;26:7904–7908. doi: 10.1038/sj.onc.1210587. [DOI] [PubMed] [Google Scholar]
- 48.Sobue S, Nemoto S, Murakami M, et al. Implications of sphingosine kinase 1 expression level for the cellular sphingolipid rheostat: relevance as a marker for daunorubicin sensitivity of leukemia cells. Int J Hematol. 2008;87:266–275. doi: 10.1007/s12185-008-0052-0. [DOI] [PubMed] [Google Scholar]
- 49.Kohno M, Momoi M, Oo ML, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nyakern M, Tazzari PL, Finelli C, et al. Frequent elevation of Akt kinase phosphorylation in blood marrow and peripheral blood mononuclear cells from high-risk myelodysplastic syndrome patients. Leukemia. 2006;20:230–238. doi: 10.1038/sj.leu.2404057. [DOI] [PubMed] [Google Scholar]
- 52.Pitson SM, Xia P, Leclercq TM, et al. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jarvis WD, Kolesnick RN, Fornari FA, Traylor RS, Gewirtz DA, Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci U S A. 1994;91:73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng EH, Kirsch DG, Clem RJ, et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Alter N, Reed JC, Borner C, Obeid LM, Hannun YA. Bcl-2 interrupts the ceramide-mediated pathway of cell death. Proc Natl Acad Sci U S A. 1996;93:5325–5328. doi: 10.1073/pnas.93.11.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amarante-Mendes GP, Naekyung Kim C, Liu L, et al. Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome C and activation of caspase-3. Blood. 1998;91:1700–1705. [PubMed] [Google Scholar]
- 57.Betito S, Cuvillier O. Regulation by sphingosine 1-phosphate of Bax and Bad activities during apoptosis in a MEK-dependent manner. Biochem Biophys Res Commun. 2006;340:1273–1277. doi: 10.1016/j.bbrc.2005.12.138. [DOI] [PubMed] [Google Scholar]
- 58.Swanton C, Marani M, Pardo O, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11:498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 59.Ng KT, Man K, Ho JW, et al. Marked suppression of tumor growth by FTY720 in a rat liver tumor model: the significance of down-regulation of cell survival Akt pathway. Int J Oncol. 2007;30:375–380. [PubMed] [Google Scholar]
- 60.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Neviani P, Santhanam R, Oaks JJ, et al. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007;117:2408–2421. doi: 10.1172/JCI31095. [DOI] [PMC free article] [PubMed] [Google Scholar]