Abstract
DNA double-strand breaks (DSBs) are repaired by non-homologous end joining (NHEJ) or homologous recombination (HR). HR requires 5′ DSB end degradation that occurs in the presence of cyclin-dependent kinase (CDK) activity. Here, we show that a lack of any of the NHEJ proteins Yku (Yku70–Yku80), Lif1 or DNA ligase IV (Dnl4) increases 5′ DSB end degradation in G1 phase, with ykuΔ cells showing the strongest effect. This increase depends on MRX, the recruitment of which at DSBs is enhanced in ykuΔ G1 cells. DSB processing in G2 is not influenced by the absence of Yku, but it is delayed by Yku overproduction, which also decreases MRX loading on DSBs. Moreover, DSB resection in ykuΔ cells occurs independently of CDK activity, suggesting that it might be promoted by CDK-dependent inhibition of Yku.
Keywords: Yku, MRX, double-strand break, checkpoint, Saccharomyces cerevisiae
Introduction
Formation of double-strand breaks (DSBs) triggers activation of the DNA damage checkpoint, and ATM and ATR in mammals and Tel1 and Mec1 in Saccharomyces cerevisiae are important in this process (Longhese et al, 2006). Moreover, DSBs can be repaired by either non-homologous end joining (NHEJ) or homologous recombination (HR). In S. cerevisiae, the core components of the NHEJ machinery are the Yku (Yku70–Yku80), MRX (Mre11–Rad50–Xrs2) and Dnl4–Lif1 complexes, which are recruited rapidly to DSBs (Daley et al, 2005), where the two Yku subunits form a ring-like structure that binds to DNA ends and initiates NHEJ (Daley et al, 2005).
If DSB ends are not rejoined by NHEJ, their 5′-to-3′ nucleolytic degradation generates 3′-ended single-stranded DNA (ssDNA) tails that are essential for downstream HR events. This DSB processing requires cyclin-dependent kinase (CDK) activity and is therefore influenced by the cell-cycle stage (Aylon et al, 2004; Ira et al, 2004). Moreover, the Tel1 checkpoint kinase is thought to recognize unprocessed DSBs (Mantiero et al, 2007), whereas ssDNA generation leads to Mec1 recruitment and subsequent Mec1-dependent checkpoint activation (Lee et al, 1998; Zou & Elledge, 2003). Here, we show that the NHEJ machinery inhibits processing of broken ends in G1, and the excess of Yku interferes with DSB processing in G2. Moreover, CDK activity is not required for DSB resection in ykuΔ cells, suggesting that it might promote DSB resection through Yku inhibition.
Results And Discussion
NHEJ inhibits DSB resection and checkpoint activation in G1
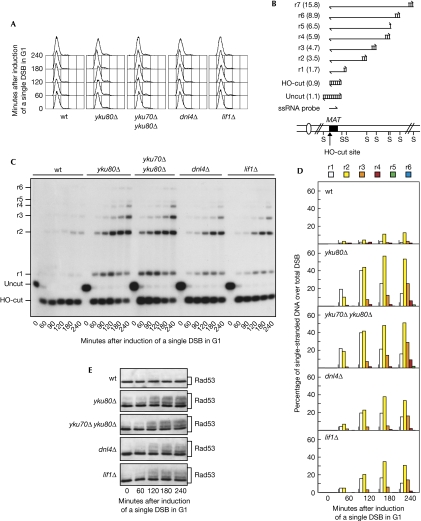
5′-to-3′ nucleolytic degradation of DSB ends is inhibited in G1 phase, when CDK activity is low, whereas it occurs in G2/M phase, when CDK activity is high (Aylon et al, 2004; Ira et al, 2004). YKU70 deletion has been shown to cause a twofold increase in the rate of 5′-to-3′ degradation of DSB ends in asynchronous cells (Lee et al, 1998). This suggests that removal of the Yku complex increases the rate of DSB resection in G2 and/or allows DSB processing in G1. In agreement with the latter possibility, yku mutants show faster degradation of DSB ends in G1 (Zhang et al, 2007; Wu et al, 2008) and increased replication protein A (RPA) focus formation following an I-SceI endonuclease-mediated DSB compared with wild type (Barlow et al, 2008). Thus, we directly measured 3′-ended ssDNA formation at a single irreparable DSB in G1-arrested cells lacking one or both Yku subunits. These cells express the site-specific HO endonuclease gene from a galactose-inducible promoter and cannot repair the HO cut by HR because they lack the homologous donor sequences HML and HMR (Lee et al, 1998). HO expression was induced by the addition of galactose to G1-arrested cell cultures (Fig 1A). Consistent with the requirement of CDK activity for DSB resection, the 3′-ended resection products (named r1–r7 in Fig 1B) at the HO cut were only barely detectable in wild-type G1 cells (Fig 1C,D). By contrast, they accumulated after HO induction in yku80Δ and yku70Δ yku80Δ G1 cells (Fig 1C,D), indicating that 5′-DSB end degradation in G1 is inhibited by Yku.
Figure 1.
The absence of non-homologous end joining allows double-strand break resection in G1. YEP+raf α-factor-arrested cell cultures of wild-type JKM139 and yku80Δ, yku70Δ yku80Δ, dnl4Δ and lif1Δ derivative strains were transferred to α-factor-containing YEP+raf+gal to induce HO expression (time zero). (A) Fluorescence-activated cell sorting analysis of DNA content. (B) Schematic representation of the system used to detect DSB resection. Gel blots of SspI-digested genomic DNA separated on alkaline agarose gel were hybridized with a single-stranded RNA probe specific for the unresected strand at the MAT locus, which shows HO-cut and uncut fragments of 0.9 and 1.1 kb, respectively. 5′-to-3′ resection progressively eliminates SspI sites located 1.7, 3.5, 4.7, 5.9, 6.5, 8.9 and 15.8 kb centromere-distal from the HO-cut site, producing larger SspI fragments (r1–r7) detected by the probe. (C) Analysis of ssDNA formation as described in (B). (D) Densitometric analysis of the representative experiment shown in (C). Three independent experiments were performed with similar results. (E) Western blot analysis of protein extracts with Rad53 antibodies. DSB, double-strand break; ssDNA, single-stranded DNA; wt, wild type; YEP, yeast extract peptone.
As the Yku complex is required for joining broken DNA ends by NHEJ, we investigated whether other NHEJ defects could increase DSB resection in G1. Processing of the HO cut was analysed in cells lacking DNA ligase IV (Dnl4) or Lif1, both of which act downstream from Yku in NHEJ (Daley et al, 2005). DSB resection was increased in both dnl4Δ and lif1Δ G1-arrested cells compared with wild type (Fig 1C,D), indicating that NHEJ interferes with DSB processing. This result is in contrast to the recent observation that dnl4Δ cells do not show an increased Rpa1 focus formation in response to an endonuclease-mediated break in G1 (Barlow et al, 2008), suggesting that detection of Rpa1 foci might require a higher amount of ssDNA than that observed in dnl4Δ cells. In any case, both dnl4Δ and lif1Δ cells accumulated lower amounts of resection products than ykuΔ cells (Fig 1C,D), implying an additional specific role of Yku in DNA end protection in G1.
Interestingly, yku80Δ, yku70Δ yku80Δ, dnl4Δ and lif1Δ G1-arrested cells accumulated mainly 1.7-, 3.5- and 4.7-kb ssDNA products (r1, r2 and r3), indicating that resection was not efficiently propagated to regions further away from the HO break (Fig 1C,D). This suggests that 5′-to-3′ nucleolytic degradation in G1, once started, occurs efficiently in the absence of NHEJ only up to a certain distance from the DNA break and then it slows down considerably. This implies that other mechanisms contribute to limit DSB processing in this cell-cycle phase.
It is well known that DSBs trigger DNA damage checkpoint activation, which requires phosphorylation of the effector kinase Rad53, which can be monitored by western blot analysis as electrophoretic mobility changes. Rad53 phosphorylation after a single DSB depends exclusively on Mec1, which recognizes 3′-ended ssDNA tails arising from DSB processing (Zou & Elledge, 2003; Mantiero et al, 2007). Consistent with the increased ssDNA generation in NHEJ-defective cells compared with wild-type cells, Rad53 phosphorylation increased 120 min after HO induction in yku70Δ, yku70Δ yku80Δ, dnl4Δ and lif1Δ G1-arrested cells, whereas it was not detected in similarly treated wild-type cells (Fig 1E). Thus, the lack of NHEJ allows DSB resection and Mec1-dependent checkpoint activation in G1.
Yku limits Mre11 recruitment at DSB ends in G1
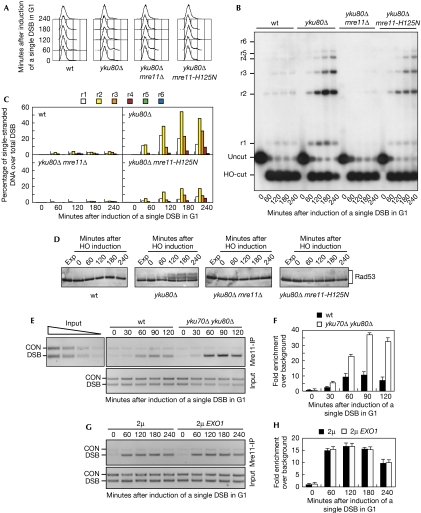
The MRX complex, which has a crucial role in DSB end processing (Clerici et al, 2006), turned out to be essential for accumulation of 3′-ended ssDNA at the HO cut in galactose-induced yku80Δ G1 cells. In fact, DSB resection was markedly reduced in yku80Δ mre11Δ cells compared with yku80Δ cells (Fig 2A–C). This MRX requirement partly depends on its nuclease activity. In fact, the amount of ssDNA in galactose-induced yku80Δ G1 cells containing the mre11-H125N mutation, which abrogates the endonuclease and exonuclease activities of Mre11 (Moreau et al, 1999), was lower than that in yku80Δ cells (Fig 2B,C). Accordingly, Mec1-dependent Rad53 phosphorylation increased after the addition of galactose in yku80Δ cells, but not in similarly treated yku80Δ mre11Δ and yku80Δ mre11-H125N cells (Fig 2D).
Figure 2.
Yku prevents Mre11-dependent double-strand break processing and efficient Mre11 recruitment to double-strand breaks in G1. (A–F) YEP+raf α-factor-arrested cell cultures of wild-type JKM139 and yku80Δ, yku80Δ mre11Δ, yku80Δ mre11-H125N, MRE11-MYC and yku70Δ yku80Δ MRE11-MYC derivative strains were transferred to α-factor-containing YEP+raf+gal (time zero). (A) Fluorescence-activated cell sorting analysis of DNA content. (B) Analysis of ssDNA formation as described in Fig 1B. (C) Densitometric analysis of the representative experiment shown in (B). Three independent experiments were performed with similar results. (D) Western blot analysis of protein extracts with Rad53 antibodies. (E) Representative ChIP time-course analysis of Mre11–DSB association. Quantitative PCRs before (Input) and after Mre11-Myc immunoprecipitation (Mre11-IP). Twofold serial dilutions of the input DNA establish the linear range of PCR. The efficiency of Mre11-Myc immunoprecipitation was similar at all time points (data not shown). (F) Quantitative analysis of Mre11–DSB association. Densitometric data from four independent experiments as in (E) were expressed as the relative fold enrichment of DSB over control (CON) signal independently for each time point and normalized to input DNA samples background. Error bars indicate s.d. (G,H) YEP+raf α-factor-arrested cell cultures of JKM139 MRE11-MYC carrying either the empty vector or the 2μ URA3 EXO1 plasmid were transferred to YEP+raf+gal to induce HO expression (time zero). (G) Representative ChIP time-course analysis of Mre11–DSB association. Quantitative PCRs before (Input) and after Mre11 immunoprecipitation (Mre11-IP) with anti-Myc antibodies. (H) Quantitative analysis of Mre11–DSB association. Densitometric data from three independent experiments as in (G) were expressed as described in (F). Error bars indicate s.d. ChIP, chromatin immunoprecipitation; DSB, double-strand break; Exp, exponentially growing cells; ssDNA, single-stranded DNA; wt, wild type; YEP, yeast extract peptone.
A previous study reported that Xrs2 association to DSBs was not influenced by the absence of NHEJ machinery, although its dissociation seemed to be delayed in both ykuΔ and dnl4Δ cells (Wu et al, 2008). Conversely, an independent study showed that Mre11 association at DSBs was reduced in both yku70Δ and dnl4Δ cells (Zhang et al, 2007). It is worth pointing out that MRX binding at DSB termini was measured in exponentially growing cell cultures in both studies. We then monitored Mre11 recruitment at an HO-induced DSB in G1-arrested wild-type and yku70Δ yku80Δ strains carrying a fully functional Myc-tagged MRE11 allele. Sheared chromatin from formaldehyde crosslinked cell samples taken at different time points after the addition of galactose was immunoprecipitated with Myc antibodies. Quantitative multiplex PCR was then used to monitor co-immunoprecipitation of DNA fragments located either 66 kb centromere-proximal to the MAT locus (CON) or 1 kb away from the HO-cut site (DSB). Mre11 association at the DSB was increased in yku70Δ yku80Δ G1 cells compared with similarly treated wild-type cells (Fig 2E,F). This enhanced Mre11 association did not seem to depend on nucleolytic DSB resection in ykuΔ cells. In fact, overproduction of the Exo1 exonuclease, which artificially triggered nuclease-dependent processing of the HO-induced DSB in G1 cells (supplementary Fig S1 online), did not increase Mre11 binding to DSB ends (Fig 2G,H). Thus, the enhanced Mre11 binding to DSBs might account for the increased efficiency of DSB end resection in ykuΔ G1 cells. However, as some MRX association at the HO cut was induced by the addition of galactose even in wild-type G1 cells (Fig 2E,F), in which DSB resection was markedly inhibited (Fig 2B,C), it is conceivable that Yku impairs 5′-end resection in G1 by inhibiting MRX activity as well as its association to DNA.
High levels of Yku influence the DSB response in G2
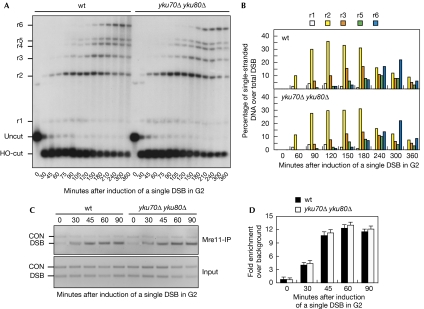
The absence of Yku does not enhance either DSB processing or Mre11 association in G2 (Fig 3). In fact, the kinetics of HO-induced DSB resection was similar in both wild-type and yku70Δ yku80Δ G2 cells (Fig 3A,B). Consistent with previous data showing that MRX recruitment to the HO cut was largely independent of Yku in exponentially growing cells (Wu et al, 2008), the extent of Mre11 association to DSBs was also similar in the two cell types (Fig 3C,D).
Figure 3.
The absence of Yku does not influence double-strand break processing and Mre11 recruitment at double-strand breaks in G2. YEP+raf nocodazole-arrested cell cultures of wild-type JKM139 and isogenic yku70Δ yku80Δ cells, expressing the MRE11-MYC allele, were transferred to nocodazole-containing YEP+raf+gal (time zero). (A) DSB resection analysis was performed as described in Fig 1B. (B) Densitometric analysis of the representative experiment shown in (A). Four independent experiments were performed with similar results. (C) Representative ChIP time-course analysis of Mre11–DSB association as described in Fig 2E. (D) Quantitative analysis of Mre11–DSB association. Densitometric data from three independent experiments as in (C) were expressed as in Fig 2F. Error bars indicate s.d. ChIP, chromatin immunoprecipitation; CON, control; DSB, double-strand break; wt, wild type; YEP, yeast extract peptone.
Conversely, accumulation of 3′-ended resection products after generation of a single HO-induced DSB was delayed by about 30 min in G2-arrested cells carrying the YKU70 and YKU80 genes on 2μ plasmids compared with otherwise isogenic cells carrying the empty vectors (Fig 4A,B). This might be due to decreased Mre11 association at the HO cut, which was lower in YKU70 YKU80-overexpressing cells than in wild-type cells (Fig 4C,D). Thus, an excess of Yku can interfere with DSB processing in G2, although the physiological amounts of Yku do not seem to inhibit it in this cell-cycle phase.
Figure 4.
Yku overproduction impairs 5′-end processing and Mre11 loading at an HO-induced double-strand break. (A–E) YEP+raf nocodazole-arrested cell cultures of JKM139 strains carrying either empty vectors or both the 2μ URA3 YKU70 and 2μ LEU2 YKU80 plasmids, all expressing the MRE11-MYC allele, were transferred to nocodazole-containing YEP+raf+gal (time zero). (A) DSB resection analysis as described in Fig 1B. (B) Densitometric analysis of the representative experiment shown in (A). Three independent experiments were performed with similar results. (C) Representative ChIP time-course analysis performed as described in Fig 2E. (D) Quantitative analysis of Mre11–DSB association. Densitometric data from three independent experiments as in (C) were expressed as in Fig 2F. Error bars indicate s.d. (E) Western blot analysis of protein extracts with Rad53 antibodies. (F) Exponentially growing YEP+raf cell cultures of mec1Δ LSY1259 (10 Ty1-HOcs-HIS3) strains transformed with 2μ URA3 plasmids, either empty or carrying both YKU70 and YKU80, were transferred to YEP+raf+gal to induce HO expression (time zero). Western blot analysis of protein extracts with Rad53 antibodies is shown. ChIP, chromatin immunoprecipitation; CON, control; DSB, double-strand break; Exp, exponentially growing cells; YEP, yeast extract peptone.
A delay in DSB resection should impair Mec1-dependent checkpoint activation and enhance Tel1 signalling activity. In fact, it is well known that Mec1 triggers Rad53 phosphorylation in response to a single processed DSB, whereas Tel1 signalling activity becomes apparent only after the generation of several DSBs and is disrupted when DSB termini are resected (Mantiero et al, 2007). As shown in Fig 4E, the amount of Rad53 phosphorylation after induction of a single HO cut was lower in YKU70 YKU80-overexpressing cells than that in wild-type cells, indicating that high levels of Yku impair Mec1 signalling activity, probably by reducing ssDNA generation. To trigger the Tel1-dependent checkpoint specifically, we used a mec1Δ strain containing ten HO recognition sites embedded within randomly dispersed Ty1 elements, in addition to the endogenous HO-cut site present at the MAT locus (Llorente & Symington, 2004; Mantiero et al, 2007). As shown in Fig 4F, Rad53 phosphorylation was induced by 11 HO cuts about 45 min after the addition of galactose in mec1Δ cells transformed with 2μ plasmids, either empty or carrying the YKU70 and YKU80 genes. Phosphorylated Rad53 then disappeared more quickly (time=180 min) in mec1Δ cells transformed with the empty vector than in Yku-overproducing mec1Δ cells, in which it was still detectable 300 min after HO induction. As this Rad53 phosphorylation was Tel1 dependent (data not shown; Mantiero et al, 2007), high levels of Yku seem to enhance Tel1 signalling activity, possibly by delaying DSB resection.
Relationships between Yku and CDKs in DSB resection
DSB resection is known to depend on CDK activity (Aylon et al, 2004; Ira et al, 2004). The finding that DSB resection in G1 is more efficient in the absence than in the presence of Yku suggests that CDKs might promote it by inhibiting Yku. If this was the case, the absence of Yku should allow ssDNA generation in G2 even when CDKs are inhibited. Thus, we monitored resection of the HO-induced DSB in G2-arrested GAL-SIC1ΔNT and GAL-SIC1ΔNT yku80Δ cells expressing high levels of a stable truncated form of the CDK inhibitor Sic1 (Sic1ΔNT; Desdouets et al, 1998). When both DSB formation and Sic1ΔNT overproduction were induced by the addition of galactose to G2-arrested cell cultures, DSB resection was markedly inhibited in GAL-SIC1ΔNT cells, as expected, whereas it was not inhibited in GAL-SIC1ΔNT yku80Δ cells (Fig 5A,B). Consistent with the increased ssDNA generation in GAL-SIC1ΔNT yku80Δ cells compared with GAL-SIC1ΔNT cells, phosphorylated Rad53 was detected in the former cells but not in the latter cells (Fig 5C). Similar to that observed in G1-arrested yku80Δ cells (Figs 1C, 2B), GAL-SIC1ΔNT yku80Δ G2 cells accumulated mainly 1.7-, 3.5- and 4.7-kb resection products (r1, r2 and r3; Fig 5A,B), whereas longer resection products were clearly detectable in similarly treated wild-type and yku80Δ G2 cells. Thus, Yku inhibition by CDKs contributes to initiate DSB resection, but other CDK targets are probably required for extensive resection.
Figure 5.
Interaction between Yku and cyclin-dependent kinase in the regulation of double-strand break resection. (A–C) YEP+raf nocodazole-arrested cell cultures of wild-type JKM139 and yku80Δ, GAL-SIC1ΔNT and GAL-SIC1ΔNT yku80Δ derivative strains were transferred to nocodazole-containing YEP+raf+gal (time zero). (A) Analysis of DSB resection as described in Fig 1B. (B) Densitometric analysis of the representative experiment shown in (A). Three independent experiments were performed with similar results. (C) Western blot analysis of protein extracts with Rad53 antibodies. (D,E) YEP+raf α-factor-arrested cell cultures of wild-type JKM139 and GAL-CLB2 or yku80Δ derivative strains were transferred to α-factor-containing YEP+raf+gal (time zero). (D) Analysis of DSB resection as described in Fig 1B. (E) Densitometric analysis of the representative experiment shown in (D). Three independent experiments were performed with similar results. (F,G) ChIP analysis of Yku70 binding to an HO-induced DSB. (F) YEP+raf α-factor- (G1) or nocodazole-arrested (G2) cell cultures of a JKM139 derivative strain expressing the YKU70-MYC allele were transferred to α-factor- or nocodazole-containing YEP+raf+gal, respectively (time zero). (G) YEP+raf α-factor-arrested cell cultures of wild-type and GAL-CLB2 isogenic strains expressing the YKU70-MYC allele were transferred to α-factor-containing YEP+raf+gal (time zero). In both (F) and (G), densitometric data from three independent experiments were expressed as in Fig 2F. Error bars indicate s.d. ChIP, chromatin immunoprecipitation; DSB, double-strand break; Exp, exponentially growing cells; wt, wild type; YEP, yeast extract peptone.
We then compared the resection kinetics of an HO-induced DSB in G1-arrested cells either lacking Yku80 or ectopically overexpressing the main mitotic cyclin gene CLB2 from the GAL promoter (GAL-CLB2). Indeed, generation of ssDNA was enhanced in both yku80Δ and GAL-CLB2 G1-arrested cells compared with wild-type cells (Fig 5D,E). Consistent with our previous finding that resection occurs efficiently only up to a certain distance from the DSB when cells lacking Yku have low levels of CDK, yku80Δ G1 cells accumulated mainly 1.7-, 3.5- and 4.7-kb resection products within 180/240 min after HO induction (Fig 5D,E). At the same time points, GAL-CLB2 G1 cells accumulated much longer ssDNA molecules, indicating that high CDK activity allows extensive DSB resection in G1 cells, in which 5′-to-3′ nucleolytic degradation is limited even in the absence of NHEJ. Therefore, although Yku/NHEJ inhibition by CDKs allows DSB resection, extensive resection is probably promoted by CDK-dependent regulation of factors other than Yku. Accordingly, DSB resection in G2 cells, in which CDK activity is high, occurred extensively independently of Yku, as it generated ssDNA fragments reaching at least 15 kb in length (Figs 3A, 5A), whereas it was limited to shorter tracts in GAL-SIC1ΔNT yku80Δ G2 cells (Fig 5A).
We also found by chromatin immunoprecipitation (ChIP) analysis that the amount of Yku70 associated at the HO-induced DSB in G1, when CDK is inactive, was similar to that in G2, when CDK is active (Fig 5F). Moreover, CLB2 overexpression in G1-arrested cells, which allows DSB resection (Fig 5D,E), did not affect Yku loading on the HO-induced DSBs (Fig 5G). Therefore, CDK does not regulate Yku loading on DSB ends, suggesting that it might modulate Yku activity and/or its ability to interact with other proteins involved in DSB processing.
How does NHEJ prevent 5′-to-3′ nucleolytic degradation of DSB ends in cells with low CDK activity? As NHEJ allows HO-induced DSB ends to be religated, defective NHEJ might increase the time available for the resection machinery to bind to DSB ends and initiate resection. The absence of Yku has a stronger effect than that of either Dnl4 or Lif1 in promoting 5′ DSB end degradation and limits Mre11 association to the break site in G1. Therefore, Yku binding at DSB ends might also directly impair the loading and/or activity of the resection machinery. Moreover, DSB resection in NHEJ-defective G1 cells occurs efficiently only up to a certain distance from the DSB, suggesting that it is limited in G1 compared with G2 even in the absence of NHEJ, and this seems to depend on the low level of CDK (Fig 5). In general, the NHEJ machinery seems to inhibit DSB processing only when CDK activity is low (G1 cells and GAL-SIC1ΔNT G2 cells) or becomes rate limiting to inhibit NHEJ (Yku-overproducing G2 cells). In this view, active CDKs in G2 cells should be sufficient to inhibit physiological amounts of Yku/NHEJ completely, but not excessive amounts of Yku. As DSB resection seems to be more efficiently propagated to DNA regions far away from the break in the presence of high CDK activity than in the absence of Yku, extensive DSB processing probably requires CDK-mediated regulation of other factors, although Yku has an important role in its control.
Finally, our evidence that the absence of Yku partly overcomes the requirement of CDK activity to promote 5′-to-3′ nucleolytic degradation suggests that CDK-dependent phosphorylation events might lead to inhibition of Yku or other NHEJ proteins. A good candidate as a CDK target might be Yku itself, as human Ku70 has been shown to interact physically with cyclin A1 and to be phosphorylated by the cyclin A1–CDK2 complex (Müller-Tidow et al, 2004). To verify whether this also applies to yeast Yku and whether this possible phosphorylation has a role in regulating DSB processing will be a challenging goal for future work.
Methods
Yeast strains. Yeast strains generated during this study were derivatives of strain JKM139 (MATa hmlΔ hmrΔ ade1 lys5 leu2-3,112 trp1∷hisG ura3-52 ho ade3∷GAL-HO) or LSY1259 (10 Ty1-HOcs-HIS3), kindly provided by J. Haber (Brandeis University, Waltham, MA, USA; Lee et al, 1998) and L. Symington (Columbia University, New York, NY, USA; Llorente & Symington, 2004), respectively. Strain genotypes are listed in supplementary Table S1 online. To induce HO expression in JKM139 and its derivative strains, cells were grown in raffinose-containing yeast extract peptone (YEP) and then transferred to raffinose- and galactose-containing YEP. HO-expressing LSY1259 and derivative strains were obtained by transformation with a centromeric plasmid carrying a GAL-HO fusion (pFH800; Llorente & Symington, 2004). Transformants were then grown in raffinose-containing synthetic medium and then transferred to YEP+raf+gal.
Other techniques. DSB end resection at the MAT locus was analysed on alkaline agarose gels as described previously (Clerici et al, 2006), by using a single-stranded probe complementary to the unresected DSB strand. Quantitative analysis of DSB processing was performed by calculating the ratio of band intensities for ssDNA and total amount of DSB products. ChIP analysis was performed as described previously (Viscardi et al, 2007). Multiplex PCRs were carried out by using primer pairs complementary to DNA sequences located 1 kb from the HO-cut site at MAT (DSB) and to DNA sequences located 66 kb from MAT (CON). Multiplex PCRs for Mre11 immunoprecipitates were carried out for 30 cycles. Gel quantification was determined by using the NIH image program. The relative fold enrichments of DSB-bound Mre11 were calculated as follows: [DSBIP/CONIP]/[DSBinput/CONinput], where IP and input represent the amount of PCR product in the immunoprecipitates and in input samples before immunoprecipitation, respectively.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank E. Alani, J. Diffley, J. Haber, S. Jackson, S. Piatti and C. Santocanale for providing yeast strains, plasmids and antibodies. This work was supported by grants from Associazione Italiana Ricerca sul Cancro to M.P.L.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aylon Y, Liefshitz B, Kupiec M (2004) The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J 23: 4868–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow JH, Lisby M, Rothstein R (2008) Differential regulation of the cellular response to DNA double-strand breaks in G1. Mol Cell 30: 73–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M, Mantiero D, Lucchini G, Longhese MP (2006) The Saccharomyces cerevisiae Sae2 protein negatively regulates DNA damage checkpoint signalling. EMBO Rep 7: 212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley JM, Palmbos PL, Wu D, Wilson TE (2005) Nonhomologous end joining in yeast. Annu Rev Genet 39: 431–451 [DOI] [PubMed] [Google Scholar]
- Desdouets C, Santocanale C, Drury LS, Perkins G, Foiani M, Plevani P, Diffley JFX (1998) Evidence for a Cdc6-independent mitotic resetting event involving DNA polymerase α. EMBO J 17: 4139–4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ira G et al. (2004) DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 431: 1011–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Moore JK, Holmes A, Umezu K, Kolodner RD, Haber JE (1998) Saccharomyces Ku70, Mre11/Rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell 94: 399–409 [DOI] [PubMed] [Google Scholar]
- Llorente B, Symington LS (2004) The Mre11 nuclease is not required for 5′ to 3′ resection at multiple HO-induced double-strand breaks. Mol Cell Biol 24: 9682–9694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhese MP, Mantiero D, Clerici M (2006) The cellular response to chromosome breakage. Mol Microbiol 60: 1099–1108 [DOI] [PubMed] [Google Scholar]
- Mantiero D, Clerici M, Lucchini G, Longhese MP (2007) Dual role for Saccharomyces cerevisiae Tel1 in the checkpoint response to double-strand breaks. EMBO Rep 8: 380–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau S, Ferguson JR, Symington LS (1999) The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol Cell Biol 19: 556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Tidow C et al. (2004) The cyclin A1–CDK2 complex regulates DNA double-strand break repair. Mol Cell Biol 24: 8917–8928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscardi V, Bonetti D, Cartagena-Lirola H, Lucchini G, Longhese MP (2007) MRX-dependent DNA damage response to short telomeres. Mol Biol Cell 18: 3047–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Topper LM, Wilson TE (2008) Recruitment and dissociation of NHEJ proteins at a DNA double-strand break in Saccharomyces cerevisiae. Genetics 178: 1237–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hefferin ML, Chen L, Shim EY, Tseng HM, Kwon Y, Sung P, Lee SE, Tomkinson AE (2007) Role of Dnl4–Lif1 in nonhomologous end-joining repair complex assembly and suppression of homologous recombination. Nat Struct Mol Biol 14: 639–646 [DOI] [PubMed] [Google Scholar]
- Zou L, Elledge SJ (2003) Sensing DNA damage through ATRIP recognition of RPA–ssDNA complexes. Science 300: 1542–1548 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information