Abstract
In most organisms, kinesin-5 motors are essential for mitosis and meiosis, where they crosslink and slide apart the antiparallel microtubule half-spindles. Recently, it was shown using single-molecule optical trapping that a truncated, double-headed human kinesin-5 dimer can step processively along microtubules. However, processivity is limited (∼8 steps) with little coordination between the heads, raising the possibility that kinesin-5 motors might also be able to move by a nonprocessive mechanism. To investigate this, we engineered single-headed kinesin-5 dimers. We show that a set of these single-headed Eg5 dimers drive microtubule sliding at about 90% of wild-type velocity, indicating that Eg5 can slide microtubules by a mechanism in which one head of each Eg5 head-pair is effectively redundant. On the basis of this, we propose a muscle-like model for Eg5-driven microtubule sliding in spindles in which most force-generating events are single-headed interactions and alternate-heads processivity is rare.
Keywords: Eg5, kinesin-5, single-head, nonprocessive, processivity
Introduction
Members of the kinesin-5 family of microtubule (MT) plus-end-directed motors are dimers-of-dimers with two motor domains at each end of a central four-stranded stalk (Kashina et al, 1996). Recently, it was shown that a truncated human Eg5 dimer, corresponding to one end of the wild-type homotetramer, moves processively under load in a single-bead optical trapping assay (Valentine et al, 2006); Eg5 dimers typically take only a few steps (∼8 steps on average) per processive run (Valentine et al, 2006; Korneev et al, 2007).
The limited processivity of Eg5 dimers is in sharp contrast to that of kinesin-1, which is a well-coordinated walking machine. Studies of the kinesin-1 coordination mechanism have shown that, (i) ATP binding to the MT-attached head unmasks the tubulin binding site on the tethered head and activates its diffusional search for the next binding site (Alonso et al, 2007), (ii) ATP binding is inhibited by rearwards strain (Rosenfeld et al, 2003) and (iii) the attachment of the forward head to the next binding site accelerates the dissociation of the rearward head by about twofold (Crevel et al, 2004a). These various gating mechanisms allow kinesin-1 to make runs of typically more than 100 steps, with each step consuming one ATP molecule. Processive stepping slows down under load, and stalls occur when the load is such that back-steps and fore-steps are equally probable (Nishiyama et al, 2002; Carter & Cross, 2005).
Why are dimeric kinesin-5 molecules so much less processive than dimeric kinesin-1 molecules? Kinetic analysis offers a possible answer. Krzysiak & Gilbert (2006) analysed the same construct used by Valentine et al (2006) in their optical trapping experiments (human Eg5-513). ATP binding, mantADP release and the dissociation of the MT–Eg5 complex were all biphasic, with the rate constants for the second phases up to 60 times slower than those for the first phases. By contrast, for kinesin-1, the corresponding first and second phases have comparable rate constants (Hackney, 1994; Ma & Taylor, 1997; Gilbert et al, 1998; Crevel et al, 1999). From the biphasic behaviour of Eg5, Krzysiak & Gilbert (2006) came to the conclusion that dimeric Eg5 goes through alternating-site catalysis. They noted however that the rate constant for the second phase of MT-activated mantADP release was too slow to be consistent with a sequential alternating-sites mechanism in which the release of mantADP from the first head and then the second head is required at each step. They also noted a discrepancy between the maximum rate of MT-activated ATP turnover by Eg-513 in solution of 0.5 s−1 and the expected rate based on the measured rate of mechanical stepping (∼100 nm s−1, which corresponds to approximately 12 steps per second for a walking model with 8 nm steps and predicts a 12 s−1 turnover rate). To explain these discrepancies, Krzysiak & Gilbert postulated that a slow (∼1 s−1) conformational change follows MT encounter of the first head and converts Eg5-513 from an initially nonprocessive state to a processive state. In this proposed processive state, coordination between the two heads is weak, but nonetheless sufficient to allow a few steps before termination of the processive run. More recently, these same authors suggested a modified model in which Eg5 dimers begin processive runs with both motor domains bound to the MT in a nucleotide-free state (Krzysiak et al, 2008). This again is in contrast with current kinesin-1 models, which propose a one-head-attached starting state (Alonso et al, 2007; Mori et al, 2007).
In summary, at present it is thought that the stepping mechanism of Eg5 differs significantly from that of kinesin-1. Eg5 dimers show limited processivity, reflecting limited coordination between the two heads of each dimer. This raises the question, to what extent is coordination between the two heads of Eg5 dimers required for Eg5-driven MT sliding?
Results And Discussion
Single-headed Eg5 dimers can drive MT sliding
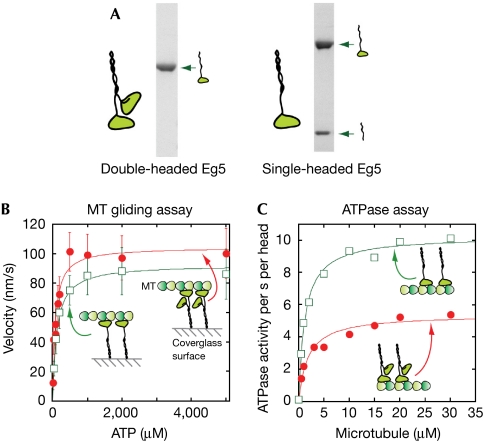
To investigate this question, we engineered a single-headed dimer based on Xenopus laevis Eg5 (Fig 1A). One chain of this dimer has an Eg5 motor domain and neck linker (residues Met 1–Thr 370), fused at its amino terminus to GST and at its carboxy terminus to a truncated kinesin-1 coiled-coil tail (Ala 337–Leu 432). The other chain consists of just the truncated kinesin-1 tail and a C-terminal 6-His tag. Here, we used a kinesin-1 dimerization domain to ensure tight dimerization (Tomishige et al, 2002) and to facilitate construction of kinesin-1–Eg5 heterodimers (Kaseda et al, 2002). The two chains were coexpressed, the single-headed dimer was purified by sequential GST-affinity and Ni-affinity chromatography, and the GST moiety was cleaved away (Fig 1A). To exclude potential trace contamination of our single-headed heterodimer Eg5 by material lacking the 6-His tag, we performed all motility assays using coverslips coated with penta-His antibody. Control experiments showed that Eg5 dimer lacking the 6-His tag does not bind to this surface sufficiently well to be able to recruit MTs to it (data not shown).
Figure 1.
Microtubule sliding and ATPase activity of Eg5 homodimers. (A) SDS–polyacrylamide gel electrophoresis of double-headed (left) and single-headed (right) Eg5. (B) Eg5-driven MT sliding velocities. Squares, single-headed Eg5; circles, double-headed Eg5. (C) MT-activated ATPase activities. Activities are expressed in terms of per second per head. MT, microtubule.
In kinesin-1, a well-coordinated walking machine, deletion of one head per head-pair substantially reduces MT sliding velocity and MT-stimulated ATPase activity to about 10% of that of wild type (Hancock & Howard, 1998, 1999). If Eg5 dimers also move by a walking mechanism, then deletion of one head per head-pair should abrogate walking and affect the sliding velocity, just as it does for kinesin-1. Instead, we found that deletion of one head per Eg5 head-pair had little effect: single-headed Eg5 dimers drove smooth, continuous MT sliding at 92±2 nm s−1, which is close to the 104±4 nm s−1 velocity of wild-type, double-headed dimers (Fig 1B). Sliding velocities were constant over the entire range of motor concentrations at which continuous sliding occurs (supplementary Fig 1 online). For both constructs, MT sliding was robust and smooth: qualitatively as well as quantitatively, single-headed and double-headed Eg5 performed similarly in MT sliding assays.
Comparison of the MT-activated ATPase of wild-type Eg5 homodimers with that of single-headed heterodimers is informative. Deletion of one head per head-pair doubles Vmax, the maximum per head steady-state MT-activated ATPase, from 5.4±0.3 to 10.1±0.2 s−1 per head. This suggests that in the wild-type Eg5 dimer, only one head can engage at a time, which is consistent with the sliding force being produced by a single-headed mechanism. If correct, this would explain why only one head of each head-pair is required to drive MT sliding at a rate close to that of wild type.
Kinesin-1–Eg5 heterodimers are nonprocessive
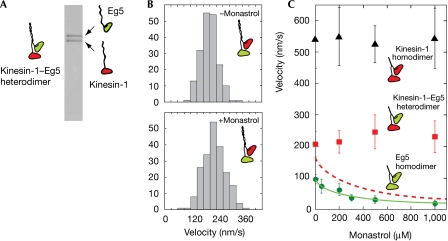
To gain further insight into the ability of Eg5 heads to coordinate, we asked whether Eg5 heads can participate in an alternate-heads walking mechanism if engineered into a suitable molecular context. To investigate this, we constructed a kinesin-1–Eg5 heterodimer (Fig 2A). In the presence of 1 mM ATP, these heterodimers slid MTs at approximately 200 nm s−1 (Fig 2B), which is considerably faster than Eg5 homodimers (∼100 nm s−1; Fig 1B) but considerably slower than processive double-headed kinesin-1 dimers (∼540 nm s−1). To test for an alternate-heads walking mechanism, we titrated in monastrol, a small-molecule inhibitor that is specific for Eg5 heads. In a model with alternate-heads walking, monastrol treatment should reduce the sliding velocity because the Eg5 heads in each molecule will be inhibited and stepping by strict alternation of the two heads would necessarily be inhibited. By contrast, in a nonprocessive model, monastrol is predicted to cause an increase in MT sliding velocity because it will relieve the molecular drag caused by the slower-cycling Eg5 heads (Crevel et al, 2004b). We found that monastrol treatment slightly increased the MT sliding velocity (Fig 2C), supporting the nonprocessive model. This interpretation depends on the Eg5 head in each kinesin-1–Eg5 heterodimer being active before the addition of monastrol and inhibited after the addition of monastrol so that it exerts less friction. To confirm that this is the case, we made mixed surfaces carrying both kinesin-1–Eg5 heterodimer and kinesin-1 homodimer. The molecular drag because of the slower-cycling Eg5 heads is then obvious, as is the marked reduction in drag caused by monastrol (supplementary Fig 2 online). This control experiment confirms that the Eg5 heads in the kinesin-1–Eg5 heterodimer are able to attach to MTs and to be inhibited by monastrol. We conclude that even when paired with a kinesin-1 head, Eg5 heads cannot participate in an alternate-heads walking mechanism. Eg5 dimers seem to be similar to kinesin-1 in that they operate on a similar one-head-at-a-time rule, but different from kinesin-1 in that they have little or no additional head–head coordination in place.
Figure 2.
Microtubule sliding by kinesin-1–Eg5 heterodimers. (A) SDS–polyacrylamide gel electrophoresis of kinesin-1–Eg5 heterodimers. Two heavy chains are present in equal amounts. (B) Histograms of MT sliding velocities driven by kinesin-1–Eg5 heterodimer in the absence (top) and presence (bottom) of 1 mM monastrol. (C) MT sliding velocities driven by kinesin-1–Eg5 heterodimer (squares), kinesin-1 homodimer (triangles) and Eg5 homodimer (circles). Monastrol inhibited MT sliding by the Eg5 homodimer. A fit (solid line) to a hyperbolic inhibition equation V=Intcpt−(amplitude × [monastrol]/(K50%+[monastrol])) indicated that 50% inhibition occurs at 256 μM monastrol. The predicted velocity of kinesin-1–Eg5 heterodimer on a model with strict alternation of the kinesin-1 and Eg5 heads, and a monastrol-dependent dwell time for the Eg5 component is shown by the dotted line. This line was generated by calculating Vpredicted=2Vk Ve/(Vk+Ve) for each monastrol concentration, where Vk is the measured MT sliding velocity for kinesin-1 and Ve is that for Eg5. The dotted line is the fit of the above inhibition equation to these calculated points. The actual behaviour (squares) is different, emphasizing that a scheme with strict alternation does not apply. MT, microtubule.
There are other examples of single-headed mechanisms in double-headed kinesins. Neurospora crassa kinesin-3 (Nckin-3) has one active head and one null head (Adio et al, 2006), whereas Kar3, a minus-end-directed kinesin-14, has one functional head paired to a heterologous, enzymatically inactive partner (Allingham et al, 2007). Ncd, another kinesin-14, forms homodimers that need both strands of their tail, but not their second head, to drive MT sliding at the rate of wild type (Endres et al, 2006), and deletion of one head per ncd dimer has little impact on the MT-activated ATPase (Endres et al, 2006). All these dimeric kinesin family proteins share with Eg5 the characteristic that motility does not require the second head of the dimer.
Our data are consistent with a predominantly nonprocessive model for the Eg5 mechanism, in which individual Eg5 molecules in an ensemble make single-headed force-generating interactions with an overlying MT and then relax (Fig 3, right column). There is evidence that relaxed Eg5•ADP molecules can remain attached to MTs in a weak binding state that slip along the MT rail whenever other motors in the ensemble generate an impulse of force (Crevel et al, 2004b; Fig 3, left column). However, optical trapping data (Valentine et al, 2006) show that kinesin-5 dimers can move processively—although only for a few steps—strongly suggesting that the two heads of the molecule can interact alternately with MTs. How can we reconcile these two apparently contradictory results?
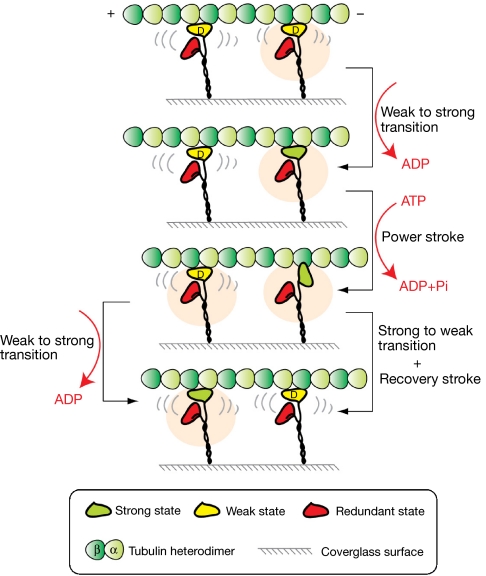
Figure 3.
Force generation with negative intramolecular cooperativity. The orange shading indicates active molecules. In any one molecule, only one of the two heads is involved in force generation, whereas the other head is redundant for the duration of the force-generating event. In subsequent cycles, the heads might re-assort and the redundant head might be active instead. For each active head, the cycle of force generation begins with ADP release, which converts the head to stable strong binding, and continues with ATP binding and Pi release, which returns the head to a weak binding Eg5•ADP state. Weakly bound Eg5•ADP heads (left column) remain attached to the MT in a low-friction state that can be readily slid along the MT by forces developed by other, active molecules in the array. D, ADP; MT, microtubule.
In the MT scheme that we favour (Fig 4), Eg5 homotetramers crosslink antiparallel MTs in spindles, much as myosin thick filaments crosslink actin filaments in a muscle. In both cases, filament sliding is driven by several independent force generators comprising one head of each head-pair. We predict that in a single Eg5 cycle, one head per head-pair releases its ADP, switches stably to strong binding, contributes an impulse of force, binds to and hydrolyses ATP, releases Pi and relaxes back into a long-lived, weakly bound Eg5•ADP state. In our model, Eg5•ADP heads tend to remain attached to MTs, but in a low-friction state (Crevel et al, 2004b) that can be readily slid along as other heads in the ensemble in turn exert force. Consistent with this, the dissociation of Eg5•ADP from MTs has been shown to be one of the slowest steps in the kinetic cycle (Cochran & Gilbert, 2005; Krzysiak & Gilbert, 2006).
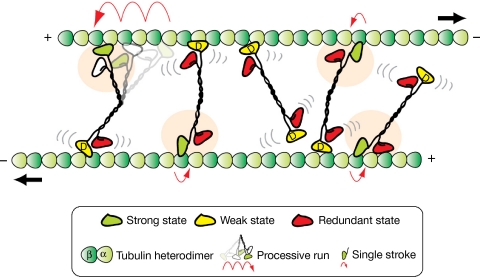
Figure 4.
Model of Eg5 homotetramer molecules in spindles. The orange shading indicates active molecules. Individual molecules contribute intermittent, single-headed force-generating interactions and then revert to the low-friction Eg5•ADP state. The collective of Eg5 molecules maintains active crosslinking of the MTs and exert consistent sliding force that drives the MTs to slide apart. Individual Eg5 molecules within the collective cycle between sticking (force-generating and force-holding) and slipping (low-friction, Eg5•ADP) states. Processive events (8 nm steps by an alternate-heads mechanism) can occur, but are rare (top left). MT, microtubule.
This muscle-like scheme accounts for our core finding that single-headed Eg5 molecules are as good as double-headed molecules at driving MT sliding, because a surface of single-headed Eg5 dimers functions almost equivalently to a surface of double-headed dimers. Why then does Eg5 have two heads? One possibility is that the resulting higher local density of motor heads produces a faster on-rate at the beginning of the crossbridge stroke. A second possibility is that Eg5 gains the ability occasionally to use a processive mechanism. We speculate that in special circumstances, the second head of an Eg5 dimer might engage and limited processivity can occur. Our scheme allows for occasional processive steps, as a consequence of negative cooperativity between the two heads of each head-pair, but with no other head–head coordination in place (Veigel et al, 2005). Single-molecule optical trapping necessarily detects only these processive events. It was recently proposed (Krzysiak et al, 2008) that Eg5 dimers undergo a slow conformational change (∼1 s−1 or slower) before the molecule starts its processive run and that a competing nonprocessive detachment pathway runs at ∼10 s−1. This suggests that a relatively small fraction of force-generating events would be processive, which is consistent with our model and with the low chemical processivity of Eg5 dimers in solution (Lockhart & Cross, 1996).
Methods
Expression constructs and protein purification. The preparation of homodimeric and heterodimeric kinesins has been described in detail previously (Kaseda et al, 2002). Double-headed constructs were engineered by using the pET17b vector (Novagen Inc., Darmstadt, Germany). DNA encoding the motor domain plus neck linker (Met 1–Thr 370) of X. laevis Eg5-2 was fused to that encoding a section of the coiled-coil tail of human kinesin-1 (Ala 337–Leu 432) with a C-terminal 6-His tag. The proteins were expressed in BLR(DE3) pLys S (Novagen Inc.) cells and purified with Ni-NTA beads (Qiagen, Crawley, West Sussex, UK). For the single-headed Eg5 heterodimer, we engineered the His-tagged kinesin-1 tail (Ala 337–Leu 432) in tandem with a motor domain–neck linker–tail construct fused at its N terminus to GST. The expressed proteins were purified first with a glutathione resin and then with an Ni-NTA-Agarose resin (Qiagen). GST was removed by thrombin (Sigma, Gillingham, Dorset, UK) treatment.
ATPase assay. The steady-state stimulation of ATPase activity by pig brain tubulin was measured at 25°C by using a pyruvate kinase/lactate dehydrogenase linked assay to measure NADH absorbance at 340 nm, in a Cary 50 spectrophotometer as described previously (Lockhart & Cross, 1994). The assay buffer was 80 mM K-PIPES, 2 mM MgCl2, 1 mM EGTA, 100 mM K-acetate, 5 mM dithiothreitol, 20 μM Taxol, pH 6.9 supplemented with 1 mg ml−1 BSA. The concentration of motor proteins used in the assays was kept as low as possible to minimize possible crowding effects on MTs (Huang & Hackney, 1994). Typically, assays were carried out at a final motor head concentration of 0.1 μM. Mixed-isomers monastrol was obtained from Sigma. The ATPase rates were least-squares fit to the Michaelis–Menten equation as a function of MT concentration to determine the Km(MT) and kcat values.
Multiple motor motility assay. This assay was performed on glass coverslips coated with penta-His antibody (Qiagen) in assay buffer augmented with 0.5 mg ml−1 casein, 1 mM ATP and oxygen scavenger system at 25±1°C (Kaseda et al, 2002). The motor concentration used was typically 1–3 μM. Taxol-stabilized pig brain MTs were visualized by using video enhanced differential interference contrast microscopy and the sliding trajectories were analysed using NJ Carter's RETRAC program (http://mc11.mcri.ac.uk/retrac.html); data are mean±s.d.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Fig 1
Acknowledgments
We thank D. Drummond and A. McAinsh for thoughtful comments. This work was funded by a Japan Society for the Promotion of Science Research Fellowship for Young Scientists and by Marie Curie Cancer Care.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adio S, Bloemink M, Hartel M, Leier S, Geeves MA, Woehlke G (2006) Kinetic and mechanistic basis of the non-processive kinesin-3 motor NcKin3. J Biol Chem 281: 37782–37793 [DOI] [PubMed] [Google Scholar]
- Allingham JS, Sproul LR, Rayment I, Gilbert SP (2007) Vik1 modulates microtubule–Kar3 interactions through a motor domain that lacks an active site. Cell 128: 1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso MC, Drummond DR, Kain S, Hoeng J, Amos L, Cross RA (2007) An ATP gate controls tubulin binding by the tethered head of kinesin-1. Science 316: 120–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter NJ, Cross RA (2005) Mechanics of the kinesin step. Nature 435: 308–312 [DOI] [PubMed] [Google Scholar]
- Cochran JC, Gilbert SP (2005) ATPase mechanism of eg5 in the absence of microtubules: insight into microtubule activation and allosteric inhibition by monastrol. Biochemistry 44: 16633–16648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevel I, Carter N, Schliwa M, Cross R (1999) Coupled chemical and mechanical reaction steps in a processive Neurospora kinesin. EMBO J 18: 5863–5872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevel IM, Nyitrai M, Alonso MC, Weiss S, Geeves MA, Cross RA (2004a) What kinesin does at roadblocks: the coordination mechanism for molecular walking. EMBO J 23: 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevel IM, Alonso MC, Cross RA (2004b) Monastrol stabilises an attached low-friction mode of Eg5. Curr Biol 14: R411–R412 [DOI] [PubMed] [Google Scholar]
- Endres NF, Yoshioka C, Milligan RA, Vale RD (2006) A lever-arm rotation drives motility of the minus-end-directed kinesin Ncd. Nature 439: 875–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SP, Moyer ML, Johnson KA (1998) Alternating site mechanism of the kinesin ATPase. Biochemistry 37: 792–799 [DOI] [PubMed] [Google Scholar]
- Hackney DD (1994) Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc Natl Acad Sci USA 91: 6865–6869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock WO, Howard J (1998) Processivity of the motor protein kinesin requires two heads. J Cell Biol 140: 1395–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock WO, Howard J (1999) Kinesin's processivity results from mechanical and chemical coordination between the ATP hydrolysis cycles of the two motor domains. Proc Natl Acad Sci USA 96: 13147–13152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TG, Hackney DD (1994) Drosophila kinesin minimal motor domain expressed in Escherichia coli. Purification and kinetic characterization. J Biol Chem 269: 16493–16501 [PubMed] [Google Scholar]
- Kaseda K, Higuchi H, Hirose K (2002) Coordination of kinesin's two heads studied with mutant heterodimers. Proc Natl Acad Sci USA 99: 16058–16063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashina AS, Baskin RJ, Cole DG, Wedaman KP, Saxton WM, Scholey JM (1996) A bipolar kinesin. Nature 379: 270–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneev MJ, Lakamper S, Schmidt CF (2007) Load-dependent release limits the processive stepping of the tetrameric Eg5 motor. Eur Biophys J 36: 675–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzysiak TC, Gilbert SP (2006) Dimeric Eg5 maintains processivity through alternating-site catalysis with rate-limiting ATP hydrolysis. J Biol Chem 281: 39444–39454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzysiak TC, Grabe M, Gilbert SP (2008) Getting in sync with dimeric Eg5: initiation and regulation of the processive run. J Biol Chem 283: 2078–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart A, Cross RA (1994) Origins of reversed directionality in the ncd molecular motor. EMBO J 13: 751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart A, Cross RA (1996) Kinetics and motility of the Eg5 microtubule motor. Biochemistry 35: 2365–2373 [DOI] [PubMed] [Google Scholar]
- Ma YZ, Taylor EW (1997) Interacting head mechanism of microtubule-kinesin ATPase. J Biol Chem 272: 724–730 [DOI] [PubMed] [Google Scholar]
- Mori T, Vale RD, Tomishige M (2007) How kinesin waits between steps. Nature 450: 750–754 [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Higuchi H, Yanagida T (2002) Chemomechanical coupling of the forward and backward steps of single kinesin molecules. Nat Cell Biol 4: 790–797 [DOI] [PubMed] [Google Scholar]
- Rosenfeld SS, Fordyce PM, Jefferson GM, King PH, Block SM (2003) Stepping and stretching. How kinesin uses internal strain to walk processively. J Biol Chem 278: 18550–18556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomishige M, Klopfenstein DR, Vale RD (2002) Conversion of Unc104/KIF1A kinesin into a processive motor after dimerization. Science 297: 2263–2267 [DOI] [PubMed] [Google Scholar]
- Valentine MT, Fordyce PM, Krzysiak TC, Gilbert SP, Block SM (2006) Individual dimers of the mitotic kinesin motor Eg5 step processively and support substantial loads in vitro. Nat Cell Biol 8: 470–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veigel C, Schmitz S, Wang F, Sellers JR (2005) Load-dependent kinetics of myosin-V can explain its high processivity. Nat Cell Biol 7: 861–869 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Fig 1