Abstract
The intracellular modification of proteins by the addition of a single O-linked N-acetylglucosamine (O-GlcNAc) molecule is a ubiquitous post-translational modification in eukaryotic cells. It is catalysed by O-linked N-acetylglucosaminyltransferase, which attaches O-GlcNAc to serine/threonine residues, and it is counter-regulated by β-N-acetylglucosaminidase, which is the antagonistic glycosidase that removes the O-GlcNAc group. O-GlcNAc modification competes with phosphorylation by protein kinases at similar sites, thereby affecting important signalling nodes. Accumulating evidence supports a central role for O-GlcNAc modifications and the corresponding enzymes in the regulation of immune cells, particularly in the activation processes of T and B lymphocytes. Here, we discuss recent advances in the field of O-GlcNAc modifications, focusing on the cells of the immune system.
Keywords: B-cell activation, O-GlcNAc, OGT, O-linked N-acetylglucosamine, T-cell activation
Introduction
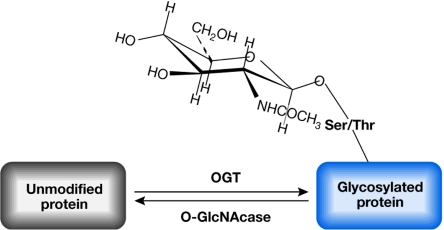
The addition of a single residue of O-linked N-acetylglucosamine (O-GlcNAc) is a post-translational modification of proteins that takes place in the nuclear and cytosolic compartments of eukaryotic cells. Since its initial discovery in 1984 by Hart and colleagues, O-GlcNAc modification has been described for a large and still increasing number of proteins, many of which are key modulators of cellular signalling. O-GlcNAc modifications are catalysed by a glycosyltransferase named O-linked N-acetylglucosaminyltransferase (OGT), and are removed by the antagonistic enzyme β-N-acetylglucosaminidase (O-GlcNAcase; Holt et al, 1987; Kreppel et al, 1997; Lazarus et al, 2006). A scheme depicting the O-GlcNAcylation of proteins and the enzymes catalysing this post-translational modification is presented in Fig 1.
Figure 1.
General scheme of O-linked N-acetylglucosamine modification. N-acetylglucosamine is added to serine/threonine (Ser/Thr) residues of target proteins by the enzyme O-linked N-acetylglucosaminyltransferase (OGT) using UDP-GlcNAc as substrate. The N-acetylglucosamine group is removed by the antagonistic activity of β-N-acetylglucosaminidase. O-GlcNAc, O-linked N-acetylglucosamine.
Accumulating evidence indicates that, analogous to phosphorylation, O-GlcNAc modification of proteins has a key role in regulating protein activity. In some cases, O-GlcNAc addition has been mapped to the same site that is modified by serine/threonine (Ser/Thr) protein kinases, implying that the two modifications might compete for the same site (Cheng et al, 2000; Cheng & Hart, 2001; Chou et al, 1995; Hart et al, 2007; Kelly et al, 1993). O-GlcNAc modification of a Ser/Thr hydroxyl group can result in quantifiable differences in the properties of the protein as compared to O-phosphate modification of the same group (Hart et al, 2007). For example, in hepatic cells, phosphorylation of the cyclic AMP-response-element-binding (CREB) coactivator (CRTC2) at Ser 70 and Ser 171 promotes its interaction with 14-3-3 proteins and the retention of CRTC2 in the cytosol. By contrast, O-glycosylation at the same sites initiates the translocation of CRTC2 into the nucleus, where this factor regulates the expression of genes involved in gluconeogenesis (Dentin et al, 2008).
O-GlcNAcylation not only has a role in normal cellular signalling, but its faulty regulation might also be involved in neurodegenerative diseases, diabetes mellitus and cancer (Hanover, 2001; Hart et al, 2007). More recently, evidence for the importance of O-GlcNAc signalling in the immune system was reported (Golks et al, 2007; Table 1). Here, we briefly summarize what is known about the enzymes controlling O-GlcNAc protein modification and their role in cellular signalling. We then focus on recent advances concerning the involvement of O-GlcNAc signalling in the regulation of the immune system.
Table 1.
O-linked N-acetylglucosamine signalling in immune cells
| Cells | Effects of O-GlcNAc modification |
|---|---|
| T cells | PMA/ionomycin or ConA treatment leads to an increase in O-GlcNAc glycoproteins (Kearse & Hart, 1991) Tissue-specific deletion of OGT in T cells leads to reduced numbers of total CD4+ T cells, but does not affect the development of CD4/CD8 thymocytes (O'Donnell et al, 2004) TCR stimulation leads to O-GlcNAc modification of transcription factors NF-κB and NFAT, and is required for T-cell activation (Golks et al, 2007) |
| B cells | BCR stimulation leads to O-GlcNAc modification of transcription factors NF-κB and NFAT, and is required for B-cell activation (Golks et al, 2007) |
| Neutrophils | Chemokine stimulation leads to an increase in O-GlcNAc (Kneass & Marchase, 2004) Neutrophil signalling and motility is dependent on O-GlcNAc cycling (Kneass & Marchase, 2005) |
BCR, B-cell receptor; ConA, concanavalin A; NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor-κB; O-GlcNAc, O-linked N-acetylglucosamine; OGT, O-linked N-acetylglucosaminyltransferase; PMA, phorbol 12-myristate 13-acetate; TCR, T-cell receptor.
Enzymes involved in O-GlcNAc signalling
O-GlcNAc modifications have been found in the intracellular compartments of multicellular organisms. The level of this reversible modification changes in response to cellular stress, extracellular stimuli and cell-cycle progression, and during cellular development. Using UDP-GlcNAc as the substrate, OGT catalyses the transfer of a single O-GlcNAc to the hydroxyl group of Ser/Thr residues in target proteins. OGT has been identified in several eukaryotic organisms including humans, rats, mice, Caenorhabditis elegans and Arabidopsis, but it is absent in Saccharomyces cerevisiae (Zachara & Hart, 2004). Only one gene for OGT has been identified and its sequence is highly conserved among the different species.
OGT transcripts are ubiquitously expressed, but they are particularly enriched in the β-cells of the islets of Langerhans in the pancreas, brain, skeletal and cardiac muscles, and fat tissue (Hanover et al, 1999; Kreppel et al, 1997; Lubas et al, 1997; Roos et al, 1998). A single copy of the OGT gene is encoded by the X chromosome in mice and humans. OGT-knockout mice that carry a generalized deletion of the gene die prenatally (Nolte & Muller, 2002; Shafi et al, 2000), indicating an important role for OGT during embryogenesis. Three splice variants of OGT have been described, which differ in their cellular localization (nuclear/cytosolic or mitochondrial) and length (Hanover et al, 2003; Love et al, 2003). The role of the mitochondria-located OGT variant is still poorly understood.
Analysis of the crystal structure of the regulatory—amino-terminal—domain revealed that OGT acts as a dimer (Jinek et al, 2004). The N terminus of OGT comprises 11 tetratricopeptide repeats (TPRs), whereas the carboxyl terminus contains the catalytic domain and a phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3)-binding domain (Kreppel et al, 1997; Lubas et al, 1997; Yang et al, 2008). TPRs are found in a domain mediating protein–protein interactions, and there is evidence that the TPR domain has a role in the binding of OGT to γ-aminobutyric acid type A (GABAA) receptor-interacting factor 1 (GRIF1), N-acetylglucosamine transferase-interacting protein 106 (OIP106) and the mouse co-repressor SIN3A (Iyer et al, 2003; Iyer & Hart, 2003; Yang et al, 2002). The TPR domain might also have a role in regulating the subcellular distribution of OGT (Iyer et al, 2003; Iyer & Hart, 2003; Yang et al, 2002). Recently, the C-terminal PtdIns(3,4,5)P3-binding domain was shown to mediate the recruitment of OGT to the plasma membrane in response to insulin stimulation and phosphatidyl-3-OH-kinase (PI(3)K) activation (Yang et al, 2008).
O-GlcNAc is removed from proteins by the hexosaminidase O-GlcNAcase, which is a 103-kDa protein (Gao et al, 2001). O-GlcNAcase co-purifies with other proteins and might form a large multi-subunit complex with other cellular components (Gao et al, 2001; Wells et al, 2002). O-GlcNAcase consists of an N-terminal hexosaminidase domain and a C-terminal histone acetyltransferase (HAT) domain (Comtesse et al, 2001; Farook et al, 2002; Gao et al, 2001; Toleman et al, 2004).
O-GlcNAcase—or at least the function mediating intracellular O-GlcNAc modifications—can be inhibited by a substance called O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc) or by another potent compound called 1,2-dideoxy-2′-methyl-α-D-glucopyranoso[2,1-d]-Δ 2′-thiazoline (NAG-thiazoline; Whitworth et al, 2007). There are no specific inhibitors for studying OGT in vivo (Haltiwanger et al, 1998); however, two recent publications identified inhibitors of OGT in biochemical assays, raising the hope that such tools might soon become available (Gross et al, 2005, 2008).
O-GlcNAc modifications in intracellular signalling
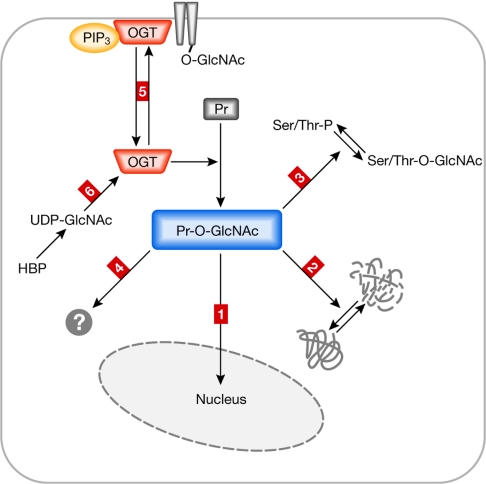
It was initially thought that the activity of OGT was solely regulated by changes in the concentration of its substrate UDP-GlcNAc (Hart et al, 2007). UDP-GlcNAc is a glucose sensor and its concentration strongly depends on the metabolic state of the cell. Nevertheless, this process is too slow to explain short-lived changes in the level of O-GlcNAc-modified proteins (Golks et al, 2007). Recently, a study performed in a brain cell line indicated that OGT is activated through phosphorylation by calcium-calmodulin-dependent protein kinase IV (CaMKIV) in response to potassium-chloride-induced depolarization (Song et al, 2007). Another study showed that the specific binding of OGT to inositol-phosphate lipids can regulate the cellular localization of OGT (Yang et al, 2008). A PI(3)K-dependent increase of PtdIns(3,4,5)P3 resulted in the enrichment of OGT at the plasma membrane, with a consequent increase of O-GlcNAc modification of enzymes involved in insulin signalling (Fig 2). This process was shown to have a strong effect on the sensitivity of insulin signalling in cellular systems. Regulatory mechanisms that affect OGT activity and the potential consequences of O-GlcNAc modification are summarized in Fig 2.
Figure 2.
Possible mechanisms controlling O-linked N-acetylglucosaminyl-transferase activity and the consequences of O-linked N-acetylglucosamine modifications. O-GlcNAc protein modifications mediated by OGT have different outcomes, some of which are summarized in this figure. OGT itself is under the control of different regulatory mechanisms. On O-GlcNAc modification, proteins (for example, transcription factors) translocate into the nucleus (outcome 1). O-GlcNAc modification can affect protein stability (outcome 2). O-GlcNAc modification occurs on serine/threonine (Ser/Thr) residues that are also targets of protein phosphorylation (outcome 3); this can result in competitive regulation of target proteins with different outcomes. O-GlcNAc modification might have additional (not yet well-characterized) effects on target proteins such as structural consequences, effects on catalytic activity and hetero-homodimer formation (outcome 4). OGT function can be regulated by cellular re-localization, such as recruitment to the plasma membrane (outcome 5). OGT activity depends on the concentration of its substrate UDP-GlcNAc, which is strongly coupled to the metabolic state of the cells (outcome 6). HBP, hexosamine biosynthetic pathway; O-GlcNAc, O-linked N-acetylglucosamine; OGT, O-linked N-acetylglucosaminyltransferase; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; Pr, protein; Pr-GlcNAc, O-GlcNAc-modified protein.
O-GlcNAc signalling in human diseases
Accumulating evidence implies that defects in the regulation of O-GlcNAc modifications are involved in human diseases, including diabetes mellitus, neurodegenerative diseases and cancer, although the biochemical details of these processes are still poorly understood (Sidebar A; Dias & Hart, 2007; Hanover, 2001). Some of the proteins that have a role in the pathology of human diseases have been shown to be directly modified by O-GlcNAc. For instance, the Ser/Thr kinase AKT, PI(3)K, insulin receptor substrate 1, glycogen synthase and endothelial nitric oxide synthase—all of which are enzymes that have a crucial role in insulin signalling—are reversibly modified by OGT. A recent study showed that the recruitment of OGT to the plasma membrane specifically prevents the phosphorylation of AKT and probably other proteins, thereby terminating insulin signalling (Yang et al, 2008). In pancreatic β-cells, glucosamine can inhibit insulin secretion, and treatment with the non-specific inhibitor of O-GlcNAcase, streptozotocin, has toxic effects, whereas the more specific O-GlcNAcase inhibitor, PUGNAc, induces insulin resistance in adipocytes and skeletal muscle cells. PUGNAc and streptozotocin both mediate an increase in the intracellular level of O-GlcNAc-modified proteins. A similar mechanism has been recently described for hepatic cells, in which chronic exposure to glucose or glucosamine results in an increase of O-GlcNAc modification of CRTC2, its translocation to the nucleus and the transcription of genes involved in gluconeogenesis. This process might lead to insulin resistance (Dentin et al, 2008). Although these experiments need to be interpreted with caution, they strengthen the view that increasing the level of O-GlcNAc modifications correlates with the development of insulin resistance, which is a characteristic of type II diabetes (Love & Hanover, 2005; Zachara & Hart, 2006).
There are indications that O-GlcNAc modifications also have a role in Alzheimer disease (Dias & Hart, 2007). Higher levels of O-GlcNAc can be detected in brain tissue and several proteins involved in neuronal signalling are modified with O-GlcNAc. Among them are the β-amyloid precursor protein, clathrin-assembly proteins and neurofilaments. In the brains of patients with Alzheimer disease, hyperphosphorylated Tau protein was modified by O-GlcNAc to a lesser extent than in healthy individuals (Dias & Hart, 2007).
Various groups have shown that some oncogenes and tumour suppressors are targets of O-glycosylation, including the SV40 T antigen and c-MYC (Chou & Hart, 2001). A detailed study of the tumour-suppressor protein p53 identified the sites that were modified by O-GlcNAc (Yang et al, 2006). The same study also showed that O-GlcNAc modification protected p53 from degradation much more efficiently than the modification of the same residue by O-phosphate (Yang et al, 2006). Finally, tumour cells have an altered glucose metabolism that is expected to produce changes in O-GlcNAc levels and to affect different signalling pathways (Fig 2).
The role of O-GlcNAc in the immune system
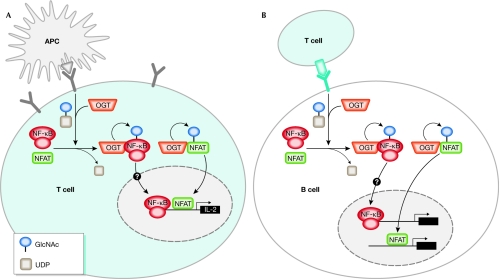
Initial evidence for the occurrence of O-GlcNAc protein modification during lymphocyte activation was published in 1991, when Kearse and Hart showed that both concanavalin A and phorbol 12-myristate 13-acetate (PMA)/ionomycin treatment of T cells lead to a rapid increase in nuclear and cytoplasmic O-GlcNAc glycoproteins (Kearse & Hart, 1991; Table 1). Around a decade later, the transient O-GlcNAc modification of the nuclear factor-κB (NF-κB) transcription factor was described (James et al, 2002). More recent work has confirmed that another important lymphocyte transcription factor, nuclear factor of activated T cells (NFAT), contains sites that are modified by O-GlcNAc. Importantly, the O-GlcNAc modification of these two factors is needed for productive T-cell activation in a T-cell receptor (TCR)-dependent manner (Golks et al, 2007). Similar effects for NFAT and NF-κB have been observed during B-cell receptor (BCR)-dependent activation of B cells. A schematic model of the role of OGT in T-cell and B-cell activation is presented in Fig 3. Knockdown of OGT with small interfering RNA (siRNA) showed reproducible and significant inhibition of T-cell activation, at a level comparable with that observed after the inactivation of the T-cell-specific tyrosine kinase Lck, which is required for T-cell activation and reprogramming. This initially surprising observation was confirmed by findings showing that activation of the TCR and BCR promoted OGT-dependent O-GlcNAc modification of NFAT and NF-κB (Golks et al, 2007). The requirement of the active OGT enzyme for productive T-cell activation is consistent with the decrease in the total number of CD4+ T cells observed in mice carrying an inactivating T-cell deletion in the OGT gene. The reduction of T cells in these transgenic animals was a consequence of an increase in apoptosis (O'Donnell et al, 2004). The abortive activation of T cells leads to anergy and apoptosis, implying that in the absence of OGT, an important component is missing for the progression of the reprogramming of lymphocytes. The role of OGT is probably restricted to mature lymphocytes, as no defect was observed in thymocytes, which have not yet undergone positive or negative selection.
Figure 3.
Model of O-linked N-acetylglucosamine modification during lymphocyte activation. (A) Activation of T lymphocytes through the T-cell receptor (TCR) leads to the association of the transcription factors known as nuclear factor of activated T cells (NFAT) and nuclear factor-κB (NF-κB) to O-linked N-acetylglucosaminyltransferase (OGT) and their O-GlcNAc modification. The transient O-GlcNAc modification enhances the nuclear translocation of NFAT (and probably also that of NF-κB). Nuclear localization and activation of NFAT and NF-κB is needed for the transcriptional activation of many genes, such as interleukin-2 (IL-2). (B) Activation of B lymphocytes through the B-cell receptor (BCR) leads to the association of the transcription factors NFAT and NF-κB to OGT, and their O-GlcNAc modification. The transient O-GlcNAc modification enhances the nuclear translocation of NFAT (and probably also that of NF-κB). Nuclear localization of NFAT and NF-κB is crucial to the reprogramming of B cells. APC, antigen-presenting cell; O-GlcNAc, O-linked N-acetylglucosamine.
In Jurkat and human primary T cells, shortly after TCR stimulation, a large increase of O-GlcNAc-modified NFAT can be detected, within 5 min in the cytosol and within 10–15 min in the nucleus. The transient increase of the level of modified NFAT lasts for 5–15 min after TCR stimulation and then decreases sharply. These preliminary results indicate that the turnover of O-GlcNAc modification, at least in lymphocytes, is controlled by a specific signalling pathway downstream from TCR signalling, and that it is not solely dependent on the glucose/UDP-GlcNAc level.
The hexosamine biosynthetic pathway (HBP), which uses up to 1–3% of the total glucose in the cell, is known to have a regulatory function in cells. Its main end-product UDP-GlcNAc is a substrate for many glycosylation processes. UDP-GlcNAc is also the substrate for OGT. When glucosamine is added to cell-culture medium, the O-GlcNAc levels in the cells increase (Parker et al, 2003; Vosseller et al, 2002). Cells of the immune systems undergo large changes in their metabolic rates, in particular when they are activated by ‘danger' signals. Some of these cells enter a phase of extensive and rapid cell proliferation. This results in changes in the level of cellular glucose concentration and is expected to affect strongly the level of O-GlcNAc-modified proteins.
In neutrophils, external stimulation with the agonistic chemotactic tripeptide formyl-methionine-leucine-phenylalanine leads to an increase in O-GlcNAc-modified proteins (Kneass & Marchase, 2004; Table 1). Neutrophils are components of the innate immune response towards infection as well as tissue damage. A change in signal transduction leads to cytoskeletal rearrangements and migration of the neutrophils to the site of inflammation. In addition, as the HBP system is expected to be affected at sites of inflammation, changes in the level of UDP-GlcNAc and, consequently, in the level of O-GlcNAc-modified proteins might influence this process. The same group published data indicating that O-GlcNAc signalling affects neutrophil motility (Kneass & Marchase, 2005). Increased levels of O-GlcNAc in neutrophils correlate not only with an enhancement of motility, but also with higher activity of the mitogen-activated protein kinases p38, p44/42, MKK3/6 and MEK1/2. These results are mostly based on the inhibition of O-GlcNAcase using PUGNAc or the increase of O-GlcNAc modification using glutamine, and still require confirmation using more specific tools. A recent study showing that the cellular re-localization of OGT responds quickly to a PI(3)K-dependent increase of PtdIns(3,4,5)P3 clearly points to the presence of mechanisms that allow the cell to rapidly raise the level of O-GlcNAc-modified proteins (Yang et al, 2008). It will be important to test whether similar mechanisms are in place in lymphocytes, and, if so, whether they are responsible for the effects on NFAT and NF-κB on TCR or BCR stimulation.
Conclusions and future directions
A factor that has hampered the progress of O-GlcNAc signalling research has been the limited availability of tools to detect O-GlcNAc-modified proteins. There is also a need to develop better methods to identify the sites and to quantify the extent of O-GlcNAc modifications. Specific and effective inhibitors for O-GlcNAcase and OGT are needed to expand our understanding of the physiological role of O-GlcNAc cycling. Recent evidence based on work with lymphocytes indicates that OGT might be activated not only by the changes in the concentration of cytosolic UDP-GlcNAc, but also by other mechanisms that need to be better defined. OGT can be activated by depolarization-dependent phosphorylation (Song et al, 2007) or, by binding to PtdIns(3,4,5)P3, it can be recruited to the plasma membrane where crucial enzymes involved in insulin signalling are located (Yang et al, 2008). These findings represent a new paradigm, which implies that O-GlcNAc modification can respond to rapid changes in intracellular signalling. It will be important to verify whether the same mechanisms are also involved in TCR-dependent and BCR-dependent O-GlcNAc modification of NFAT and NF-κB in lymphocytes. Future work should focus on how the enzymes that are involved in O-GlcNAc cycling are regulated, and on which additional signalling components are involved (Sidebar A). As indicated by the recent work in lymphocytes, OGT forms protein complexes with NFAT and NF-κB, but it is not known whether additional cellular components, such as scaffolding proteins or other enzymes, are also present in the complexes (Golks et al, 2007).
The analysis of O-GlcNAc modification and its regulation will certainly provide new insights into the pathology of diseases such as diabetes, neurodegeneration and cancer, and it will also improve our knowledge of lymphocyte activation, providing potentially new and exciting ways in which to intervene in diseases stemming from defective immune regulation, such as autoimmune diseases. Future research efforts should be aimed at better elucidating the crosstalk between phosphorylation and O-GlcNAcation, as well as the effect of O-GlcNAc modification on protein stability, enzymatic activity and cellular localization.
Sidebar A | In need of answers.
What are the structural consequences of O-linked N-acetylglucosamine (O-GlcNAc) protein modification?
How are O-linked N-acetylglucosaminyltransferase (OGT) and β-N-acetylglucosaminidase (O-GlcNAcase) function, activity and cellular localization regulated?
What is the impact of O-GlcNAcation on human diseases such as diabetes mellitus, cancer, neurodegenerative diseases and autoimmunity?
What are the effects of O-GlcNAc modification on the subpopulations of mature, activated and memory T and B cells?

Alexander Golks

Danilo Guerini
References
- Cheng X, Hart GW (2001) Alternative O-glycosylation/O-phosphorylation of serine-16 in murine estrogen receptor beta: post-translational regulation of turnover and transactivation activity. J Biol Chem 276: 10570–10575 [DOI] [PubMed] [Google Scholar]
- Cheng X, Cole RN, Zaia J, Hart GW (2000) Alternative O-glycosylation/O-phosphorylation of the murine estrogen receptor beta. Biochemistry 39: 11609–11620 [DOI] [PubMed] [Google Scholar]
- Chou TY, Hart GW (2001) O-linked N-acetylglucosamine and cancer: messages from the glycosylation of c-Myc. Adv Exp Med Biol 491: 413–418 [DOI] [PubMed] [Google Scholar]
- Chou TY, Hart GW, Dang CV (1995) c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem 270: 18961–18965 [DOI] [PubMed] [Google Scholar]
- Comtesse N, Maldener E, Meese E (2001) Identification of a nuclear variant of MGEA5, a cytoplasmic hyaluronidase and a beta-N-acetylglucosaminidase. Biochem Biophys Res Commun 283: 634–640 [DOI] [PubMed] [Google Scholar]
- Dentin R, Hedrick S, Xie J, Yates J, Montminy M (2008) Hepatic glucose sensing via the CREB coactivator CRTC2. Science 319: 1402–1405 [DOI] [PubMed] [Google Scholar]
- Dias WB, Hart GW (2007) O-GlcNAc modification in diabetes and Alzheimer's disease. Mol Biosyst 3: 766–772 [DOI] [PubMed] [Google Scholar]
- Farook VS, Bogardus C, Prochazka M (2002) Analysis of MGEA5 on 10q24.1-q24.3 encoding the beta-O-linked N-acetylglucosaminidase as a candidate gene for type 2 diabetes mellitus in Pima Indians. Mol Genet Metab 77: 189–193 [DOI] [PubMed] [Google Scholar]
- Gao Y, Wells L, Comer FI, Parker GJ, Hart GW (2001) Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J Biol Chem 276: 9838–9845 [DOI] [PubMed] [Google Scholar]
- Golks A, Tran TT, Goetschy JF, Guerini D (2007) Requirement for O-linked N-acetylglucosaminyltransferase in lymphocytes activation. EMBO J 26: 4368–4379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross BJ, Kraybill BC, Walker S (2005) Discovery of O-GlcNAc transferase inhibitors. J Am Chem Soc 127: 14588–14589 [DOI] [PubMed] [Google Scholar]
- Gross BJ, Swoboda JG, Walker S (2008) A strategy to discover inhibitors of O-linked glycosylation. J Am Chem Soc 130: 440–441 [DOI] [PubMed] [Google Scholar]
- Haltiwanger RS, Grove K, Philipsberg GA (1998) Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate. J Biol Chem 273: 3611–3617 [DOI] [PubMed] [Google Scholar]
- Hanover JA (2001) Glycan-dependent signaling: O-linked N-acetylglucosamine. FASEB J 15: 1865–1876 [DOI] [PubMed] [Google Scholar]
- Hanover JA, Lai Z, Lee G, Lubas WA, Sato SM (1999) Elevated O-linked N-acetylglucosamine metabolism in pancreatic beta-cells. Arch Biochem Biophys 362: 38–45 [DOI] [PubMed] [Google Scholar]
- Hanover JA, Yu S, Lubas WB, Shin SH, Ragano-Caracciola M, Kochran J, Love DC (2003) Mitochondrial and nucleocytoplasmic isoforms of O-linked GlcNAc transferase encoded by a single mammalian gene. Arch Biochem Biophys 409: 287–297 [DOI] [PubMed] [Google Scholar]
- Hart GW, Housley MP, Slawson C (2007) Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446: 1017–1022 [DOI] [PubMed] [Google Scholar]
- Holt GD, Snow CM, Senior A, Haltiwanger RS, Gerace L, Hart GW (1987) Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol 104: 1157–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SP, Hart GW (2003) Roles of the tetratricopeptide repeat domain in O-GlcNAc transferase targeting and protein substrate specificity. J Biol Chem 278: 24608–24616 [DOI] [PubMed] [Google Scholar]
- Iyer SP, Akimoto Y, Hart GW (2003) Identification and cloning of a novel family of coiled-coil domain proteins that interact with O-GlcNAc transferase. J Biol Chem 278: 5399–5409 [DOI] [PubMed] [Google Scholar]
- James LR, Tang D, Ingram A, Ly H, Thai K, Cai L, Scholey JW (2002) Flux through the hexosamine pathway is a determinant of nuclear factor kappaB- dependent promoter activation. Diabetes 51: 1146–1156 [DOI] [PubMed] [Google Scholar]
- Jinek M, Rehwinkel J, Lazarus BD, Izaurralde E, Hanover JA, Conti E (2004) The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat Struct Mol Biol 11: 1001–1007 [DOI] [PubMed] [Google Scholar]
- Kearse KP, Hart GW (1991) Lymphocyte activation induces rapid changes in nuclear and cytoplasmic glycoproteins. Proc Natl Acad Sci USA 88: 1701–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WG, Dahmus ME, Hart GW (1993) RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc. J Biol Chem 268: 10416–10424 [PubMed] [Google Scholar]
- Kneass ZT, Marchase RB (2004) Neutrophils exhibit rapid agonist-induced increases in protein-associated O-GlcNAc. J Biol Chem 279: 45759–45765 [DOI] [PubMed] [Google Scholar]
- Kneass ZT, Marchase RB (2005) Protein O-GlcNAc modulates motility-associated signaling intermediates in neutrophils. J Biol Chem 280: 14579–14585 [DOI] [PubMed] [Google Scholar]
- Kreppel LK, Blomberg MA, Hart GW (1997) Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem 272: 9308–9315 [DOI] [PubMed] [Google Scholar]
- Lazarus BD, Love DC, Hanover JA (2006) Recombinant O-GlcNAc transferase isoforms: identification of O-GlcNAcase, yes tyrosine kinase, and tau as isoform-specific substrates. Glycobiology 16: 415–421 [DOI] [PubMed] [Google Scholar]
- Love DC, Hanover JA (2005) The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE 2005: re13. [DOI] [PubMed] [Google Scholar]
- Love DC, Kochan J, Cathey RL, Shin SH, Hanover JA (2003) Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J Cell Sci 116: 647–654 [DOI] [PubMed] [Google Scholar]
- Lubas WA, Frank DW, Krause M, Hanover JA (1997) O-linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J Biol Chem 272: 9316–9324 [DOI] [PubMed] [Google Scholar]
- Nolte D, Muller U (2002) Human O-GlcNAc transferase (OGT): genomic structure, analysis of splice variants, fine mapping in Xq13.1. Mamm Genome 13: 62–64 [DOI] [PubMed] [Google Scholar]
- O'Donnell N, Zachara NE, Hart GW, Marth JD (2004) Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol 24: 1680–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GJ, Lund KC, Taylor RP, McClain DA (2003) Insulin resistance of glycogen synthase mediated by O-linked N-acetylglucosamine. J Biol Chem 278: 10022–10027 [DOI] [PubMed] [Google Scholar]
- Roos MD, Xie W, Su K, Clark JA, Yang X, Chin E, Paterson AJ, Kudlow JE (1998) Streptozotocin, an analog of N-acetylglucosamine, blocks the removal of O-GlcNAc from intracellular proteins. Proc Assoc Am Physicians 110: 422–432 [PubMed] [Google Scholar]
- Shafi R, Iyer SP, Ellies LG, O'Donnell N, Marek KW, Chui D, Hart GW, Marth JD (2000) The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA 97: 5735–5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Kim HS, Park JM, Kim SH, Kim IH, Ryu SH, Suh PG (2007) o-GlcNAc transferase is activated by CaMKIV-dependent phosphorylation under potassium chloride-induced depolarization in NG-108-15 cells. Cell Signal 20: 94–104 [DOI] [PubMed] [Google Scholar]
- Toleman C, Paterson AJ, Whisenhunt TR, Kudlow JE (2004) Characterization of the histone acetyltransferase (HAT) domain of a bifunctional protein with activable O-GlcNAcase and HAT activities. J Biol Chem 279: 53665–53673 [DOI] [PubMed] [Google Scholar]
- Vosseller K, Wells L, Lane MD, Hart GW (2002) Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci USA 99: 5313–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A, Hart GW (2002) Dynamic O-glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J Biol Chem 277: 1755–1761 [DOI] [PubMed] [Google Scholar]
- Whitworth GE, Macauley MS, Stubbs KA, Dennis RJ, Taylor EJ, Davies GJ, Greig IR, Vocadlo DJ (2007) Analysis of PUGNAc and NAG-thiazoline as transition state analogues for human O-GlcNAcase: mechanistic and structural insights into inhibitor selectivity and transition state poise. J Am Chem Soc 129: 635–644 [DOI] [PubMed] [Google Scholar]
- Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, Cho JW (2006) Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol 8: 1074–1083 [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang F, Kudlow JE (2002) Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell 110: 69–80 [DOI] [PubMed] [Google Scholar]
- Yang X et al. (2008) Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature 451: 964–969 [DOI] [PubMed] [Google Scholar]
- Zachara NE, Hart GW (2004) O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta 1673: 13–28 [DOI] [PubMed] [Google Scholar]
- Zachara NE, Hart GW (2006) Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta 1761: 599–617 [DOI] [PubMed] [Google Scholar]