Abstract
Exotoxin A (ExoA) from Pseudomonas aeruginosa is an important virulence factor that belongs to a class of exotoxins that are secreted by pathogenic bacteria which cause human diseases such as cholera, diphtheria, pneumonia and whooping cough. We present the first crystal structures, to our knowledge, of ExoA in complex with elongation factor 2 (eEF2) and intact NAD+, which indicate a direct role of two active-site loops in ExoA during the catalytic cycle. One loop moves to form a solvent cover for the active site of the enzyme and reaches towards the target residue (diphthamide) in eEF2 forming an important hydrogen bond. The NAD+ substrate adopts a conformation remarkably different from that of the NAD+ analogue, βTAD, observed in previous structures, and fails to trigger any loop movements. Mutational studies of the two loops in the toxin identify several residues important for catalytic activity, in particular Glu 546 and Arg 551, clearly supporting the new complex structures. On the basis of these data, we propose a transition-state model for the toxin-catalysed reaction.
Keywords: mono-ADP-ribosyltransferase toxins, protein–protein interactions, mutagenesis, X-ray crystallography
Introduction
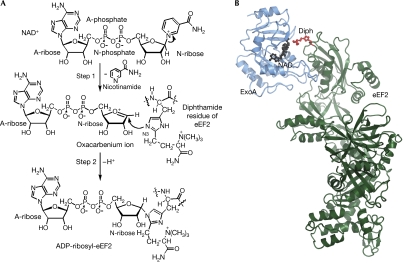
Bacterial exotoxins, including exotoxin A (ExoA), diphtheria toxin (DT), cholera toxin (CT), C3 exoenzyme and pertussis toxin, are an important class of deadly virulence factors (Popoff, 2005; Yates et al, 2006). Most of them are A-B toxins that bind to the eukaryotic target cell membrane with a receptor-binding domain (B subunit) and deliver a second moiety (A subunit) into the host cytoplasm. One large group of A-B toxins is known as the mono-ADP-ribosyltransferase (mART) family, which invades eukaryotic cells and causes cell death by covalent modification of a protein essential for the host organism (Corda & Di Girolamo, 2003). Despite having different targets, toxins in the mART family catalyse the same enzymatic reaction and share a common structural core that contains the NAD+-binding site. The toxins can be divided into two groups: the DT group composed of DT, ExoA and cholix toxin (Jorgensen et al, 2008), and the CT group comprising CT, C3 exoenzyme and pertussis toxin, among others. The substrate target of the DT group of toxins is a unique post-translationally modified histidine residue, diphthamide, located in domain IV of the essential ribosomal elongation factor 2 (eEF2) protein, which is inactivated on covalent transfer of ADP-ribosyl moiety of NAD+ to N3 atom of the diphthamide imidazole (Fig 1A; Van Ness et al, 1980). The ADP-ribosylation reaction follows a random third-order SN1 mechanism and NAD+ cleavage results in the formation of an oxacarbenium ion intermediate with a positively charged nicotinamide (N)-ribose (Beattie et al, 1996). The known catalytic residues in ExoA include Glu 553, His 440, Tyr 481 and Tyr 470. In ExoA, Glu 553 forms a hydrogen bond with the 2′OH of N-ribose of NAD+ and orientates the dinucleotide substrate for nucleophilic attack by the diphthamide residue (Wilson & Collier, 1992). Previous structures of the Michaelis complex of eEF2 and the catalytic domain of ExoA (ExoAc, residues 399–609; Jorgensen et al, 2005) showed that the contact surface in the complex is surprisingly small and that the changes are not large on binding and catalysis. These structures also showed that the interaction between the diphthamide and the N-phosphate of NAD+ might help to trigger the transferase reaction. Furthermore, the eEF2–ExoAc complex suggested the involvement of two active-site loops, loop 1 (L1, residues 457–464) and loop 3 (L3, 546–551), in ExoA during the catalytic cycle but failed to show the specific function of the two loops.
Figure 1.
Reaction mechanism and structure of ExoA. (A) Schematic representation of the reaction catalysed by ExoA. ExoA catalyses the transfer of A-ribose from NAD+ to N3 of the diphthamide imidazole, impairing eEF2 function and causing inhibition of protein synthesis. (B) Ribbon representation of the eEF2–ExoAc–NAD+ complex. The ExoAc (blue) is bound to the diphthamide-containing domain of eEF2 (green) with the diphthamide (Diph; red ball and stick) pointing towards the NAD+ (black ball and stick). A-ribose, ADP ribose; A-phosphate, adenine phosphate; eEF2, elongation factor 2; ExoA, exotoxin A; N-ribose, nicotinamide ribose.
Here, we investigate the function of L1 and L3 by site-directed mutagenesis of the ExoAc sequence. Enzyme activities of both ADP-ribosyltransferase (ADPRT) and glycohydrolysis (GH), as well as the NAD+ substrate-binding ability of the toxin mutants, were measured. Furthermore, to gain structural insights into the molecular basis of L1 and L3 functions, we solved the crystal structures of wild-type (WT) ExoAc, as well as three ExoAc mutants in complex with eEF2 and NAD+.
Results And Discussion
Mutational scanning of L1 and L3
To investigate the involvement of L1 and L3 in the ADPRT mechanism, we scanned these loops by introducing single-point mutations in ExoAc and then measured the relative ADPRT, GH and NAD+-binding activities. This showed that two residues in L3 are crucial for ADPRT activity (Glu 546 and Arg 551; Table 1). Glu 546 and Arg 551 have not been implicated previously in the catalytic mechanism for the DT group of mART toxins, as these residues are not strictly conserved among toxin family members, unlike the crucial catalytic residue Glu 553 (Glu 148 in DT and Glu 581 in cholix toxin). Cholix toxin contains a Glu (Glu 574) that corresponds to Glu 546 in ExoAc, but has another Glu (Glu 579) at the Arg 551 site. DT does not show conservation at either of these two sites with ExoAc and, consequently, Ala-scanning mutagenesis of L3 in DT failed to uncover other active-site residues (data not shown). Remarkably, E546A (ExoAc) was not impaired in NAD+ substrate binding or GH activity, unlike the effect of Ala substitution at Glu 553, indicating that the Glu 546 and Glu 553 residues participate in different aspects of the catalytic mechanism (Table 1). This correlates well with the CT group in the mART family, where a second catalytic Glu/Gln in what corresponds to L3 has been proposed to be involved in the coordination of the nucleophile, but not in the GH of NAD+ (Tsuge et al, 2003), and/or possibly in the stabilization of the transition-state structure, as suggested for vegetative insecticidal protein 2 (Han et al, 1999). The replacement of Glu 546 with Gln could only partly rescue the ADPRT activity, whereas the Glu-to-Asp replacement produced an even more defective enzyme than the Gln replacement, indicating the importance of position/orientation of the carboxylic group of Glu 546 in the reaction mechanism. Multiple substitutions at Arg 551 showed that the ADPRT activity of ExoAc is sensitive to the nature of the substitution for Arg (Ala>Lys>Gln>Glu>His>Cys). Evidence for the concerted involvement of both Glu 546 and Arg 551 in the catalytic mechanism of ExoAc was found in the effect of the double Ala mutant, E546A/R551A, which showed almost complete loss of ADPRT activity, yet showed near WT activity for NAD+ binding and GH activity. The more conservative double-replacement mutant, E546D/R551K, and the residue reversal mutant, E546R/R551E, did little to restore the lost ADPRT activity, indicating the orientation-specific role of these residues in the catalytic mechanism of this toxin. Mutational scanning of L1 from Gly 453 to Asp 463 showed three ExoAc mutants with impaired ADPRT activity, G454A, R458A and Q460A (supplementary Table 1 online). Multiple substitutions at R458H showed that the ADPRT activity was sensitive to the nature of the substitution (Gln>Lys>Trp>Ala>His). The R458H mutant showed a 56-fold reduction in ADPRT activity, a 53-fold reduction in GH and a 31-fold increase in KD, indicating that Arg 458 participates in NAD+ substrate docking and orientation during catalysis.
Table 1.
Relative ADP-ribosyltransferase, glycohydrolysis and NAD+-binding activity of Pseudomonas aeruginosa ExoAc wild type and L3 mutants
| ExoAc | Relative kcat (ADPRT)* | Relative kcat (GH)‡ | Relative KD (NAD+)§ |
|---|---|---|---|
| Wt | 1.00±0.09 | 1.00±0.02 | 1.00±0.09 |
| E546Aa | 0.0012±0.00002 | 0.24±0.008 | 1.77±0.34 |
| E547Aa | 0.795±0.077 | 0.83±0.14 | 1.97±0.77 |
| E548Aa | 1.669±0.298 | 0.76±0.01 | 2.69±0.11 |
| G549Aa | 0.770±0.076 | 0.30±0.005 | 8.00±0.94 |
| G550Aa | 0.562±0.166 | 0.41±0.04 | 6.60±0.54 |
| R551Aa | 0.380±0.024 | 0.32±0.005 | 2.69±0.03 |
| L552Aa | 2.365±0.527 | 0.58±0.002 | 4.43±0.57 |
| E553Aa | 0.0015±0.00005 | 0.07±0.009 | 2.57±0.86 |
| E546Db | 0.00025±0.00005 | 0.45±0.002 | 3.83±0.89 |
| E546Fd | 0.007±0.0002 | 0.69±0.05 | 1.97±0.11 |
| E546Hb | 0.0012±0.0002 | 0.59±0.009 | 1.03±0.09 |
| E546Nb | 0.0010±0.00009 | 0.67±0.03 | 2.14±0.26 |
| E546Qb | 0.011±0.002 | 0.80±0.04 | 2.31±0.71 |
| R551Eb | 0.011±0.005 | 0.09±0.001 | 5.03±0.51 |
| R551Hb | ≈0∣∣ | 0.41±0.002 | 2.29±0.46 |
| R551Kb | 0.317±0.024 | 0.69±0.03 | 1.29±0.03 |
| R551Qb | 0.185±0.014 | 0.38±0.01 | 2.63±0.39 |
| R551Cb | ≈0∣∣ | 0.10±0.006 | 5.57±0.91 |
| E546A/R551Ac | 0.0001±0.0001 | 0.84±0.04 | 1.11±0.03 |
| E546D/R551Kc | 0.0009±0.0013 | 0.42±0.0002 | 2.71±0.74 |
| E546R/R551Ec | 0.00001±0.000002 | 0.38±0.01 | 1.29±0.49 |
| ADPRT, ADP-ribosyltransferase; ExoA, exotoxin A; GH, glycohydrolysis; Wt, wild type. *The relative ADPRT activity was set at 1.00 for the wild-type ExoAc enzyme (a746±18 min−1; b628±8 min−1; c847±21 min−1; d900±20 min−1). Briefly, various concentrations of toxin (5–500 nM) were mixed with 12 μM elongation factor 2 (eEF2) and different amounts of ɛ-NAD substrate in 20 mM Tris–HCl buffer, pH 7.9, at 25°C (70 μl) and the fluorescence time-based data were collected for 300 s. ‡The relative GH activity was measured as described above except without eEF2 and the time course was for a period of 60 min. The relative GH activity was set at 1.00 for the wild-type ExoAc and ranged from 0.062 to 0.13 min−1. §The NAD+-binding ability of wild-type and mutant ExoAc proteins was measured by the quenching of the intrinsic Trp fluorescence caused by the binding of NAD+ to the active site. In this case, the toxin concentration was 1.5 μM. The KD of wild-type protein was 35±3 μM. ∣∣The ADPRT activity could not be accurately measured as it was nearly zero and close to the background signal. The kinetic and equilibrium binding data represent the mean±s.d. from four independent experiments. |
Structures of the eEF2–ExoAc–NAD+ complex
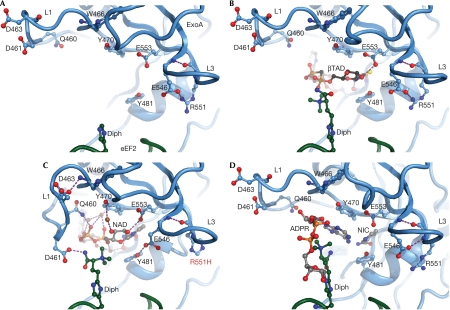
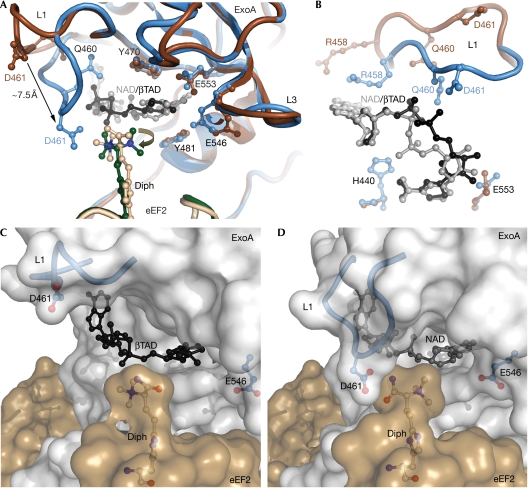
We determined the crystal structures of ExoAc WT and three mutant proteins (E546A, E546H and R551H) in complex with NAD+ and eEF2 at 2.35–3.0 Å resolution (Fig 1B; supplementary Table 2 online). To prevent NAD+ hydrolysis in the eEF2–ExoAc WT complex, NAD+ was soaked into the crystal in a cryo-protection buffer adjusted to pH 6.0. This pH has been shown to minimize ADPRT activity in ExoAc (Armstrong & Merrill, 2001). The mutant enzymes were significantly weaker catalysts for the ADPRT reaction and, therefore, NAD+ could be added to the protein crystals at pH 7.2 before being flash frozen in liquid N2. The structures were solved by molecular replacement using the eEF2–ExoAc–PJ34 structure (Jorgensen et al, 2005) and, as for the previous structures, contained three complexes in the asymmetric unit. In all the structures, the densities for NAD+, the L1 residues, the replacement residues in L3 and, to some extent, the diphthamide are well defined (supplementary Fig 1A–E online). In the previously solved eEF2–ExoAc–apo structure, the NAD+ binding pocket is solvent exposed, with L1 in an open conformation and the diphthamide too flexible to be modelled (Fig 2A). Binding of βTAD (β-methylene thiazole-4-carboxamide adenine dinucleotide) to the toxin active site fixes the diphthamide but fails to activate L1 (Fig 2B). In all three complexes within the asymmetric unit of the new crystal structures with bound NAD+, L1 flips towards the diphthamide residue of eEF2 (Fig 2C; supplementary Fig 2A–C online). The L1 flip is caused by the hinged action of Ala 457 and Ala 464 with the Cα of Asp 461 migrating more than 7 Å (Fig 3A). In one of the three complexes in each of the structures (chains C and D), the diphthamide amide forms hydrogen bonds with the Asp 461 side chain of ExoAc and the trimethyl ammonium (TMA) substituent on the diphthamide has rotated about 105° compared with the crystal structure with βTAD. Moreover, the new L1 conformation places the amide of the catalytically important Gln 460 within hydrogen-bonding distance of the adenine (A)-phosphate of NAD+. In addition, the δ-guanido group of the Arg 458 side chain has moved from a solvent-exposed position in the βTAD complex to form van der Waals interactions with the adenine base of the NAD+ substrate (Fig 3B), supporting its important role in binding NAD+ (supplementary Table 1 online). Finally, after ADP-ribosylation of the diphthamide, the ADP-ribose leaves the binding pocket and L1 returns to its original position (Fig 2D). Thus, it seems that L1 interacts with NAD+ and forms a main solvent cover for the dinucleotide-binding pocket (Fig 3C,D) that helps to orientate the Michaelis complex for the ADPRT reaction and to stabilize the NAD+ in the active-site cleft. During the ADPRT reaction, L1 might also act to protect the highly reactive oxacarbenium species from being quenched by water, while intensifying dipole–dipole interactions in the heart of the enzyme reaction centre.
Figure 2.
Sequence of events for the ADP-ribosylation of eEF2. (A) The structure of the eEF2–ExoAc complex before NAD+ binding. ExoAc is shown in blue, eEF2 in green and hydrogen bonds in purple. Residues surrounding the NAD+ binding pocket are shown as a ball-and-stick representation (Protein Data Bank accession code 1ZM3; Jorgensen et al, 2005). (B) Structure of the eEF2–ExoAc–βTAD complex with the trimethyl ammonium group of the diphthamide (Diph; green) reaching towards the nicotinamide phosphate of βTAD (black carbons; Protein Data Bank accession code 1ZM4; Jorgensen et al, 2005). (C) The structure of the eEF2–ExoAc(R551H)–NAD+ complex with L1 hydrogen bonding to the NAD+ (grey carbons) and the diphthamide. Several water molecules (brown spheres) mediate contact between NAD+ and ExoAc. (D) The ADP-ribosyltransferase reaction product, the ADPR–eEF2–ExoAc complex (Protein Data Bank accession code 1ZM2; Jorgensen et al, 2005). The ADP-ribose (grey carbons) has been transferred from NAD+ (donor) to N3 of the imidazole ring of the diphthamide residue. The nicotinamide is docked into the binding pocket. ExoA, exotoxin A; eEF2, elongation factor 2; L, loop.
Figure 3.
The NAD+ binding pocket. (A) A comparison of the NAD+-binding site in the eEF2–ExoAc–βTAD complex and the eEF2–ExoAc(R551H)–NAD+ mutant complex. In the eEF2–ExoAc–βTAD complex, ExoAc is shown in dark brown, eEF2 in light brown and βTAD in black. In the eEF2–ExoAc(R551H)–NAD+ complex, ExoAc is shown in blue, eEF2 in dark green and NAD+ in grey. Residues involved in coordinating NAD+ are shown as a ball-and-stick representation. (B) Conformation of L1, and the NAD+ and βTAD legends in the NAD+-binding site as viewed from the diphthamide residue. When compared with (A), the view is rotated −90° around the x axis and 90° around the z axis. Residue and legend colours are as shown in (A). (C) Transparent surface representation of the complex. Open conformation of L1 (blue cartoon with Asp 461 (D 461) in a ball-and-stick representation) in the eEF2–ExoAc–βTAD complex. The βTAD (black), the diphthamide (white carbons) and the ExoAc E551H (blue) are shown as a ball-and-stick representation. (D) Closed conformation of L1 in the eEF2–ExoAc(E551H)–NAD+ complex. The colouring is as in (C), except for NAD+ (grey ball and stick). ExoA, exotoxin A; eEF2, elongation factor 2; L, loop.
In the structure of apo–ExoA, the NAD+-binding site is occluded by several residues in L1 (Wedekind et al, 2001). Previous structures of ExoAc (Li et al, 1995, 1996; Yates et al, 2005) and DT (Bell & Eisenberg, 1996) have shown a disordered L1 on NAD+ binding. Moreover, when superimposing the catalytic domains of ExoAc, DT, CT and C3 exoenzyme using their bound NAD+ substrates and the critically conserved catalytic Glu (corresponding to Glu 553 in ExoA) as the reference point, then L1 in ExoAc and DT, the active-site loop in CT and α3 in C3 overlap almost perfectly (supplementary Fig 3 online). In the structure of full-length CT (no substrate), the active-site loop (residues 47–56) corresponding to L1 in ExoAc also occludes the binding site, whereas in the CT–ARF6–GTP (ARF6 for ADP-ribosylation factor 6) complex, the loop swings out into an open conformation, thereby allowing NAD+ binding (O'Neal et al, 2005). The active-site loop in the open conformation of CT forms a ‘knob', which is positioned just next to the ‘ADP-ribosylating turn-turn' motif known to be important for target protein recognition (Han & Tainer, 2002). Furthermore, NAD+ binding to the C3 exoenzyme from Clostridium botulinum and Staphylococcus aureus induces several conformational changes, which, among others, bring residues in helix α3 within hydrogen bonding distance of the NAD+ (Evans et al, 2003). This suggests that the movement of L1 and its interaction with the target protein substrate during the ADPRT reaction is universal to this class of toxins.
In the complex structures without bound NAD+, L3 is stabilized by hydrogen bonds formed between the side chains, as well as the backbones of Arg 551 and Glu 546 (Fig 2A,B,D). However, the side-chain hydrogen bond between Glu 546 and Arg 551 is broken in the ExoAc and ExoAc(R551H) complexes with bound NAD+ and the side chain of Glu 546 has rotated towards the N-ribose (Figs 2C, 3A; supplementary Fig 2C online). In this orientation, Glu 546 is now less than 4 Å from the 2′OH and C1 of the N-ribose, and it now forms a hydrogen bond with the conserved and catalytically important Tyr 481. This potentially prevents any bound water molecules near the labile C–N bond of NAD+ from quenching the reaction as seen in the E546H complex structure between Tyr 481, His 546 and the 2′OH of N-ribose (supplementary Fig 2B online). Glu 553 maintains its hydrogen bond to the 2′OH on the N-ribose in spite of a side-chain rotation caused by the Glu 546 movement. Thus, the reduced ADPRT activity of the two ExoAc(E546A/H) mutant enzymes might be due to an inability to ‘seal' the binding pocket, thereby allowing the aqueous solvent to seep through to the unstable oxacarbenium cation and prevent ADP-ribose transfer to the diphthamide nucleophile.
Ligand binding to the active site
An important lesson from this study is that the N-ribose and the two NAD+ phosphates of NAD+ have adopted new conformations compared with βTAD in previous ExoAc–βTAD structures (Li et al, 1996; Jorgensen et al, 2005; Fig 3A,B). The N-phosphate has flipped towards His 440 of ExoAc compared with the βTAD structure and is now buried deeper into the NAD+ binding pocket (Fig 3B). The correct conformation of NAD+ seems to be a prerequisite for the triggering of L1 movements. Furthermore, the specific interactions of L1 with the NAD+ substrate and the diphthamide testify to the need for keeping the substrate coordinated and stabilized during the ADPRT reaction. It is possible that the interaction of Gln 460 in ExoAc with the A-phosphate of NAD+ is a means of protecting the labile phosphoanhydride and phosphoester bonds of NAD+ during catalysis to prevent the unintended formation (hydrolysis side reaction) of AMP or ADP, as seen in previous toxin–NAD+ crystal structures (Li et al, 1995, 1996; Menetrey et al, 2002). In addition, the rotation (105°) of the diphthamide TMA group indicates that the imidazole ring must be precisely positioned in its role as the attacking nucleophile in the ADPRT reaction (Fig 3A).
Transition state of the ADP-ribosylation reaction
In our latest structures, the diphthamide N3 nucleophilic atom is still about 10 Å from the electrophilic C1 centre of the N-ribose and therefore at least one additional step is necessary for the consummation of the nucleophilic substitution reaction. As L1 caps the substrate-binding pocket, it is unlikely that the C1 atom (electrophile) of the NAD+ will migrate much closer to the eEF2 diphthamide (nucleophile). In addition, when soaking NAD+ into eEF2–ExoAc crystals at pH 7.2, the reaction readily occurs with the crystals remaining intact and able to diffract to some extent (Jorgensen et al, 2005), which argues against any major domain movements during catalysis. Notably, a previous transition-state model, based on kinetic isotope effect measurements in the absence of eEF2 substrate, implied an electrophile migration of about 2 Å leaving a considerable gap between the electrophile and nucleophile (Parikh & Schramm, 2004). The diphthamide is housed in a loop with a small 310 helix flanked by three conserved Gly residues that make the loop potentially very flexible (Fig 4A). The diphthamide would need to migrate only about 6 Å towards the electrophilic reaction centre to position the imidazole N3 atom sufficiently close to the N-ribose C1 for the substitution reaction to occur, as postulated from kinetic isotope effect studies. The surface charge of the inner walls of the NAD+ binding pocket formed by L1 along with the two NAD+ phosphates shows selected patches of negative charge, which might provide an enticing environment to attract the positively charged diphthamide moiety during the transferase reaction (Fig 4B). In addition, the solvent-restricted binding pocket, guarded by L1 in the toxin active site, clearly leaves sufficient space for the diphthamide TMA group to electrostatically ratchet along the NAD+ phosphates poising the imidazole N3 for the chemical attack. Such a movement would orient the diphthamide TMA in a position adjacent to the NAD+ phosphates, similar to that observed in the structures of the ADP-ribose eEF2 product (Jorgensen et al, 2004, 2005; Fig 2D). In our transition-state model, the nucleophilic N3 atom of the diphthamide imidazole is within hydrogen bonding distance of both the Glu 546 (rotated conformation) and the hydroxyl group of the Tyr 481 residue (Fig 4A). Such interactions could potentially result in a net transfer of H+ from the diphthamide imidazolium moiety, thereby making N3 more nucleophilic.
Figure 4.
Transition-state model for the ADP-ribosyltransferase reaction. (A) The imidazole ring of the diphthamide residue (Diph; dark green; N3 nucleophile, cyan) migrates towards the nicotinamide ribose as the TMA group of the diphthamide moves between L1 of the toxin and the NAD+ phosphates. The original diphthamide position from the eEF2–ExoAc(R551H)–NAD+ structure is shown in light brown with Gly 701, Gly 702 and Gly 703 in red. Active-site residues of ExoAc are shown as light blue ball and sticks, with the position of Glu 546 (before roamer rotation) and Arg 551 shown in brown. ADP-ribose and nicotinamide carbons are shown in grey (C1 electrophile, dark grey). Known hydrogen bonds are shown in purple, whereas potential interactions during transition-state formation are indicated in yellow. (B) The electrostatic molecular surface of the binding pocket of the eEF2–ExoAc(R551H)–NAD+ complex. Charges were assigned to ExoAc during electrostatic calculations and the position of NAD+ (grey) is shown as a ball-and-stick representation. Asp 461 and Glu 546 (blue), and the diphthamide (white) are shown as a ball-and-stick representation. ExoA, exotoxin A; eEF2, elongation factor 2; L, loop; TMA, trimethyl ammonium.
Conclusion
These new X-ray structures provide a breakthrough in the understanding of the ADPRT reaction mechanism. Together with the previous complex structures, we now have a detailed sequence of events involving the opening and closing of the NAD+ binding pocket, the coordination of both the NAD+ and the diphthamide substrates, as well as the stabilization of the reactive oxacarbenium during ADPRT reaction by L1 and L3. The function of L1 might, in fact, be universal to this family of toxins, as corresponding loops in other toxins show equally flexible properties. These data suggest that for DT-specific toxins, the diphthamide loop in eEF2 is a likely candidate as the vehicle to propel the nucleophile into position near the C1 atom (electrophile) of the NAD+ substrate. Indisputably, a deeper understanding of the ADPRT reaction mechanism of this important family of toxins is fundamental for the development of new and more potent therapeutics to aid in the treatment of bacterial diseases and infections.
Methods
Sample preparation. Purification of yeast eEF2 and ExoAc was completed as described previously (Armstrong et al, 2002; Jorgensen et al, 2002). All the ExoAc mutants were prepared by the standard QuikChange™ mutagenesis protocol. The plasmid containing the gene encoding the catalytic domain for ExoAc was used to prepare the single-site mutants and cumulative (double) mutants were made by using the single mutant plasmid DNA as the template. The folded integrity of WT and mutant ExoAc enzymes was monitored by both Trp fluorescence λ emission maxima (Trp λem,max) and circular dichroism spectroscopy (results not shown), and none of the mutant proteins was shown to be significantly misfolded.
Measurement of enzyme activity and substrate binding. The NAD+-dependent ADPRT activity, GH and NAD+ binding of the various mutant proteins for both ExoAc, and DT were tested as reported previously (Yates & Merrill, 2004).
Structure solution. The preparation, crystallization and structural solution of the eEF2–ExoAc complexes were carried out as described previously (Jorgensen et al, 2005; see the supplementary information online for a more detailed description). All the structures are highly isomorphous and, as in previous structures of the complex, the asymmetric unit contains three complexes (complex 1: chains A and B; complex 2: chains C and D; complex 3: chains E and F). In addition, in all the structures, the complexes are highly ordered around the interaction area of ExoAc and domain IV (559–729, 801–842) of eEF2. However, in complex 2, eEF2 domains G′ (219–328) and III (482–558) are mobile, and, in complex 3, all eEF2 domains except for domain IV are mobile. The lower pH in the cryo-protection buffer used for the eEF2-ExoAc crystal did not result in any visible changes in the conformation of the structure. Moreover, the conformation of L1, L3 and NAD+ in all three complexes in each of the four structures is highly isomorphous. The diphthamide could be modelled in chain C of all the structures and also in chain E of the E546H/A mutant complexes. The diphthamide-containing loop in the transition-state model was modelled in O (Jones et al, 1991) using Lego_Ca and Refi_Zone to maintain correct backbone bond lengths and angles; however, the positions of the side chains were ignored.
Coordinates. The atomic coordinates and structure factors have been deposited at the Protein Data Bank with the accession codes 3B8H, 3B82, 3B78 and 2ZIT.
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Information
Acknowledgments
We thank G. Prentice for excellent technical assistance and for the purification of yeast eEF2 protein. We are indebted to D. White for preparing some of the toxin mutants. We thank G.R. Andersen, M. Kimber, N. Oppenheimer and A.L. Schwan for helpful discussions. We also thank the beamline scientists at the Advanced Photon Source and the Canadian Light Source for help with X-ray data collection. This work was funded by the Canadian Institutes of Health Research, the Canadian Cystic Fibrosis Foundation, the Villum Kann Rasmussen Foundation and Carlsbergfondet.
Footnotes
The authors declare that they have no conflict of interest.
References
- Armstrong S, Merrill AR (2001) Application of a fluorometric assay for characterization of the catalytic competency of a domain III fragment of Pseudomonas aeruginosa exotoxin A. Anal Biochem 292: 26–33 [DOI] [PubMed] [Google Scholar]
- Armstrong S, Yates SP, Merrill AR (2002) Insight into the catalytic mechanism of Pseudomonas aeruginosa exotoxin A. Studies of toxin interaction with eukaryotic elongation factor-2. J Biol Chem 277: 46669–46675 [DOI] [PubMed] [Google Scholar]
- Beattie BK, Prentice GA, Merrill AR (1996) Investigation into the catalytic role for the tryptophan residues within domain III of Pseudomonas aeruginosa exotoxin A. Biochemistry 35: 15134–15142 [DOI] [PubMed] [Google Scholar]
- Bell CE, Eisenberg D (1996) Crystal structure of diphtheria toxin bound to nicotinamide adenine dinucleotide. Biochemistry 35: 1137–1149 [DOI] [PubMed] [Google Scholar]
- Corda D, Di Girolamo M (2003) Functional aspects of protein mono-ADP-ribosylation. EMBO J 22: 1953–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HR, Sutton JM, Holloway DE, Ayriss J, Shone CC, Acharya KR (2003) The crystal structure of C3stau2 from Staphylococcus aureus and its complex with NAD. J Biol Chem 278: 45924–45930 [DOI] [PubMed] [Google Scholar]
- Han S, Tainer JA (2002) The ARTT motif and a unified structural understanding of substrate recognition in ADP-ribosylating bacterial toxins and eukaryotic ADP-ribosyltransferases. Int J Med Microbiol 291: 523–529 [DOI] [PubMed] [Google Scholar]
- Han S, Craig JA, Putnam CD, Carozzi NB, Tainer JA (1999) Evolution and mechanism from structures of an ADP-ribosylating toxin and NAD complex. Nat Struct Biol 6: 932–936 [DOI] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard M (1991) Improved methods for building protein models in electron-density maps and the location of errors in these models. Acta Crystallogr A 47: 110–119 [DOI] [PubMed] [Google Scholar]
- Jorgensen R, Carr-Schmid A, Ortiz PA, Kinzy TG, Andersen GR (2002) Purification and crystallization of the yeast elongation factor eEF2. Acta Crystallogr D 58: 712–715 [DOI] [PubMed] [Google Scholar]
- Jorgensen R, Yates SP, Teal DJ, Nilsson J, Prentice GA, Merrill AR, Andersen GR (2004) Crystal structure of ADP-ribosylated ribosomal translocase from Saccharomyces cerevisiae. J Biol Chem 279: 45919–45925 [DOI] [PubMed] [Google Scholar]
- Jorgensen R, Merrill AR, Yates SP, Marquez VE, Schwan AL, Boesen T, Andersen GR (2005) Exotoxin A–eEF2 complex structure indicates ADP ribosylation by ribosome mimicry. Nature 436: 979–984 [DOI] [PubMed] [Google Scholar]
- Jorgensen R, Purdy AE, Fieldhouse RJ, Kimber MS, Bartlett DH, Merrill AR (2008) Cholix toxin, a novel ADP-ribosylating factor from Vibrio cholerae. J Biol Chem 283: 10671–10678 [DOI] [PubMed] [Google Scholar]
- Li M, Dyda F, Benhar I, Pastan I, Davies DR (1995) The crystal structure of Pseudomonas aeruginosa exotoxin domain III with nicotinamide and AMP: conformational differences with the intact exotoxin. Proc Natl Acad Sci USA 92: 9308–9312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Dyda F, Benhar I, Pastan I, Davies DR (1996) Crystal structure of the catalytic domain of Pseudomonas exotoxin A complexed with a nicotinamide adenine dinucleotide analog: implications for the activation process and for ADP ribosylation. Proc Natl Acad Sci USA 93: 6902–6906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menetrey J, Flatau G, Stura EA, Charbonnier JB, Gas F, Teulon JM, Le Du MH, Boquet P, Menez A (2002) NAD binding induces conformational changes in Rho ADP-ribosylating Clostridium botulinum C3 exoenzyme. J Biol Chem 277: 30950–30957 [DOI] [PubMed] [Google Scholar]
- O'Neal CJ, Jobling MG, Holmes RK, Hol WG (2005) Structural basis for the activation of cholera toxin by human ARF6-GTP. Science 309: 1093–1096 [DOI] [PubMed] [Google Scholar]
- Parikh SL, Schramm VL (2004) Transition state structure for ADP-ribosylation of eukaryotic elongation factor 2 catalyzed by diphtheria toxin. Biochemistry 43: 1204–1212 [DOI] [PubMed] [Google Scholar]
- Popoff MR (2005) Bacterial exotoxins. Contrib Microbiol 12: 28–54 [DOI] [PubMed] [Google Scholar]
- Tsuge H, Nagahama M, Nishimura H, Hisatsune J, Sakaguchi Y, Itogawa Y, Katunuma N, Sakurai J (2003) Crystal structure and site-directed mutagenesis of enzymatic components from Clostridium perfringens iota-toxin. J Mol Biol 325: 471–483 [DOI] [PubMed] [Google Scholar]
- Van Ness BG, Howard JB, Bodley JW (1980) ADP-ribosylation of elongation factor 2 by diphtheria toxin. NMR spectra and proposed structures of ribosyl-diphthamide and its hydrolysis products. J Biol Chem 255: 10710–10716 [PubMed] [Google Scholar]
- Wedekind JE, Trame CB, Dorywalska M, Koehl P, Raschke TM, McKee M, FitzGerald D, Collier RJ, McKay DB (2001) Refined crystallographic structure of Pseudomonas aeruginosa exotoxin A and its implications for the molecular mechanism of toxicity. J Mol Biol 314: 823–837 [DOI] [PubMed] [Google Scholar]
- Wilson BA, Collier RJ (1992) Diphtheria toxin and Pseudomonas aeruginosa exotoxin A: active-site structure and enzymic mechanism. Curr Top Microbiol Immunol 175: 27–41 [DOI] [PubMed] [Google Scholar]
- Yates SP, Merrill AR (2004) Elucidation of eukaryotic elongation factor-2 contact sites within the catalytic domain of Pseudomonas aeruginosa exotoxin A. Biochem J 379: 563–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates SP, Taylor PL, Jorgensen R, Ferraris D, Zhang J, Andersen GR, Merrill AR (2005) Structure–function analysis of water-soluble inhibitors of the catalytic domain of exotoxin A from Pseudomonas aeruginosa. Biochem J 385: 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates SP, Jorgensen R, Andersen GR, Merrill AR (2006) Stealth and mimicry by deadly bacterial toxins. Trends Biochem Sci 31: 123–133 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information