Abstract
Aging is associated with an inability to mount protective antibody responses to vaccines and infectious agents. This decline is associated with acquisition of defects in multiple cellular compartments, including B cells. While peripheral B-cell numbers do not decline with aging, the composition of the compartment appears to change, with loss of naïve follicular B cells, accumulation of antigen-experienced cells, and alteration of the antibody repertoire. The underlying cause of this change is unknown. We tested the hypothesis that aging-associated repertoire changes can be attributed directly to decreased B lymphopoiesis. Using an Ig transgenic model to report changes in the B-cell repertoire, we show that the reduced B-cell generative capacity of “aged” long-term reconstituting hematopoietic stem cells (LT-HSCs) alters the representation of antigen specificities in the peripheral B-cell repertoire. Further, we show that reconstitution using suboptimal numbers of fully functional LT-HSCs results in the generation of a similarly altered B-cell repertoire. This may be an important factor to consider when deciding the number of bone marrow cells to transplant in the clinical setting. In conclusion, when B lymphopoiesis is limited peripheral B-cell homeostasis is altered. This is reflected in reduced diversity of the B-cell repertoire, which likely reduces the protective quality of the immune response.
Follicular (FO) lymphocytes are the largest B-cell subpopulation in peripheral lymphoid organs and display the maximum breadth of germline-encoded antibody specificity for foreign antigens. Because of the breadth of this repertoire, these naïve FO cells are uniquely suited to give rise to protective primary immune responses and to be selected for high-affinity antibody production.
Aging is associated with decreased efficacy of vaccination in both humans and mice, increased susceptibility to infection, and increased rate of cancer (1–4). In mice, where it can be examined, aging is associated with a reduction in FO B cells, but not total B-cell numbers (5, 6). This reduction in FO B-cell numbers is further associated with a reduction in immunoglobulin (Ig) diversity within the B-cell pool and an increase in the frequency of antigen-experienced cells, including marginal zone, B1, and memory cells. In young animals the sizes of these compartments is independently regulated (7). Thus, it seems plausible that the humoral immune defects seen in aging may be the consequence of reduced frequency of FO B cells bearing high-affinity receptors for offensive pathogens.
FO B cells are short-lived (estimates ranging from 40 to 120 d) and nonself-renewing and therefore must be constantly replenished by new B cells produced in hematopoietic organs, e.g., adult bone marrow (BM) (8, 9). For reasons that are unclear, transplanted bone marrow stem cells from older adult human donors often do not give rise to B cells in recipients (10). Similarly, LT-HSCs from aged mice, while more numerous than in young animals, are selectively impaired in the ability to reconstitute the B-cell compartment of irradiated recipients (11, 12). Finally, autoreconstitution of B cells following lymphoablation is impaired in aged mice (13, 14). On the basis of these findings we hypothesize that the poor quality of the primary antibody response seen in the aged results from a decline in naive follicular B-cell numbers, which is in turn the result of the decreased ability of LT-HSCs from aged animals to replenish the follicular compartment. Studies described in this report test this hypothesis.
A major obstacle to studying the effects of aging on the B-cell compartment is the difficulty of resolving B-cell repertoire changes in wild type (WT) mice where B cells exhibit diverse Ig specificity. Therefore, to address the relationship between the age-associated decline in B lymphopoiesis and the peripheral B-cell repertoire, we used the 3-83 μδ Ig transgenic (Tg) mouse model. In young 3-83 μδ mice, ∼93% of B cells express transgene encoded Igs (tgIg) specific for the MHC class I antigen H-2Kk/b, an exotic antigen in the H-2Kd mice (B10.D2) used in this study (15). The remaining B cells in these mice express B-cell receptors encoded by endogenous Ig (enIg) genes. As reported previously, immature B cells in the bone marrow of aged 3-83 μδ mice maintain this ratio of 93% tgIg:7% enIg (6). Importantly however, in these same aged mice the frequency of peripheral B cells (B cells in blood, spleen, and lymph nodes) expressing tgIg decreases, and the compartment becomes dominated by cells expressing enIg that appear, on the basis of surface phenotype, to be antigen experienced (memory, marginal zone, and CD5+ B1-like). By 18 months of age, ∼90% of 3-83 μδ mice express a B-cell compartment skewed in favor of enIg. In ∼75% of these mice, >60% of B cells express enIg (6). Furthermore, this enIg population is highly enriched in cells specific for auto- and environmental antigens, a hallmark of aging. Finally in aged 3-83 μδ mice, naive FO B cells are reduced in number and uniformly express transgene-encoded receptors. Thus, in this model system a shift in the peripheral B-cell repertoire away from tgIg and toward enIg expression is an easily measured indicator of immunologic aging.
Using this model, we show that although LT-HSCs increase in number as mice age, these LT-HSCs lose functionality, resulting in decreased ability to generate B cells. This decreased output of naive B cells from the BM determines the aged peripheral repertoire. Furthermore, artificial limitation of HSC function in young animals mimics aging, generating a B-cell repertoire skewed toward nonfollicular cell types.
Results and Discussion
Aging-Associated B-Cell Repertoire Changes Are Caused by Stem-Cell Defects.
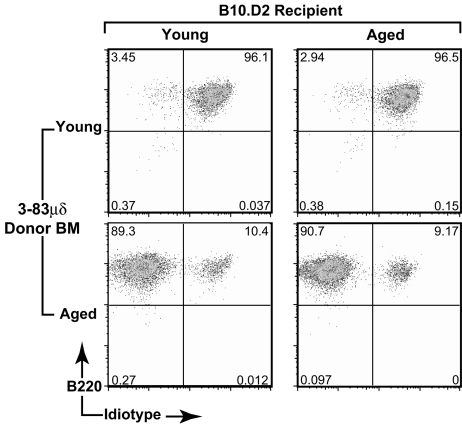
To assess the stem-cell contribution to aging-associated changes in the peripheral B-cell repertoire, we analyzed the ability of bulk HSC populations from prescreened immunologically aged, i.e., mice in which >60% of B cells express enIg, 3-83 μδ mice to recapitulate, in young lethally irradiated WT recipients, the repertoire changes seen in the HSC donors. Furthermore, to assess the role played by the BM microenvironment in the development of the aged B-cell repertoire, the converse experiment, in which bulk HSCs from young 3-83 μδ mice were transferred into aged lethally irradiated recipients, was also performed. B-cell reconstitution was assessed 6 weeks posttransfer by flow cytometric analysis of the splenic B-cell compartment. As shown in Fig. 1, lymphocyte-depleted BM from young 3-83 μδ mice reconstituted a young-like (predominantly tgIg, idiotype positive) repertoire in both young and old recipients (top panels), whereas lymphocyte-depleted BM from aged 3-83 μδ mice reconstituted an aged-like (predominantly enIg, idiotype negative) repertoire in both young and old recipients (bottom panels). These data suggest that acquired stem-cell autonomous defects determine development of the aged repertoire and that this defect is not determined by the microenvironment.
Fig. 1.
Aging-associated decline in B lymphopoiesis is B-cell/stem cell autonomous. Young or aged WT (B10.D2) mice were lethally irradiated, and lymphocyte-depleted bone marrow cells from young or prescreened immunologically aged 3-83μδ animals were transferred by i.v. injection. Donor BM was pooled from multiple animals before depletion, and BM equivalents were transferred at a ratio of one donor equivalent to four recipients. Reconstitution of the peripheral B-cell compartment was assessed 6 weeks posttransfer. Splenocytes were gated on live, CD19+ B cells, and 3-83μδ idiotype and total IgM expression was evaluated. Data are representative of analyses of a minimum of three individual animals in each category.
We next sought to exclude the possibility that passenger cells (non-HSC) in the stem-cell preparation contributed to the observed results. To further enrich stem cells, young and aged 3-83 μδ donor mice were treated with 5-Fluorouracil (5-FU) to deplete proliferating cells and to facilitate increased production of HSCs. HSCs from 5-FU-treated mice have an activated phenotype, similar to fetal liver stem cells and transiently repopulating stem cells found in normal BM (16, 17). BM was harvested 5 d after treatment and transferred to lethally irradiated recipients as before. Analysis of animals 9 weeks later revealed a pattern of B-cell reconstitution equivalent to that seen following transfer of lymphocyte-depleted BM cells (Fig. 2C).
Fig. 2.
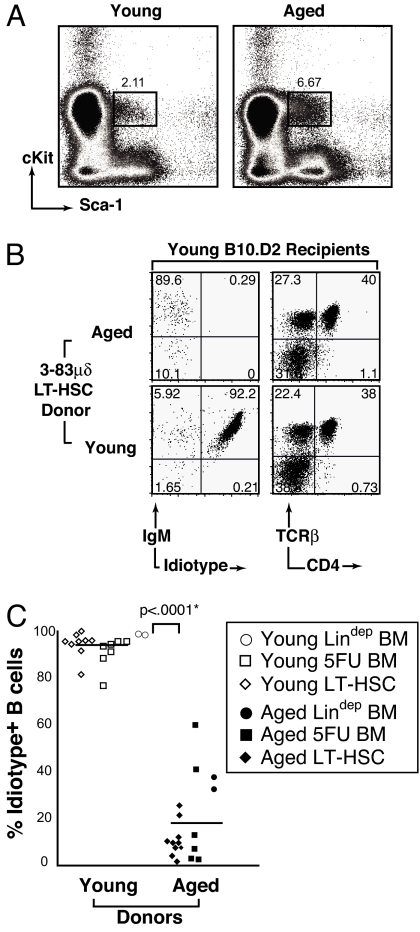
LT-HSCs from aged animals exhibit reduced B-cell generative capacity associated with repertoire alteration. Untreated whole BM was harvested from four young or four aged mice, RBCs were lysed, and LT-HSCs purified as described in experimental procedures. (A) Sorting profile of LT-HSCs from young and aged 3-83μδ lineage-depleted BM. (B) Reconstitution of young WT recipients 10 weeks posttransfer of 5000 LT-HSCs (Lin−Sca-1+cKit+Flk-2−CD34−) from young or aged 3-38μδ plus 3 × 105 Rag2−/− whole BM carrier cells. Total cells recovered were equivalent among recipients. Shown on the left are cytograms demonstrating IgM and 3-83μδ idiotype expression by CD19+ pregated splenocytes (B-cell plots). Shown on the right are cytograms illustrating T-cell antigen receptor (TCRβ) and CD4 expression by total splenocytes. Data are representative of analyses of a minimum of four individual animals in each category. (C) Summary of the percentage of idiotype-negative B cells present in the spleens of animals reconstituted with lineage-depleted (Fig. 1), 5-FU-treated, and flow-purified LT-HSC preparations (Fig. 2 A and B) derived from either young or aged donors. Means (horizontal bars) and statistically significant P < 0.0001* values are indicated.
Finally, we transferred to irradiated young recipients, 5000 highly purified sorted LT-HSCs (lineage marker negative (Lin−), Sca-1+cKit+Flk-2−CD34−) (18–21) from aged or young untreated 3-83 μδ donor mice along with 3 × 105 whole BM carrier cells from Rag-2−/− mice. Consistent with published findings in WT mice (11, 22), aged 3-83 μδ donor BM contained an increased frequency of LT-HSCs (Fig. 2A, compare boxed populations). The splenic B-cell compartments of LT-HSC recipients were analyzed 10 weeks posttransfer to provide additional assurance that we were assaying LT-HSC activity, rather than that of short-term (ST) reconstituting HSCs. The latter are capable of peripheral reconstitution for only 6–8 weeks (23). It should be noted that the HSC population transferred should contain no ST-HSCs because it contained no CD34 positive cells. The LT-HSC populations from aged and young mice gave rise to similar numbers of CD4+ T cells (Fig. 2B, right) and other lineages (data not shown). In contrast, the LT-HSC population from aged 3-83 μδ mice gave rise to 30-fold fewer CD19+ B cells (5.3 × 105/spleen from aged vs. 14.2 × 106/spleen from young) than LT-HSCs from young 3-83 μδ mice, and the B cells detected in spleen were idiotype negative (Fig. 2B, left).
Fig. 2C compiles data from various reconstitution experiments using lineage-depleted bone marrow, bone marrow from 5-FU-treated animals or purified LT-HSCs and reinforces the conclusion that repertoire changes seen in aged animals are directly ascribable to stem cell intrinsic defects. Collectively, the data presented in Fig. 2 confirm earlier studies of WT mice (12) demonstrating that while capable of generating T cells and cells of other hematopoietic lineages, LT-HSCs from immunologically aged 3-83 μδ animals are defective in B-cell genesis. Our data extend this observation by showing that the few B cells produced from these aged stem cells are highly skewed toward enIg- (3-83 μδ idiotype negative) expressing cells. This is consistent with selective preservation of long-lived, antigen-experienced cells in an effort to fill the B-cell compartment (6).
Limitation of Functional HSCs in Young Mice Reduces Total Splenic B-Cell Numbers and Alters the B-Cell Repertoire.
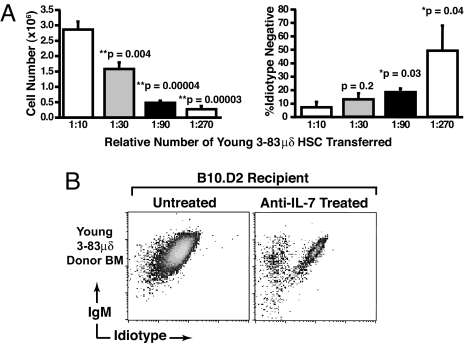
The observed defect in HSCs from immunologically aged animals could simply reflect a lower frequency of fully competent stem cells among LT-HSCs enriched on the basis of surface phenotype. To begin to address this question, we examined the correlation between stem cell input, B-cell reconstitution, and repertoire shift. Young 3-83 μδ donors were treated with 5-FU as before, HSCs harvested, and transferred (in decreasing doses) to lethally irradiated young WT recipients. Six weeks posttransfer, immunofluorescence was used to assess total splenic CD19+ B-cell numbers, and 3-83 μδ idiotype expression. As shown in Fig. 3A, when the capacity of the transferred cells to completely reconstitute the B-cell compartment was exceeded (∼1:30 femur equivalent), we began to see selective accumulation of enIg-expressing cells. Furthermore, this accumulation of enIg-expressing cells became more pronounced as the capacity to generate B cells was further restricted (Fig. 3A). Thus, the “artificial” reduction of fully competent stem cells from young animals mimicked the effects of aging on the HSC compartment, resulting in both reduced B lymphopoiesis and a shift in the peripheral B-cell repertoire.
Fig. 3.
Limitation of HSC function in young mice reduces total splenic B-cell numbers and alters the B-cell repertoire. (A) BM was harvested from 5-FU-treated young 3-83μδ mice and RBC lysed. Cells were then pooled, serially diluted (represented as BM equivalents) in PBS, and each dilution spiked with 3 × 105 whole BM carriers from Rag2−/− mice. Cell mixtures were transferred to lethally irradiated young WT recipients by i.v. injection. Six weeks posttransfer, live CD19+ splenic B cells were analyzed in terms of absolute number recovered and 3-83 μδ idiotype and IgM expression. BM equivalents transferred are indicated below each bar in the graphs and P-values provided. Statistics were generated from a minimum of three mice in each category, error bars represent SEM. P-values were determined by comparing the 1:10 dilution to each subsequent dilution in turn using the t-test: two samples assuming equal variances. (B) Effect of anti-IL-7 inhibition of B lymphopoiesis on repertoires development in young lethally irradiated WT mice that were reconstituted with stem cells from 5-FU-treated young 3-83 μδ mice. Control mice were sham treated by injection of PBS. Splenocytes were analyzed after the last antibody injection (6 weeks posttransfer) for 3-83 μδ idiotype and IgM expression. Splenocytes were pregated on live, CD19+ cells. Data are representative of analyses of a minimum of four individual animals in each category.
To further address the apparent connection between reduced B lymphopoiesis and the peripheral B-cell repertoire, we specifically suppressed B lymphopoiesis in young recipients (that received a full complement of HSCs from young 3-83 μδ donors) by treatment with anti-IL7 antibodies (24). B-cell recovery from spleens of recipients treated with anti-IL7 was reduced ∼90% compared to PBS sham controls (data not shown). Importantly, antibody treatment also induced a shift in the B-cell repertoire toward enIg-expressing cells (Fig. 3B, right, boxed). These data further support the hypothesis that BM-derived B-cell generative capacity determines the peripheral B-cell repertoire, i.e., reduced generative capacity selects for antigen-experienced cells.
Quantitation of the Relative B-Cell Generative Capacity of LT-HSC-Enriched Cell Populations from Young and Immunologically Aged Animals.
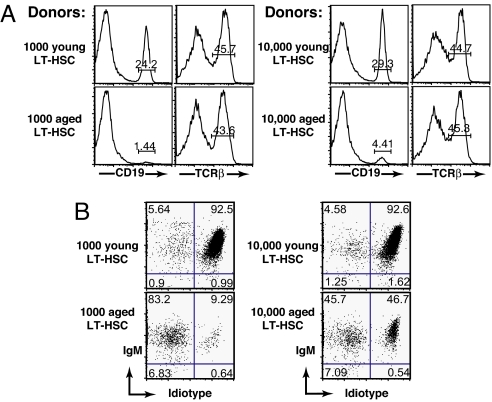
The experiments described above provide qualitative evidence of the reduced B-cell generative capacity of stem cells from aged animals and its impact on the peripheral B-cell repertoire. Realizing that individual animals must vary in terms of the extent of this defect, we nonetheless wished to quantitate this defect more precisely. To accomplish this we sorted LT-HSCs from young and immunologically aged animals (lineage marker negative (Lin−), Sca-1+cKit+Flk-2−CD34−), and transferred 1000 or 10,000 cells along with 3 × 105 bone marrow cells from Rag-2−/− mice to irradiated young recipients. We then quantified the ability of these innocula to give rise to a normal or skewed B-cell repertoire by analysis of the splenic B-cell compartment 10 weeks later. As shown in Fig. 4A, transfer of either 1000 (top, far left) or 10,000 (top, middle right) LT-HSCs from young animals reconstituted a splenic compartment in which ∼24–29% of cells in the viable scatter gate were CD19+ B cells. Notably, CD19+ B cells represent 30–35% of splenocytes in intact, nonmanipulated B10.D2 animals. In contrast, stem cells from aged animals gave rise to far fewer B cells, ∼1% per 1000 HSCs (bottom, far left) and ∼4% per 10,000 HSCs (bottom, middle right). This represents a ∼60-fold reduction in the B-cell generative capacity of stem cells from aged animals. Importantly, LT-HSCs from aged and young mice appear similarly competent to give rise to T cells (Fig. 4A, middle left and far right). It should be noted that because thymic reconstitution was not measured in this study, one cannot conclude that the T-cell generative capacity of HSCs from aged animals is equivalent to young animals.
Fig. 4.
Reconstitution with increasing numbers of aged LT-HSCs increases B-cell genesis and partially restores a naive peripheral repertoire. Young WT (B10.D2) mice were lethally irradiated and reconstituted with 1000 or 10,000 LT-HSCs (Lin−Sca-1+cKit+Flk-2−CD34−) sorted from young or aged 3-83 μδ donors plus 3 × 105 whole BM carriers from Rag2−/− mice. Reconstitution was assessed at 10 weeks posttransfer. (A) Histograms show frequency of CD19+ B cells and TCRβ+ T cells relative to total splenocytes recovered from recipient mice. Histogram regions containing stained populations are marked with a bar and percentages within that region are indicated. (B) Cytograms showing 3-83 μδ idiotype and IgM expression (gated on CD19+ splenocytes). Percentages of B cells falling into each quadrant are indicated. Data are representative of analyses of a minimum of four individual animals in each category.
As expected, because B-cell representation in the splenic lymphoid compartment was nearly normal in animals receiving LT-HSCs from young donors, the ratio of tgIg (idiotype positive) to enIg (idiotype negative) was maintained at ∼93:7% as observed in intact, nonmanipulated young 3-83 μδ animals (Fig. 4B, top). However, consistent with data presented in Fig. 2, a shift in the B-cell repertoire was seen in animals receiving LT-HSCs from prescreened immunologically aged animals (Fig. 4B, bottom). Significantly, increasing the number of aged LT-HSCs administered from 1000 to 10,000 partially corrected B-cell repertoire skewing. One thousand aged LT-HSCs resulted in only ∼10% of splenic B cells expressing tgIg (Fig. 4B, bottom left, boxed), while administration of 10,000 LT-HSCs resulted in ∼50% of splenic B cells expressing tgIg (Fig. 4B, bottom right, boxed). B-cell ratios of 50% tgIg:50% enIg exceeded that of the donor animals, which were selected to be <40% tgIg:>60% enIg. It is highly likely that administration of increasing numbers of aged LT-HSCs would further increase B-cell genesis and thus a fully competent naive peripheral repertoire (>93% IgTg). Interestingly, we found in subsequent experiments that as few as 50 LT-HSCs from young animals could give rise to a young-like B-cell compartment in adoptive recipients (data not shown).
As discussed above, in many individuals aging is associated with a progressive decline in the quality of immune responses to newly encountered pathogens and vaccines. Our findings demonstrate that this acquired deficiency is likely caused by the loss of B-cell generative capacity of the aged stem cell compartment.
Repertoire changes in aging could reflect a decrease in the number of functional LT-HSCs in immunologically aged animals, decreased competence of individual LT-HSCs, or both. Resolution of this question will require the ability to enumerate and isolate pure LT-HSCs, which has not yet been achieved. On the basis of studies employing both the conventionally accepted LT-HSC marker set used in this study and supplemental use of the SLAM family markers CD150 and CD 48 indicate that aged animals contain 2- to 3-fold more stem cells than young animals (25). Interestingly however, LT-HSCs isolated from young mice using SLAM family markers showed 3-fold higher frequency of cells competent to reconstitute multiple lineages than cells from aged mice. These findings do not allow firm conclusions to be drawn regarding the degree to which acquired defects in aging reflect decreased HSC frequency or decreased HSC functionality. However, they strongly suggest that decreased functionality of individual LT-HSCs is a component of the problem. Further studies using refined markers will be required to resolve this issue.
While the LT-HSC population, as defined on the basis of widely accepted surface phenotypic markers, is larger in aged animals, the B-cell generative capacity appears reduced 10- to 100-fold compared to LT-HSCs from young animals. Further, it is possible to mimic the immunologically aged phenotype in young animals simply by limiting the functional output of HSCs, reenforcing the conclusion that HSC output determines repertoire. Taken together, our data suggest that the tipping point at which reduced B-cell output forces a change in the peripheral repertoire (reflected by the accumulation of antigen-experienced cells) is at a ∼90% reduction of normal output. It appears that at this point, homeostatic mechanisms activated by B-cell lymphopenia drive selective filing of the peripheral compartment with memory, marginal zone, and CD5+ B1-like cells, i.e., antigen-experienced cells.
These findings may have implications in the context of clinical bone marrow transplantation. A general finding in recipients of marrow transplants, particularly from haploidentical donors, is poor reconstitution of the B-cell compartment (26). These patients often require protracted passive antibody treatment to afford protective immunity. In addition, transplantation often leads to selective population of peripheral blood with normally rare CD5 positive cells that are thought to be equivalent to antigen-experienced B1 cells in mice (27). Thus clinical BM transplantation leads to development of an aged-like B-cell compartment. We submit that this may result simply from transplantation of too few LT-HSCs and suggest that this possibility should be explored and considered in the development of best practices for BM transplantation.
While peripheral B-cell homeostasis is poorly understood, it appears largely controlled by availability of the survival factor BAFF (BLys etc.). Importantly, marginal-zone B cells appear more dependent on BAFF for survival than follicular B cells and high BAFF levels are associated with increased accumulation of MZ cells (28, 29). It seems likely that in aged animals, B lymphopenia caused by reduced bone marrow output of naive B cells may lead to increased BAFF availability. Increased availability would be the consequence of reduced consumption, i.e., competition for BAFF, rather than increased production. This increased BAFF may skew the repertoire by enabling accumulation of MZ cells and perhaps other antigen-experienced cells. At points of extremely limited B-cell production, follicular cells may virtually disappear.
Factors that determine the selective decline in HSC competence to produce B cells remain poorly defined. Recent studies using DNA-array approaches indicate that stem cells from young and aged animals exhibit numerous differences in gene expression (12, 30). These presumably reflect epigenetic changes, which through resultant alterations in gene expression bias HSC output toward the myeloid and away from the B-lymphoid lineage. Of particular note are several factors involved in B-lymphocyte development/function, e.g., Flt3, Ebi3, Runx1, and CD79b (30). The decreased expression of these genes seen in aged LT-HSCs may somehow dictate reduced commitment to the lineage.
Interestingly, Igκ light-chain gene expression is increased in aged LT-HSCs (30). κ-chain expression is normally silenced by methylation in HSC. Its expression in aged HSCs may suggest a propensity toward loss of allelic exclusion in aging and this could affect repertoire. However, we do not believe aberrant allelic exclusion is occurring in our model. B cells retain normal allelic exclusion in bone marrow (6), and alterations in repertoire are detected only in peripheral lymphoid organs, thus supporting a homeostatic mechanism.
In conclusion, our data indicate that maintenance of a naive B-cell repertoire, necessary for generation of protective humoral immunity, is directly dependent on maintenance of LT-HSC B-cell generative capacity. Strategies that focus on replenishing/maintaining functional LT-HSCs in the elderly may be our best hope for improving vaccine efficacy and provide insight for improving therapeutic stem cell transplantation in the elderly. Similarly, use of HSCs of appropriate quality and in appropriate number to reconstitute the follicular B-cell compartment should be a consideration in clinical BM transplantation.
Materials and Methods
Mice.
3-83 μδ tgIg mice on a B10.D2 background, B10.D2 and Rag-2−/− B10.D2 mice were bred and housed in the National Jewish Medical and Research Center animal care facility (Denver, CO). Rag-2−/− BM cells used as filler cells cannot give rise to lymphocytes (31). In all cases, recipient animals were B10.D2 males.
Selection of Immunolgically Aged Mice.
3-83 μδ animals aged ≥18 months were prescreened for signs of immunologic aging via fluorescence-activated cell sorting (FACS) analysis of peripheral blood (PB) as described (6). TgIg positive cells were detected on the basis of staining with the anti-3-83 idiotype antibody 54.1. Except where noted, animals were selected as aged HSC donors only when >60% of B cells present in their peripheral blood expressed enIg (idiotype negative).
Cell Purification.
Bone marrow was flushed from the femurs and tibias of young or aged donor mice into IMDM medium, single-cell suspensions were prepared, and red blood cells lysed using ACK (150 mM NH4Cl, 10 mM KHCO3, 100 mM Na2 EDTA). Depletion of committed lymphocytes from whole BM was accomplished by negative sorting using beads conjugated with antibodies against CD19, IgM, and CD3 (Miltenyi Biotec, Auburn, CA). Depleted BM was examined by FACS to confirm the absence of the desired cell types and injected intravenously into lethally irradiated recipients. Highly purified LT-HSCs were isolated by lineage depletion of whole BM using PE-conjugated antibodies (CD3, CD4, CD5, CD8, B220, CD19, IgM, Gr-1, Mac-1, and Ter119) and anti-PE MACs beads (Miltenyi Biotec). Cells were run through a depleting program on the AutoMACs sorter (Miltenyi Biotec), yielding cell purities of >95%. Lineage-depleted cells were stained with Sca-1 FITC, cKit APC, Flk-2 PE (eBiosciences, San Diego, CA), CD34 PE (BD PharMingen), and Strepavidin PE. Cells were sorted on a MoFLOW cell sorter (Cytomation, Fort Collins, CO), gating out the PE channel and selecting for Sca-1+cKit+. After isolation, cells were washed twice with 1× sterile PBS (Mediatech). Rag2−/− BM carriers (3 × 105 cells per recipient) were mixed with donor LT-HSCs and injected intravenously into lethally irradiated recipients.
5-FU Treatment, Irradiation, and Antibody Treatment.
Young or immunologically aged mice were injected i.p. with 1 mg/100 μl of 5-Fluorouracil (5-FU) and stem-cell-enriched BM harvested 5 d later as described above. BM cells (5 × 105) from 5-FU donors were transferred into young (6–8 weeks old) or aged (>18 months old) lethally irradiated recipients. Recipient mice were lethally irradiated in a split dose 800R + 400R with 3 h between doses and donor cell populations transferred 18 h later. One milligram of anti-IL-7 (clone M25) was injected intravenously into reconstituted young or aged recipients every 3 d for 6 weeks. Untreated mice were sham operated by i.v. injection with 1× PBS (Mediatech).
Antibodies for Cell Surface Staining.
The following Abs (BD PharMingen, San Diego, CA) were used: anti-CD19 (6D5) PE, anti-B220 (RA3–6B2) PE, anti-CD5 (53–7.2) PE, anti-IgM (R6–60.2) PerCp-Cy5.5, anti-CD4 (RM4–5) PE, anti-CD8a (53–6.7), anti-Mac-1/Cd11b (M1/70) PE, anti-GR-1 (RB6–8C5) PE, anti-CD34 (Ram34) PE, anti-CD117/cKit (2B8) APC, and anti-Sca-1 (D7) PE. Also used were anti-IgD (RPE 11–26c) PE (Southern Biotech, Birmingham, AL), anti-Flk-2/CD135 (A2F10) PE (eBiosciences) and anti-3-83 μδ idiotype-alexa647 or -FITC (54.1) (prepared from hybridoma supernatants as described) (Lane, 1998).
Acknowledgments.
The authors acknowledge Sandra Duran for assistance in the preparation of this manuscript. This work was supported by National Institutes of Health (NIH) grant RO1AG013983. L.G. is supported by NIH grant T32 AG00279.
Footnotes
The authors declare no conflict of interest.
References
- 1.LeMaoult J, Szabo P, Weksler M E. Effect of age on humoral immunity, selection of the B-cell repertoire and B-cell development. Immunol Rev. 1997;160:115–126. doi: 10.1111/j.1600-065x.1997.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 2.Bell DR, Van Zant G. Stem cells, aging, and cancer: Inevitabilities and outcomes. Oncogene. 2004;23:7290–7296. doi: 10.1038/sj.onc.1207949. [DOI] [PubMed] [Google Scholar]
- 3.Nicoletti C, Borghesi-Nicoletti C, Yang XH, Schulze DH, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. II. Phosphorylcholine-antibody in young and aged mice differ in both VH/VL gene repertoire and in specificity. J Immunol. 1991;147:2750–2755. [PubMed] [Google Scholar]
- 4.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 5.Weksler ME. Changes in the B-cell repertoire with age. Vaccine. 2000;18:1624–1628. doi: 10.1016/s0264-410x(99)00497-1. [DOI] [PubMed] [Google Scholar]
- 6.Johnson SA, Rozzo SJ, Cambier JC. Aging-dependent exclusion of antigen-inexperienced cells from the peripheral B cell repertoire. J Immunol. 2002;168:5014–5023. doi: 10.4049/jimmunol.168.10.5014. [DOI] [PubMed] [Google Scholar]
- 7.Agenes F, Rosado MM, Freitas AA. Peripheral B cell survival. Cell Mol Life Sci. 2000;57:1220–1228. doi: 10.1007/PL00000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tough DF, Sprent J. Lifespan of lymphocytes. Immuol Res. 1995;14:1–12. doi: 10.1007/BF02918494. [DOI] [PubMed] [Google Scholar]
- 9.Hao Z, Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J Exp Med. 2001;194:1151–1164. doi: 10.1084/jem.194.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koehne G, Zeller W, Stockschlaeder M, Zander AR. Phenotype of lymphocyte subsets after autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1997;19:149–156. doi: 10.1038/sj.bmt.1700624. [DOI] [PubMed] [Google Scholar]
- 11.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 12.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci USA. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson KM, Owen K, Witte PL. Aging and developmental transitions in the B cell lineage. Int Immunol. 2002;14:1313–1323. doi: 10.1093/intimm/dxf092. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Jin F, Freitas A, Szabo P, Weksler ME. Impaired regeneration of the peripheral B cell repertoire from bone marrow following lymphopenia in old mice. Eur J Immunol. 2001;31:500–505. doi: 10.1002/1521-4141(200102)31:2<500::aid-immu500>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Russell DM, Dembic Z, Morahan G, Miller JF, Burki K, Nemazee D. Peripheral deletion of self-reactive B cells. Nature. 1991;354:308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randall TD, Weissman IL. Phenotypic and functional changes induced at the clonal level in hematopoietic stem cells after 5-fluorouracil treatment. Blood. 1997;89:3596–3606. [PubMed] [Google Scholar]
- 17.Serrano F, Varas F, Bernad A, Bueren JA. Accelerated and long-term hematopoietic engraftment in mice transplanted with ex vivo expanded bone marrow. Bone Marrow Transplant. 1994;14:855–862. [PubMed] [Google Scholar]
- 18.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: The paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 20.Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(−)ScaI(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 21.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: A simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Haan G, Van Zant G. Dynamic changes in mouse hematopoietic stem cell numbers during aging. Blood. 1999;93:3294–3301. [PubMed] [Google Scholar]
- 23.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 24.Grabstein KH, Waldschmidt TJ, Finkelman FD, Hess BW, Alpert AR, Boiani NE, Namen AE, Morrissey PJ. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med. 1993;178:257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer A. Thirty years of bone marrow transplantation for severe combined immunodeficiency. N Engl J Med. 1999;340:559–561. doi: 10.1056/NEJM199902183400711. [DOI] [PubMed] [Google Scholar]
- 27.Antin JH, Ault KA, Rappeport JM, Smith BR. B lymphocyte reconstitution after human bone marrow transplantation. Leu-1 antigen defines a distinct population of B lymphocytes. J Clin Invest. 1987;80:325–332. doi: 10.1172/JCI113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JP, Stadanlick JE, Cancro MP. Space, selection, and surveillance: Setting boundaries with BLyS. J Immunol. 2006;176:6405–6410. doi: 10.4049/jimmunol.176.11.6405. [DOI] [PubMed] [Google Scholar]
- 30.Chambers SM, Goodell MA. Hematopoietic stem cell aging: Wrinkles in stem cell potential. Stem Cell Rev. 2007;3:201–211. doi: 10.1007/s12015-007-0027-1. [DOI] [PubMed] [Google Scholar]
- 31.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]