Systemic vasculitis is a heterogeneous group of diseases characterized by inflammation and necrosis of the blood vessel walls. Depending on the size of the involved vessels (small-, medium-, or large-vessel vasculitis), different systems can be affected; patients might, therefore, present with a wide variety of clinical signs and symptoms.1
Henoch-Schönlein purpura (HSP) is a rare nonthrombocytopenic lgA–mediated small-vessel vasculitis of autoimmune hypersensitivity. In 2006 a consensus conference involving important stakeholders established a new set of criteria for the case definition of HSP, outlined in Table 1.2
Table 1. Criteria for case definition of Henoch-Schönlein purpura.
Palpable purpuric eruption and at least one additional criteria are required for diagnosis.
| REQUIRED CRITERION | ADDITIONAL CRITERIA |
|---|---|
| Palpable purpuric eruption | Diffuse abdominal pain |
| Arthritis or arthralgia | |
| Renal involvement (hematuria or proteinuria) | |
| A biopsy showing predominant IgA deposition, mostly in the arterioles, capillaries, and venules of the skin, GI tract, or kidneys |
GI—gastrointestinal.
Adapted from Dillon.2
Henoch-Schönlein purpura mainly affects children, with a male-to-female ratio of 2:1. In children the prognosis is good, as HSP typically resolves rapidly and without complication. In adults and infants younger than 2 years of age, however, HSP tends to have atypical clinical presentations; a higher rate of severe, atypical gastrointestinal problems; and delayed renal complications. Lung and central nervous system presentation is rare. Although the etiology of the vasculitides is unknown, it is postulated that certain triggers act as antigens that induce activation of the alternative complement pathway, while altered genetic markers determine susceptibility to HSP and predict disease severity.3
Case description
A 7-year-old female child had a sore throat and felt unwell. Over the next 24 hours she became febrile and developed nonitchy erythematous spots on her lower extremities. A pediatrician saw her on the third day of her illness. He diagnosed scarlet fever and started her on amoxicillin.
Within 12 hours the rash became more prominent, with distinctly palpable purple-red edematous papules of varying dimensions and configurations. She also developed a nonpruritic, nonpitting, and asymmetrical swelling of the lower third portion of her legs, which extended to her ankles and the dorsum of her feet. The right side was distinctly more swollen and painful to light touch than the left (Figure 1). She quickly developed ankle pain and was not ambulant.
Figure 1.
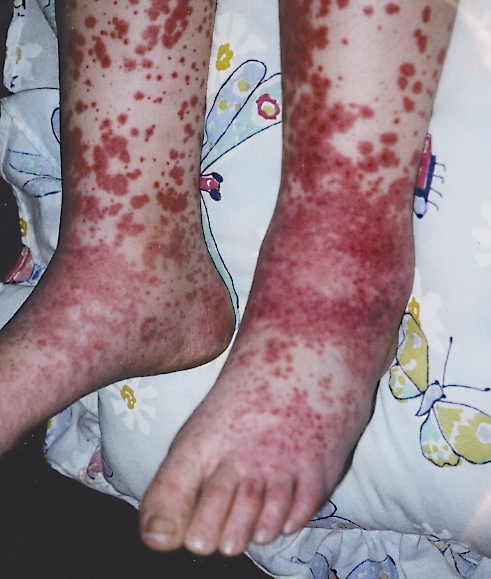
Rash and swelling of the feet caused by Henoch-Schönlein purpura
On arrival at the emergency room, the patient had incapacitating pain in both ankles. She complained of vague gastrointestinal pain but had no diarrhea. The emergency room physician noted the presence of a palpable purpuric eruption with swelling around both ankles. The lesions measured between 1 and 10 cm in diameter. The child’s vital signs, including blood pressure, were normal.
Except for a slightly elevated monocyte count of 1.04 × 109/L (normal monocyte count is 0.05 to 0.80 × 109/L) and slightly elevated bands at 0.07 × 109/L (normal range from 0.00 to 0.01 × 109/L), the complete blood count, including platelets, was normal. The urinalysis showed mild microscopic hematuria. Her blood sugar, creatinine, electrolyte, and blood gas levels were normal. She was discharged 8 hours later with a diagnosis of HSP, and placed in the care of her pediatrician.
Within 5 days, the swelling and pain in both ankles subsided. The patient was fully ambulatory within 2 weeks and was able to resume school within 15 days of the onset of the illness. Within 3 weeks, the rash had completely subsided. Results of follow-up urinalyses, at 1 and 2 weeks postdischarge from the emergency room, were normal.
Discussion
This case differs from those reported in the literature in that it had a rapid and dramatic onset (2 days instead of the usual 1 to 3 weeks), without any major prodromal symptoms and without known triggers or associations such as streptococcal or viral infection. The early maculoerythematous rash, which lasted 12 to 18 hours before the appearance of the palpable purpura, led to the erroneous diagnosis of scarlet fever and the prescribing of antibiotics.
Together with the rash, the rapid development of nonpitting edema and disabling arthralgia in both ankle joints was the dominant feature of the illness. The resolution of the clinical picture was equally dramatic (within 7 days instead of the usual 3 to 6 weeks), and was without recurrences or sequelae.
The unusual presentation and course of this case prompted me to conduct a MeSH literature search. The search included the term purpura, Schöenlein-Henoch and all its variant names. Those terms were then combined with each of the following adjuncts: prevalence, diagnosis, and management. Additional specific searching combined the general term Schöenlein-Henoch purpura with the following types of research: clinical trial, meta-analysis, randomized controlled trial, and reviews.
Epidemiology
The literature in Canada on HSP is almost entirely confined to case reports.4–11 Epidemiologic data are available only from the United States,12 Europe,13 and the Middle East.14,15 All literature indicates that the incidence and prevalence of HSP varies by age, sex, country, ethnicity, and season; there are also important differences in the epidemiologic and clinical features of HSP in adults compared with those in children.
Clinical picture
When there is palpable purpura, gastrointestinal symptoms, and arthralgia, with or without hematuria or proteinuria, the diagnosis of HSP is relatively simple. Atypical presentations, severe and varied complications, and recurrences are more common in adults and children younger than 2 years of age.
Dermatologic findings
The typical rash of HSP is palpable and purpuric, and has a distinctive distribution and morphology. It initially appears as maculopapular erythematous lesions, 1 to 10 mm in diameter, which quickly coalesce into palpable, purpuric, and ecchymotic lesions.
An atypical cutaneous manifestation might be mistaken for papular urticaria, systemic lupus erythematosus, meningococcemia, and dermatitis herpetiformis.11 It also might be mistaken for acute hemorrhagic edema of infancy, which many experts consider to be a variant of HSP; it presents with purpuric lesions, affecting the face, ears, extremities, and scrotum in infants younger than 2 years of age who are otherwise nontoxic with normal serologic studies. Acute hemorrhagic edema can also be confused with child abuse.16 Because of the various differential diagnoses, a skin biopsy is often needed to confirm the diagnosis of HSP in children younger than 2 years old and in adults.17
Gastrointestinal findings
Periumbilical pain, vomiting, and abdominal distension are usually mild and rarely severe enough to be confused with an acute abdomen leading unnecessarily to laparotomy. Rare gastrointestinal manifestations include intestinal perforation, pancreatitis, and pseudomembranous colitis requiring endoscopic evaluation.18
Arthritis or arthralgia findings
The arthritis or arthralgia associated with HSP are nonmigratory, transient, and nondestructive, and usually involve the ankles and knees. The pain responds readily to nonsteroidal anti-inflammatory agents. In 25% of cases, the arthritis or arthralgia precede the appearance of the rash.19
Renal findings
Renal involvement rarely precedes the appearance of the purpura and usually occurs within the first 3 weeks of the illness. It ranges from isolated microscopic hematuria, proteinuria, or nephriticnephrotic syndrome (> 3 g/24 h in adults and > 40 mg/m2/h in children) to acute, rapidly progressive glomerulonephritis. It can be associated with either high blood pressure or renal failure, or both. Depending on the diagnostic criteria, the frequency of renal involvement varies from 20% to 100%. (More severe prognoses are reported in nephrologic research series.) Overall, an estimated 2% of children with HSP experience renal failure. In one study, the frequency of chronic kidney disease in adults was estimated to be 1% and that of end-stage renal disease to be < 1%. The frequency of renal failure was reported to be 10% to 20% in adults and 1% in children.20
Other rare manifestations and complications
Involvement of the central and peripheral nervous system is rare. Pulmonary complications are also rare. Henoch-Schönlein purpura tends be more severe when associated with familial Mediterranean fever or thalassemia major. Genital involvement is usually confined to scrotal and perineal edema, but rarely testicular torsion; priapism, secondary to thrombosis of the dorsal penile vein, can occur. During pregnancy the third trimester can be complicated by pregnancy-induced hypertension, pre-eclampsia, and eclampsia.
Laboratory investigations
Results of most of the routine laboratory investigations remain within normal limits. A normal platelet count rules out idiopathic thrombocytopenic purpura. A normal platelet count and normal coagulation study results rule out thrombotic thrombocytopenic purpura. A normal lipase level makes acute pancreatitis unlikely. Antinuclear factor testing, serum immunoelectrophoresis, antineutrophil cytoplasm antibody testing, complement C3 and C4 testing, and genetic typing are performed only when overlap syndromes are suspected.
Treatment
Most patients with HSP require only supportive care. Analgesics and nonsteroidal anti-inflammatory drugs can relieve joint and soft tissue discomfort. Corticosteroid therapy does not implicatively alter the likelihood of recurrence; however, if given early on in the course of the illness, corticosteroids appear to produce consistent benefits for several clinically relevant HSP outcomes.21
The results of treatment with other immunosuppressive drugs (eg, cyclophosphamide, cyclosporine, azathioprine), with or without corticosteroids or prednisolone pulse therapy, are contradictory. There are several prospective studies under way, which are testing different combinations of immunosuppressants and immunomodulators.3
Points to take home
Henoch-Schönlein purpura is a rare, systemic, non-thrombocytopenic vasculitis, affecting mostly children between the ages of 2 and 10 years old. In this age group, the outcome is almost always excellent and requires only supportive care.
In other age groups atypical presentations are common. Renal complications and, to a lesser extent, gastrointestinal, pulmonary, and neurologic complications can be severe and difficult to diagnose without a biopsy of the affected system.
Most epidemiologic studies were performed in Europe and the Middle East. They demonstrate global variability in incidence, prevalence, and risk factors. The outcomes seem to depend on the prevalence of local congenital diseases. This genetic polymorphism might hold the key to a better “understanding of the predisposing and protective factors in relation to complication.”3
The treatment results of HSP complications are controversial and contradictory. Several prospective, double-blind studies are under way to clarify this issue.
Acknowledgment
I thank Mr David Le Sauvage at the Canadian Library of Family Medicine for his thorough, enthusiastic, and diligent help with the literature search for this article.
EDITOR’S KEY POINTS
In its typical form, Henoch-Schönlein purpura presents with palpable purpura, arthralgia or arthritis, and mild gastrointestinal symptoms. Twenty-five percent of children will have symptoms in the joints before development of the rash.
Renal involvement (eg, proteinuria or hematuria) generally occurs after the rash develops. A small percentage of those affected (eg, 2% of children) will develop renal failure.
Adults and children younger than 2 years of age are more likely to present with atypical symptoms and signs, as well as severe complications.
In typical cases, investigations are minimal and often include platelet count, coagulation studies, and lipase tests to rule out pancreatitis.
Treatment is usually supportive, with analgesics and nonsteroidal anti-inflammatory drugs. Corticosteroids are often used in severe cases.
POINTS DE REPÈRE DU RÉDACTEUR
Dans sa forme habituelle, la maladie de Henoch-Schönlein se présente avec un purpura palpable, de l’arthralgie ou de l’arthrite et de légers symptômes gastro-intestinaux. Chez 25% des enfants, les symptômes aux articulations apparaissent avant le rash.
Les symptômes rénaux (p. ex. protéinurie ou hématurie) se produisent généralement après l’apparition du rash. Un faible pourcentage des personnes atteintes (p. ex. 2% des enfants) développeront une insuffisance rénale.
Les adultes et les enfants de < 2 ans sont plus susceptibles de présenter des symptômes et des signes atypiques et d’avoir des complications graves.
Dans les cas typiques, les investigations nécessaires sont minimes et comportent souvent la numération plaquettaire, des études de coagulation, ainsi qu’une analyse de la lipase pour écarter la possibilité d’une pancréatite.
On administre habituellement un traitement de soutien au moyen d’analgésiques et d’anti-inflammatoires non stéroïdiens. Les corticostéroïdes sont souvent utilisés dans les cas graves.
Footnotes
Competing interests
None declared
Cet article a fait l’objet d’une révision par des pairs.
This article has been peer reviewed.
References
- 1.D’Arsigny C. Small vesselvasculitis and the pulmonary-renal syndrome. [Accessed 2008 Jun 11];Ont Thoracic Rev. 2006 18(3):1, 4–7. Available from: www.on.lung.ca/Health-Care-Professionals/Ontario-horacic-Society/downloads/OTR-Fall2006-Vol18-No03.pdf.
- 2.Dillon MJ. Henoch-Schönlein purpura: recent advances. Clin Exp Rheumatol. 2007;25(1 Suppl 44):S66–8. [PubMed] [Google Scholar]
- 3.Brogan PA. What’s new in the aetio-pathogenesis of vasculitis? Pediatr Nephrol. 2007;22(8):1083–94. doi: 10.1007/s00467-007-0450-1. Epub 2007 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer JI. Acute testicular pain: Henoch-Schönlein purpura versus testicular torsion. Pediatr Emerg Care. 1922;8(1):51–3. doi: 10.1097/00006565-199202000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Gow KW. Multiple entero-entero fistulae: an unusual complication of Henoch-Schönlein purpura. J Pediatr Surg. 1996;31(6):809–11. doi: 10.1016/s0022-3468(96)90139-6. [DOI] [PubMed] [Google Scholar]
- 6.Bissonnette R. Perforation of large and small bowel in Henoch-Schonlein purpura. Int J Dermatol. 1997;36(5):361–3. doi: 10.1111/j.1365-4362.1997.tb03098.x. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen-Ho P. Hemorrhagic intestinal Henoch-Schönlein purpura complicated by cytomegalovirus infection. Can J Gastroenterol. 1998;12(1):71–4. doi: 10.1155/1998/896945. [DOI] [PubMed] [Google Scholar]
- 8.Woolfenden AR. Encephalopathy complicating Henoch-Schönlein purpura: reversible MRI changes. Pediatr Neurol. 1998;19(1):74–7. doi: 10.1016/s0887-8994(98)00027-7. [DOI] [PubMed] [Google Scholar]
- 9.Karagozian R. Henoch-Schonlein purpura presenting with ileal involvement in an adult. Dig Dis Sci. 2004;49(10):1722–6. doi: 10.1023/b:ddas.0000043392.64347.ea. [DOI] [PubMed] [Google Scholar]
- 10.Leung AK. Hemorrhagic bullous lesions in a child with Henoch-Schönlein purpura. Pediatr Dermatol. 2006;23(2):139–41. doi: 10.1111/j.1525-1470.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 11.Freiman A. Dermacase. Henoch-Schönlein purpura. Can Fam Physician. 2005;51:511–2. [PMC free article] [PubMed] [Google Scholar]
- 12.Saulsbury FT. Epidemiology of Henoch-Schönlein purpura. Cleve Clin J Med. 2002;69(Suppl 2):SII87–9. doi: 10.3949/ccjm.69.suppl_2.sii87. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen HE. Epidemiology of Schönlein Henoch-purpura. Acta Paediatr Scand. 1988;77(1):125–31. doi: 10.1111/j.1651-2227.1988.tb10610.x. [DOI] [PubMed] [Google Scholar]
- 14.Akl K. Childhood Henoch Schonlein purpura in Middle East countries. [Accessed 2008 Jun 11];Saudi J Kidney Dis Transpl. 2007 18(2):151–8. Available from: www.sjkdt.org/article.asp?issn=1319-2442;year=2007;volume=18;issue=2;spage=151;epage=158;aulast=Akl. [PubMed]
- 15.Gershoni-Baruch R. Prevalence and significance of mutations in the familial Mediterranean fever gene in Henoch-Schönlein purpura. J Pediatr. 2003;143(5):658–61. doi: 10.1067/S0022-3476(03)00502-X. [DOI] [PubMed] [Google Scholar]
- 16.Crowe MA. Acute hemorrhagic edema of infancy. Omaha, NE: eMedicine; 2006. [Accessed 2008 Jun 11]. Available from: www.emedicine.com/derm/topic613.htm. [Google Scholar]
- 17.Gedalia A. Henoch-Schönlein purpura. Curr Rheumatol Rep. 2004;6(3):195–202. doi: 10.1007/s11926-004-0068-2. [DOI] [PubMed] [Google Scholar]
- 18.Akbar DH. Fatal complication of Henoch-Schonlein purpura: case report and literature review. [Accessed 2008 Jun 11];Saudi J Gastroenterol. 2000 6(3):165–8. Available from: www.saudijgastro.com/article.asp?issn=1319-3767;year=2000;volume=6;issue=3;spage=165;epage=168;aulast=Akbar. [PubMed]
- 19.Saulsbury FT. Henoch-Schönlein purpura in children. Report of 100 patients and review of the literature. Medicine. 1999;78(6):395–409. doi: 10.1097/00005792-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Calviño MC. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine. 2001;80(5):279–90. doi: 10.1097/00005792-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Weiss PF. Corticosteroid efficacy in Henoch Schönlein purpura: a systematic review. [Accessed 2008 Jul 9];Pediatrics. 2007 120(5):1079–87. doi: 10.1542/peds.2007-0667. Available from: www.pediatric-generalists.org/files/weiss/Corticosteroid_Efficacy_HSP.doc. [DOI] [PMC free article] [PubMed] [Google Scholar]


