Abstract
The low Ca2+ concentration of mammalian endolymph in the inner ear is required for normal hearing and balance. We reported [Yamauchi et al. Biochem Biophys Res Commun, 2005] that the epithelial Ca2+ channels TRPV5 and TRPV6 are expressed in the vestibular system and that TRPV5 expression is stimulated by 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), as also reported in kidney. TRPV5/6 channels are known to be inhibited by extracellular acidic pH. Endolymphatic pH, [Ca2+] and transepithelial potential of the utricle (UP) were measured in Cl-/ exchanger pendrin (SLC26A4) knockout mice in vivo. Slc26a4-/- mice exhibit reduced pH and UP and increased [Ca2+]. Monolayers of primary cultures of rat semicircular canal duct (SCCD) cells were grown on permeable supports and cellular uptake of 45Ca2+ was measured individually from the apical and basolateral sides. Net uptake of 45Ca2+ was greater after incubation with 1,25(OH)2D3. Net 45Ca2+ absorption was dramatically inhibited by low apical pH and was stimulated by apical alkaline pH. Gadolinium, lanthanum and ruthenium red reduced apical uptake. These observations support the notion that one aspect of vestibular dysfunction in Pendred syndrome is a pathological elevation of endolymphatic [Ca2+] due to luminal acidification and consequent inhibition of TRPV5/6-mediated Ca2+ absorption.
Keywords: Epithelial Ca Channel, vitamin D, SLC26A4, HCO3- secretion, TRPV5, TRPV6
INTRODUCTION
Pendrin (PDS or SLC26A4) is an anion exchanger that is capable of transporting iodide, chloride, formate, nitrate and bicarbonate and functions as a -secreting mechanism in kidney (30; 31; 33). SLC26A4 is expressed in kidney (29), the inner ear, thyroid (7), mammary gland (26), uterus (34), testis (16), vas deferens (3) and placenta (2). Mutation or deletion of the SLC26A4 gene leads to acidification of the urine (15) and to Pendred syndrome, which is an autosomal recessive disorder characterized by sensorineural hearing loss and goiter (21; 25).
The availability of SLC26A4 knockout mice (Slc26a4-/-) makes it possible to perform a direct study of the inner ear defects that occur in the absence of SLC26A4 (6). Slc26a4-/- mice are completely deaf and also display signs of vestibular dysfunction (6). In the vestibular system, pendrin is expressed in the apical membrane of vestibular transitional cells in the utricle and ampullae (40). If SLC26A4 secretes in the vestibule, and if there are no strong compensatory mechanisms, it can be predicted that there may be an acidification of endolymph when SLC26A4 is deleted. An altered endolymphatic pH can be expected to affect other ion transport processes in the luminal membrane of epithelial cells bordering the lumen, since several ion channels are known to be highly sensitive to extracellular pH.
The [Ca2+] of vestibular endolymph (ca. 250 μM) is lower than perilymph (ca. 1 mM) and it has a critical role in sensory transduction through hair cells (20). A Ca2+ absorption system in inner ear epithelial cells must be present to maintain the low [Ca2+] of vestibular endolymph. Recently, we reported that the epithelial Ca2+ channels TRPV5 and TRPV6 are expressed in the semicircular canal duct (SCCD) of the vestibular system and expression of TRPV5 is regulated by 1,25(OH)2D3, as in some other systems (9; 36; 44). TRPV5 and TRPV6 belong to the transient receptor potential (TRP) family of channels and are the only two highly Ca2+-selective TRP channels (12; 22). TRPV5 is expressed in specific tissues such as kidney, placenta and bone and plays a major role in Ca2+ transport and is localized at the apical membrane of epithelial cells or at the ruffled border membrane of osteoclasts (10; 11; 37). Recently, the molecular mechanisms of TRPV5/6 inhibition by both intra- and extracellular acidic pH were reported (45).
Due to the presence of the TRPV5/6 Ca2+-absorptive pathway in the vestibular system and its known inhibition by extracellular acid in other systems, we hypothesized that part of the vestibular dysfunction observed in Pendred syndrome may be due to an acidification of endolymph (loss of secretion), which in turn would lead to an elevation of luminal [Ca2+]. We therefore sought to measure the luminal pH and [Ca2+] in wild type (Slc26a4+/+), heterozygous (Slc26a4+/-) and knockout (Slc26a4-/-) mice and to determine if Ca2+ uptake across the apical and basolateral membranes of vestibular Ca2+-absorbing epithelia were sensitive to apical acidification and to known TRPV5/6 blockers.
We indeed observed a reduced pH and increased [Ca2+] in the vestibular lumen of Slc26a4-/- mice. Further, 1,25(OH)2D3 increased 45Ca2+ absorption and TRPV5/6 inhibitors reduced apical uptake of 45Ca2+. 45Ca2+ absorption was inhibited by apical acid pH and was stimulated by apical alkaline pH, consistent with the notion that one aspect of vestibular dysfunction in Pendred syndrome is a pathological elevation of endolymphatic [Ca2+] due to luminal acidification and consequent inhibition of TRPV5/6-mediated Ca2+ absorption.
METHODS
Utricular endolymphatic potential (UP), pH and [Ca2+]
Adult Slc26a4-/- and Slc26a4+/- mice were obtained from a colony at Kansas State University that was established with breeders kindly provided by Dr. Susan Wall. The mouse strain 129Sv/Ev (Taconic, Germantown, NY) was used as the source of Slc26a4+/+ mice, since Slc26a4-/- mice were generated in this background. Young adult mice 30-142 days old were deeply anesthetized with Tribromoethanol (640 mg/kg i.p.; Fluka 90710) in 0.9% NaCl. The Institutional Animal Care and Use Committee of Kansas State University approved all experimental protocols.
UP, pH and [Ca2+] were measured with double barreled microelectrodes (one side pH- or Ca2+- sensitive and the other for voltage; see below for details) using procedures developed by modifying previously described protocols (18; 19). For both pH and Ca2+ electrodes, two pieces of glass tubing containing a glass filament (World Precision Instruments 1B100F-4, Sarasota, FL) were cut to 81 mm and 60 mm and pulled using a micropipette puller (Narishige PD-5, Tokyo, Japan). After heating pulled capillaries at 180 °C for 2 h, the ion-selective barrel was silanized by placing the open end of that barrel through a hole in the lid of a beaker at 210 °C in which we put 0.08 ml Dimethyldichlorosilane (Fluka 40136) for 90 s. The reference barrel was protected from silanization by sealing the open end with Parafilm (Alcan Packaging). After heating electrodes at 180 °C for 3 h, the tips were broken to ~3 μm OD.
For pH electrodes, the reference barrel was filled with 1 M KCl and the ion-selective barrel was filled at the tip with Hydrogen ionophore II - cocktail A (Fluka 95297) and back-filled with buffer solution (KCl 500 mM, HEPES 20 mM, pH7.34). For Ca2+ electrodes, the reference barrel was filled with 150 mM KCl and the ion-selective barrel was filled at the tip with Calcium ionophore I - cocktail A (Fluka 21048) and back-filled with 500 mM CaCl2. Connection to each barrel was made with a Ag-AgCl wire. Each electrode was connected to an input of a dual electrometer (World Precision Instruments FD223) and buffered outputs were led to a Data Acquisition System (Axon Instruments DIGIDATA 1322A) and recorded with AxoScope 9 (Axon Instruments). A pulse generator was used to inject current pulses (1 nA) through the reference barrel to monitor the resistance of the electrode.
pH electrodes were calibrated at three different pH values (composition in mM): pH 6 (130 NaCl, 20 MES), pH 7 (130 NaCl, 20 HEPES) and pH 8 (130 NaCl, 20 Tricine) and had an average slope of 56.6 ± 0.7 mV/pH (n=16). Ca2+ electrodes were calibrated at three Ca2+ concentrations (composition in mM): 10 μM Ca2+ (0.121 CaCl2, 150 KCl, 10 HEPES, 1 C6H7NO6Na2 (Nitrilotriacetic acid; Sigma N-0128), pH 7.4), 100 μM Ca2+ (0.1 CaCl2, 150 KCl, 10 HEPES, pH7.4) and 1 mM Ca2+ (1 CaCl2, 150 KCl, 10 HEPES, pH 7.4) and had an average slope of 26.2 ± 0.8 mV/decade concentration (n=13). Data were analyzed using custom software written by P.W. in LabTalk (Origin 6.0, OriginLab, Northampton, MA).
To reduce the diffusion of CO2 through the exposed surface of the perilymph to the ambient air, which leads to higher pH compared to unexposed perilymph, we put liquid Sylgard 184 (Dow Corning Corporation, Midland, MI) at the fluid surface after placing the electrodes in the perilymph. The electrode was maintained below the surface of the perilymph solution after the measurement of inner ear fluids and the first calibration point was taken in situ in the bulla pocket of the temporal bone. The pH 7 buffer for calibration was placed on the Sylgard layer and the electrode retracted up into this buffer from the perilymph surrounding the utricle. Approximately 85% of the electrodes survived the travel through the Sylgard layer; outcomes from experiments in which electrodes failed this maneuver were excluded from the data set. The remaining calibration points to determine slope sensitivity were obtained by placing calibration solutions in a small conducting Ringer- agarose (Fisher Scientific, BP164) cup that rested on the exposed neck muscles. This procedure was used to preclude voltage offsets introduced through movement of the reference electrode. In some cases the electrode failed after moving to the calibration cup. In these few cases, the pre-experiment electrode slope was used in conjunction with the in situ calibration point. Calibration of Ca2+ electrodes was identical except that the first point was taken by flushing the perilymph space with 1 mM Ca2+ solution and raising the electrode into a region of the pool remote from the tissues.
Perilymph pH, [Ca2+] and UP were corrected for the liquid junction potentials of 1.3 mV (pH electrode) and 4 mV (Ca2+ electrode) between the voltage barrels in calibrating solution and in perilymph and between the voltage electrode in perilymph and endolymph.
Cellular uptake measurements
Primary cultures of Wistar rat SCCD were prepared and incubated in DMEM/F-12 (Invitrogen, #12500-062) supplemented with 5% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml) as described previously (44). Primary cultures of SCCD cells from rat were used to study transport function since murine SCCD cells have been observed to not proliferate in culture. Briefly, SCCD epithelial cells from neonatal rats were cultured on 12 mm diameter Snapwell permeable supports (0.4 μm pore, Costar 3801, Corning, NY). Confluence of primary cultures (10-14 days after seeding) was verified visually and by measurement of transepithelial electrical resistance using an Endohm meter (World Precision Instruments). Cultures were incubated further for 24 h in the presence or absence of 1,25(OH)2D3 (DM-200, Biomol, Plymouth Meeting, PA) (44).
The method for cellular 45Ca2+ uptake determination was modified from Den Dekker et al. (5). Cells were washed twice on both sides with pre-warmed Ca2+-free solution (in mM: 145 NaCl, 4 KCl, 1 MgCl2, 20 HEPES, 5 glucose, pH 7.4). Cells were then incubated in this medium at 37 °C and 5% CO2 for 45 – 60 min prior to treatment. Cells tested with Ca2+ channel blockers or different pH buffers had the apical medium changed after 45 minutes of pre-treatment, allowing 15 min incubation with the appropriate blocker or pH. The blocker medium was Ca2+-free solution with an added mixture of known inhibitors of TRPV5/6 (10 μM Ruthenium Red, 100 μM LaCl3 and 100 μM GdCl3) or each blocker in the same concentration by itself. The various pH buffers were similar to Ca2+-free solution except the pH 5.5 contained 20 mM MES as a buffer in place of HEPES and the pH of each was adjusted with HCl or NaOH as appropriate. The pre-treatment buffer on either the apical or basolateral side was replaced with 45Ca2+ uptake buffer (in mM: 145 NaCl, 4KCl, 1 MgCl2, 0.1 CaCl2, 20 HEPES, 5 glucose with 10 μM Verapamil, pH 7.4 and at least 1 μCi 45Ca2+/ml) for 15 min. Verapamil was utilized to preclude measuring Ca2+ uptake via L-type Ca2+ channels, which play important roles in some epithelia (27). Verapamil at the concentration used here is sufficient for a virtually complete block of Ca2+ current through these channels (13). The radiotracer was purchased as 1 mCi (37 MBq) 45Ca2+ as CaCl2 in 17 μl H2O (Perkin Elmer Life Sciences, Boston, #NEZ013001MC).
The cells were then rinsed 3 times on both sides with ice cold wash buffer (in mM: 145 NaCl, 4KCl, 1 MgCl2, 0.5 CaCl2, 1.5 LaCl3, 20 HEPES, 5 glucose, pH 7.4). The wash buffer was removed from both compartments and 200 μL of Lysis buffer (Lysis Reagent 1 from cAMP kit RPN225, Amersham Biosciences, Piscataway NJ) was added to the apical compartment. After 5 minutes the lysate was collected and radioactivity was determined with a liquid scintillation counter (Packard Tri-Carb 2100TR, Meriden, CT). The difference in uptake of 45Ca2+ from the apical and basolateral sides was taken as a relative measure of net flux.
Statistics
Data are presented as means ± SE from n observations. Net uptakes are the difference of the mean apical and basolateral uptakes; the SE was pooled from the variance of the apical and basolateral uptake data, as calculated from the variances with the Java applet at http://home.ubalt.edu/ntsbarsh/Business-stat/otherapplets/Pooled.htm. Significance between the UP, pH and Ca2+ control and knockout mouse data and the significance of Ca2+ uptake data were calculated with an unpaired t-test. The comparison of endolymph values to the corresponding perilymph values (* within bars, Fig. 2) were performed with a paired t-test. P < 0.05 represents a significant difference.
Figure 2.
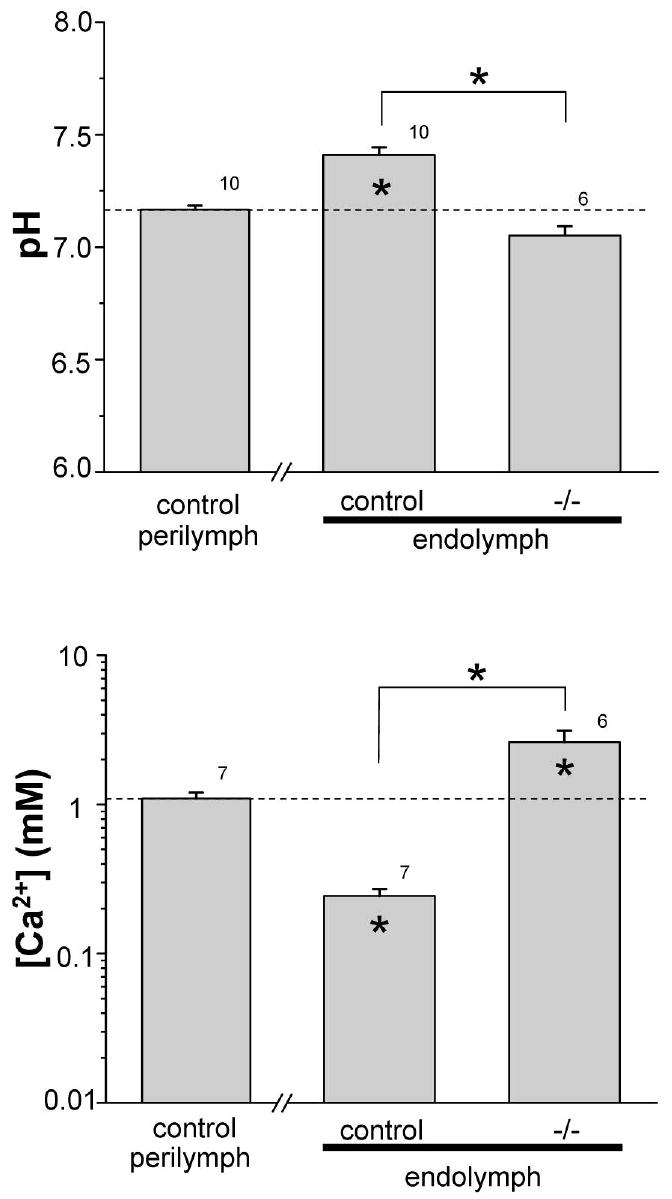
Endolymphatic pH and [Ca2+]. (Top panel) endolymphatic pH of control and knockout (-/-) genotypes compared to control perilymphatic pH (dotted line). (Bottom panel) endolymphatic [Ca2+] of control and knockout (-/-) genotypes compared to control perilymphatic pH (dotted line). * inside bars, P < 0.05 versus perilymphatic pH or [Ca2+]; * above bars, P < 0.05. Number of measurements at each bar.
RESULTS
UP, pH and [Ca2+] in the utricle
Across all measurements, Slc26a4+/- mice showed no significant differences in UP, pH and [Ca2+] compared with Slc26a4+/+ mice, consistent with an autosomal recessive trait. There were no differences between Slc26a4+/+ mice compared with Slc26a4+/- mice in the transepithelial potential (UP; -1.5 ± 0.5 mV (n=8) vs -1.5 ± 0.3 mV (n=9)), perilymphatic pH (7.14 ± 0.02 (n=5) vs 7.19 ± 0.02 (n=5)), endolymphatic pH (7.42 ± 0.02 (n=5) vs 7.40 ± 0.06 (n=5)), perilymphatic [Ca2+] (1.62 ± 0.10 mM (n=3), vs 1.51 ± 0.28 mM (n=4)), endolymphatic [Ca2+] (266 ± 51 μM (n=3) vs 230 ± 28 μM (n=4)). The data and the SE were pooled between the two genotypes and taken as “control” reference values against which the knockout mice were evaluated.
There was a small, but statistically significant, decrease of about 3 mV in the UP of slc26a4-/- mice compared to control mice (Fig. 1). No significant differences between the control and knockout mice were observed in perilymphatic pH and [Ca2+] (Fig. 1). The pH was higher in endolymph of control mice than in the perilymph, consistent with secretion of by pendrin. Endolymphatic pH was significantly decreased in Slc26a4-/- mice, compared with control mice (Fig. 2). Further, endolymphatic [Ca2+] was less than in perilymph of control mice and was significantly increased in Slc26a4-/- mice to a level higher than in perilymph (Fig. 2). These abnormalities of endolymphatic potential, pH and [Ca2+] in Slc26a4-/- mice are compatible with their vestibular dysfunction, exhibited as circling behavior and head-tilting (6).
Figure 1.
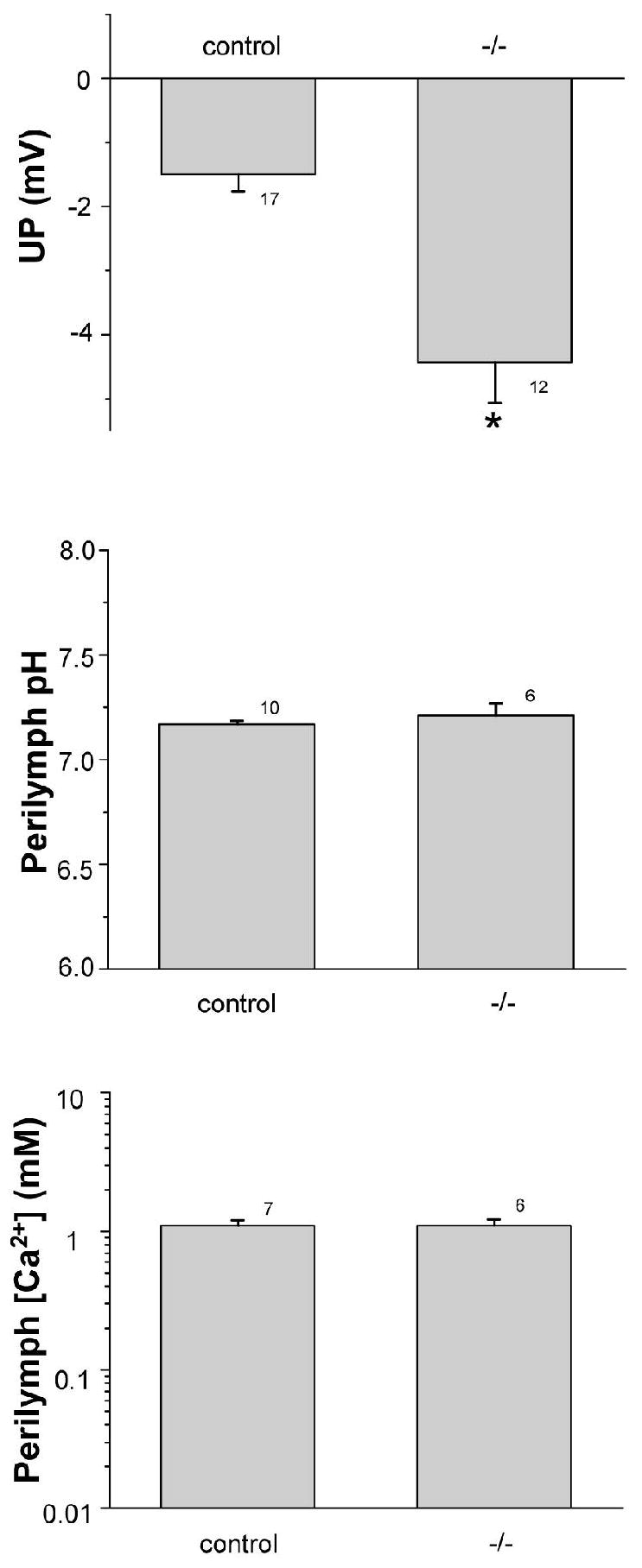
Utricular endolymphatic potential (UP), perilymphatic pH and [Ca2+]. (Top panel) UP of pooled (Control) wild-type (Slc26a4+/+; n=8) and heterozygous (Slc26a4+/-; n=9) and of knockout (Slc26a4-/-) mice. *, P < 0.05 . (Middle panel) perilymphatic pH of control and knockout mice; no significant difference (P > 0.05). (Bottom panel) perilymphatic [Ca2+] of control and knockout mice; no significant difference (P > 0.05). Number of measurements at each bar.
The high endolymphatic [Ca2+] in Slc26a4-/- mice would be consistent with altered otoconia, as reported earlier (41). Indeed we consistently observed an absence of the normal otoconia but observed a single giant crystal (Fig. 3), assumed to be CaCO3, which is the compound of calcium observed in otoconia.
Figure 3.
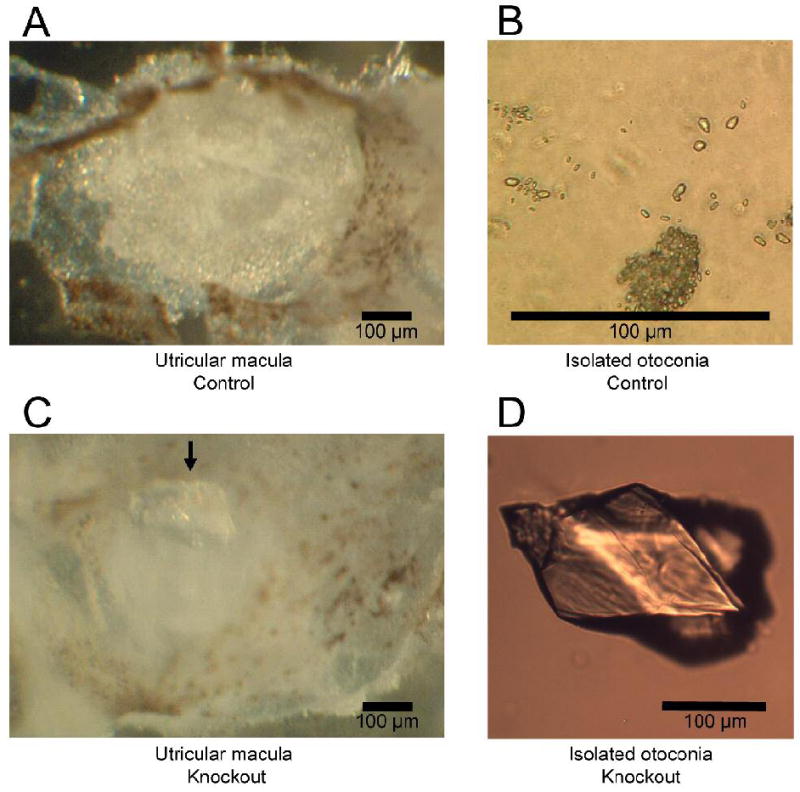
Otoconia from Control (Slc26a4+/-) and knockout (Slc26a4-/-) mice. A: Normal otoconia situated in Utricle from Slc26a4+/- mouse. B: Normal otoconia isolated from Slc26a4+/- utricle at higher magnification. C: Utricle from Slc26a4-/- mouse with giant crystal (arrow). D: Giant crystal isolated from Slc26a4-/- utricle at higher magnification.
Increased Ca2+ absorption by 1,25(OH)2D3
We tested whether Ca2+ absorption is stimulated by exposure to vitamin D since transcript expression of two genes involved in epithelial Ca2+ absorption, TRPV5 and calbindin, is upregulated (44). Stimulation of transport would therefore serve as part of the fingerprint for the involvement of this Ca2+ transport system in vestibular function.
Apical-to-cell 45Ca2+ uptake and basolateral-to-cell 45Ca2+ uptake in primary SCCD cells were measured separately. Net uptake was obtained by subtraction of the mean basolateral-to-cell 45Ca2+ uptake from the mean apical-to-cell 45Ca2+ uptake. Without any added inhibitors of TRPV5/6, cultured cells exhibited a net 45Ca2+ uptake consistent with an absorptive flux from the apical to the basolateral side (Fig. 4). This direction corresponds to endolymph (normally, low [Ca2+]) to perilymph (normally, high [Ca2+]). Incubation with 1,25(OH)2D3 for 24 h significantly increased net Ca2+ uptake by 71% (Fig. 4).
Figure 4.
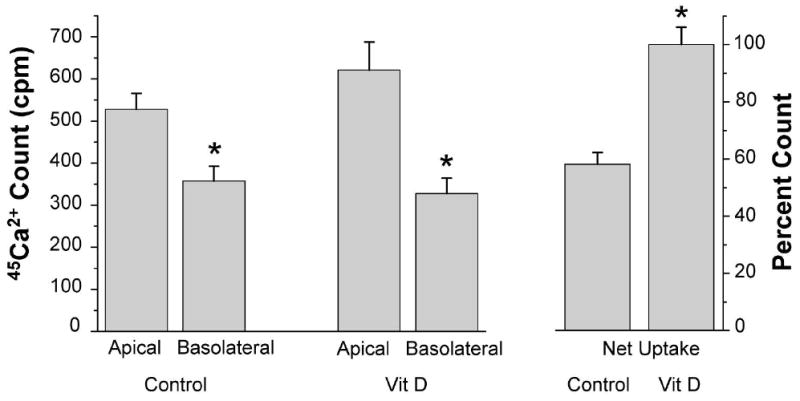
Upregulation of 45Ca2+ uptake by 1,25(OH)2D3 across SCCD cells grown on permeable supports. (left) apical-to-cell 45Ca2+ uptake (Apical) and basolateral-to-cell 45Ca2+ uptake (Basolateral) of cells incubated with and without 1,25(OH)2D3 (Vit D, 100 nM, 24 hr). (right) net 45Ca2+ uptake was calculated by subtraction of the mean basolateral-to-cell 45Ca2+ uptake from the mean apical-to-basolateral 45Ca2+ uptake; SEM was pooled. Net 45Ca2+ uptake of cells with 1,25(OH)2D3 was regarded as 100% (n = 4). Uptakes are expressed as count per minute (cpm). * P < 0.05.
Regulation by pH
The pH of the apical solution was varied over a wide range to test the dependence of Ca2+ uptake on apical pH of the SCCD cells. Decreases of the apical pH from 8.2 to 7.4 and 5.5 each significantly reduced net 45Ca2+ uptake by 37% and 96%, respectively (Fig. 5).
Figure 5.
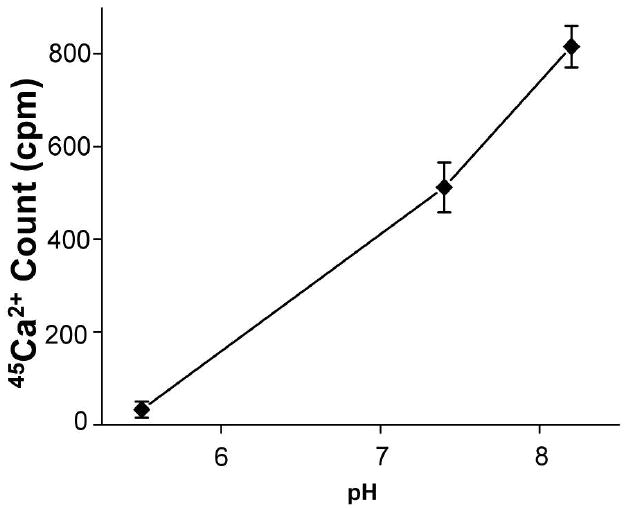
Net 45Ca2+ uptake under the different apical pH. Measured after a 15 minute incubation with the pH of 5.5, 7.4, 8.2. All cells were incubated with 1,25(OH)2D3 (100 nM, 24 hr) before the measurement (n = 6). Uptakes are expressed as count per minute (cpm).
Inhibition of Ca2+ uptake by inhibitors
Adding a mixture of agents known to block TRPV5 and TRPV6 Ca2+ channels (in μM: 10 ruthenium red, 100 LaCl3, 100 GdCl3) 15 min after 1,25(OH)2D3 treatment stopped the net uptake completely (Fig. 6-A). Both apical-to-cell and basolateral-to-cell uptakes were significantly reduced (Fig. 6-A). Since the mixture of Ca2+ inhibitors completely blocked Ca2+ net uptake, individual blockers were examined. 10 μM ruthenium red significantly decreased net Ca2+ uptake by 31% (Fig. 6-B). 100 μM LaCl3 and 100 μM GdCl3 showed stronger effects than ruthenium red (Fig. 6-C).
Figure 6.
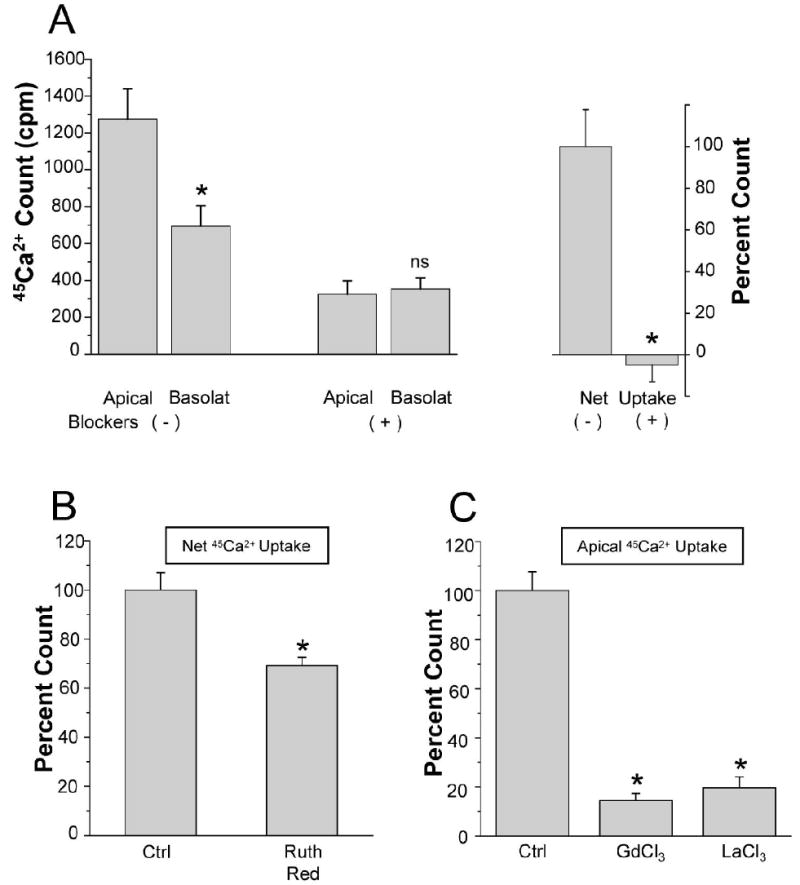
Inhibition of 45Ca2+ uptake into SCCD cells by TRPV5/6 inhibitors SCCD cells were incubated with 1,25(OH)2D3 (100 nM, 24 hr) before the measurement. A: (left) apical-to-cell 45Ca2+ uptake (Apical) and basolateral-to-cell 45Ca2+ uptake (Basolat) of cells with and without the mixture of Ca2+ inhibitors (in μM: 10 ruthenium red, 100 GdCl3, 100 LaCl3). (right) net 45Ca2+ uptake was calculated by subtraction of the mean basolateral-to-cell 45Ca2+ uptake from the mean apical-to-basolateral 45Ca2+ uptake; SEM was pooled. Net 45Ca2+ uptake of cells without the mixture of Ca2+ inhibitors was taken as 100% (n = 6). B: Net 45Ca2+ uptake of control and ruthenium red-treated cells (n = 4). 45Ca2+ uptake of cells without inhibitor was taken as 100%.C: Apical-to-cell 45Ca2+ uptake of control, gadolinium (GdCl3) and lanthanum (LaCl3) treated cells (n = 4). 45Ca2+ uptake of cells without inhibitor was taken as 100%. Concentrations for individual inhibitors are same as described in A. cpm, count per minute; Ctrl, control; Ruth Red, ruthenium red. * P < 0.05 ; ns, not significant.
DISCUSSION
We report for the first time that Slc26a4-/- mice posses a markedly lower pH and higher [Ca2+] in the luminal fluid, endolymph, than in wild-type and heterozygous mice. We also present the first evidence of net Ca2+ absorption by SCCD epithelium in the vestibular system.
SLC26A4 is expressed in several discrete areas in the inner ear, including the apical membrane of vestibular transitional cells of the utricle and ampullae of the semicircular canals (8; 40). These observations, along with the absence of pendrin transcript in the SCCD (Raveendran & Marcus, unpublished observation) suggest that pH homeostatic contributions by the Cl-/ exchanger pendrin occurs mainly outside of the SCCD.
In spite of the physical separation between the sites of secretion and of calcium absorption, it is expected that there is no significant gradient of pH among the utricle, ampullae and canal ducts since the lumen has a relatively large cross-sectional area that favors good diffusion, unlike most smaller tubular epithelial structures such as the nephron. The volume of the utricle is almost as large as the SCCD (32) and convection of endolymph through the SCCD is one of the mechanisms of vestibular perception (14; 39). It is thus reasonable to assume that both the utricle and the SCCD have similar ion concentrations and pH by diffusion and convection. The TRPV5/6 channels in the SCCD would therefore be exposed on their extracellular face to the relatively acidic conditions in the Slc26a4-/- mice.
Regulation of luminal pH by SLC26A4 could conceivably influence transport of a number of ions that are actively moved by vestibular epithelial cells. However, the deletion of SLC26A4 had no effect on luminal (endolymphatic) [K+] in the utricle (28), suggesting that neither the K+ secretory channel in the apical membrane of vestibular dark cells nor K+ exit pathways are strongly sensitive to luminal pH. However, the negative shift of the UP in Slc26a4-/- mice is consistent with an increase in apical membrane conductance of highly polarized epithelial cells, such as the hair cells (17), as opposed to low-voltage K+ -secretory dark cells (41). Increased apical conductance would increase the fractional contribution of the negative intracellular potential to the observed transepithelial potential, the UP (17). A candidate for the apical acid-activated conductance is the ASIC1b channel identified in the apical membrane of vestibular hair cells (35), although this channel in an oocyte expression system was only transiently activated by acid; ASIC1b inactivated during exposure to a step-change in acid with a time constant on the order of 1 sec (1).
Previously, we reported the expression of the epithelial Ca2+ channels TRPV5 and TRPV6 in primary cultures of SCCD epithelial cells (44). Because of the known sensitivity of the TRPV5/6 channels to external pH (46), we hypothesized that low pH of vestibular endolymph due to absence of the Cl-/ exchanger pendrin inhibits TRPV5 and TRPV6, and consequently leads to higher [Ca2+] in endolymph.
The normal [Ca2+] of vestibular endolymph (ca. 250 μM; (42)) is known to be necessary for optimal hair cell function (20) and is likely important in the utricle for proper otoconial formation, which contains precipitated CaCO3. The otoconial crystals comprise the inertial mass that is coupled to the sensory stereocilia of the utricular hair cells and are relatively fine “stones” under normal conditions, but form giant crystals in Slc26a4-/- mice (Fig. 3, (40)). The low [Ca2+] of normal endolymph points to the existence of one or more Ca2+ absorption pathways in the vestibular system. However, in addition to absorption it is known that there are also Ca2+ secretory processes in the inner ear. The plasma membrane Ca2+ ATPase PMCA2 is expressed in the apical membrane (stereocilia) of hair cells and mutation of PMCA2 leads to an absence of otoconia, a lowered [Ca2+] and to vestibular dysfunction (43).
Our results here of elevated [Ca2+] in the endolymph of Slc26a4-/- mice compared to the endolymph of wild-type mice is consistent with inhibition of the Ca2+ absorbing function of the TRPV5/6 channels by acidic pH in those mice. Interestingly, endolymphatic [Ca2+] was also higher than the abluminal fluid, perilymph, in Slc26a4-/- mice. That elevation of endolymphatic [Ca2+] (2.6 mM compared to 1.1 mM in perilymph) is even higher than the equilibrium concentration of 1.5 mM that could be accounted for by passive distribution of Ca2+ at the UP of -4.4 mV in knockout mice. These observations point to continued active secretion of Ca2+ in the vestibular system in the face of inhibited Ca2+ absorption.
TRPV5 is predominantly expressed and controlled by 1,25(OH)2D3 in the kidney, while TRPV6 is predominantly expressed and controlled by 1,25(OH)2D3 in the intestine (9; 36). Although the SCCD epithelium expresses transcripts for both TRPV5 and TRPV6, only TRPV5 is responsive to 1,25(OH)2D3 in SCCD primary cultures (44) and expression of TRPV6 is about 10-fold less in the native epithelium (Yamauchi, Raveendran & Marcus; unpublished observations). Our observation of increased Ca2+ absorption by SCCD is consistent with observations in kidney and with the increased expression of TRPV5 transcript by 1,25(OH)2D3 in SCCD.
Both intra- and extra-cellular acid pH inhibits TRPV5 and they each enhance the other’s effect (45). Binding of extracellular protons to glutamate-522 in the pore region of TRPV5 causes conformational changes and closure of the channel (46). TRPV6 has a histidine at the position equivalent to glutamate-522 in TRPV5, which predicts similar pH sensitivity for both TRPV5 and TRPV6 (46). The very steep dependence of activity of these channels and of SCCD Ca2+ uptake on pH is strong evidence for the functional expression of the TRPV5/6 channels in the apical membrane of SCCD epithelium.
In addition, TRPV5 is known to be inhibited by ruthenium red, gadolinium and lanthanum (12; 38). TRPV6 is also inhibited by ruthenium red but with less potency [IC50 0.12 μM rabbit TRPV5 vs 9 μM mouse TRPV6 (12)]. Each of the multivalent blockers have poor specificity, but the effectiveness of all of these conditions to decrease Ca2+ absorption in SCCD epithelium is strong support for the notion that a major part of the Ca2+ absorption from the semicircular canal is via the TRPV5/6 channels. Ruthenium red is an inhibitor of a wide range of Ca2+ channels, including voltage-gated and epithelial Ca2+ channels (4; 12). The net Ca2+ uptake in the SCCD was inhibited only 31% by 10 μM ruthenium red in spite of the 100x lower IC50 mentioned above for TRPV5 and IC50 for TRPV6 similar to the concentration used. This result suggests either that there is a significant contribution of another Ca2+-permeable channel in this epithelium or, more likely, points to a combination of species and condition differences to the cited studies.
The trivalent cations La3+ and Gd3+ are also effective blockers of TRPV5 and TRPV6 (23; 38), but are also not specific enough to discriminate TRPV5 and TRPV6 from other Ca2+ channels. In our study, a mixture of ruthenium red, La3+ and Gd3+ produced a complete block of net Ca2+ uptake.
Vectorial Ca2+ absorption occurs by well-characterized processes (10). Ca2+ enters the cell from the lumen down an electrochemical gradient through TRPV5/6 in the apical cell membrane. Cytotoxic accumulation of Ca2+ in the cytosol is prevented by immediate binding of Ca2+ to Ca2+-binding proteins, calbindin-9K and/or calbindin-28K, which carry the Ca2+ in a bound state by diffusion to the basolateral cell membrane. Ca2+ is then extruded from the cell by an ATP-dependent Ca2+-ATPase (PMCA) and a Na+/Ca2+ exchanger (NCX). In this way, influx at the apical membrane and apical to basolateral flux are correlated in a 1:1 fashion (24). The radiotracer accumulation in the cells from the basolateral side predominantly indicates the basolateral-to-cell unidirectional cycle of the NCX. As the net flux is reduced, this coupled basolateral uptake will also be reduced, as seen in Figure 6.
In conclusion, we have demonstrated an acid-sensitive vectorial Ca2+ absorption by the SCCD that can account for the pathological endolymph composition observed in mice with the SLC26A4 mutation. These observations increase our understanding of the etiology of Pendred syndrome.
Acknowledgments
We thank Dr. Daisuke Yamauchi and Dr. Tao Wu for technical advice with endolymphatic pH and [Ca2+] measurements. This work was supported by NIH grants R01-DC00212 (DCM), R01-DC01098 (APW) and PO1 DK 061521, project 2 (SMW) and USDA 2003-35206-14157 (BDS).
Reference List
- 1.Bässler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Gründer S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem. 2001;276:33782–33787. doi: 10.1074/jbc.M104030200. [DOI] [PubMed] [Google Scholar]
- 2.Bidart JM, Lacroix L, Evain-Brion D, Caillou B, Lazar V, Frydman R, Bellet D, Filetti S, Schlumberger M. Expression of Na+/I- symporter and pendred syndrome genes in trophoblast cells. J Clin Endocrinol Metab. 2000;85:4367–4372. doi: 10.1210/jcem.85.11.6969. [DOI] [PubMed] [Google Scholar]
- 3.Carlin RW, Sedlacek RL, Quesnell RR, Pierucci-Alves F, Grieger DM, Schultz BD. PVD9902, a porcine vas deferens epithelial cell line that exhibits neurotransmitter-stimulated anion secretion and expresses numerous HCO3- transporters. Am J Physiol Cell Physiol. 2006;290:C1560–C1571. doi: 10.1152/ajpcell.00468.2005. [DOI] [PubMed] [Google Scholar]
- 4.Cibulsky SM, Sather WA. Block by ruthenium red of cloned neuronal voltage-gated calcium channels. J Pharmacol Exp Ther. 1999;289:1447–1453. [PubMed] [Google Scholar]
- 5.Den Dekker E, Schoeber J, Topala CN, Van de Graaf SF, Hoenderop JG, Bindels RJ. Characterization of a Madin-Darby canine kidney cell line stably expressing TRPV5. Pflugers Arch. 2005;450:236–244. doi: 10.1007/s00424-005-1409-3. [DOI] [PubMed] [Google Scholar]
- 6.Everett LA, Belyantseva IA, Noben-Trauth K, Cantos R, Chen A, Thakkar SI, Hoogstraten-Miller SL, Kachar B, Wu DK, Green ED. Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum Mol Genet. 2001;10:153–161. doi: 10.1093/hmg/10.2.153. [DOI] [PubMed] [Google Scholar]
- 7.Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nature Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 8.Everett LA, Morsli H, Wu DK, Green ED. Expression pattern of the mouse ortholog of the Pendred’s syndrome gene (Pds) suggests a key role for pendrin in the inner ear. Proc Natl Acad Sci USA. 1999;96:9727–9732. doi: 10.1073/pnas.96.17.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoenderop JG, Muller D, Van der Kemp AW, Hartog A, Suzuki M, Ishibashi K, Imai M, Sweep F, Willems PH, Van Os CH, Bindels RJ. Calcitriol controls the epithelial calcium channel in kidney. J Am Soc Nephrol. 2001;12:1342–1349. doi: 10.1681/ASN.V1271342. [DOI] [PubMed] [Google Scholar]
- 10.Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- 11.Hoenderop JG, Van der Kemp AW, Hartog A, Van de Graaf SF, Van Os CH, Willems PH, Bindels RJ. Molecular identification of the apical Ca2+ channel in 1, 25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem. 1999;274:8375–8378. doi: 10.1074/jbc.274.13.8375. [DOI] [PubMed] [Google Scholar]
- 12.Hoenderop JG, Vennekens R, Muller D, Prenen J, Droogmans G, Bindels RJ, Nilius B. Function and expression of the epithelial Ca2+ channel family: comparison of mammalian ECaC1 and 2. J Physiol. 2001;537:747–761. doi: 10.1111/j.1469-7793.2001.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu XD, Qian JQ. DDPH inhibited L-type calcium current and sodium current in single ventricular myocyte of guinea pig. Acta Pharmacol Sin. 2001;22:415–419. [PubMed] [Google Scholar]
- 14.Kassemi M, Oas JG, Deserranno D. Fluid-structural dynamics of ground-based and microgravity caloric tests. J Vestib Res. 2005;15:93–107. [PubMed] [Google Scholar]
- 15.Kim YH, Verlander JW, Matthews SW, Kurtz I, Shin W, Weiner ID, Everett LA, Green ED, Nielsen S, Wall SM. Intercalated cell H+/OH- transporter expression is reduced in Slc26a4 null mice. Am J Physiol Renal Physiol. 2005 doi: 10.1152/ajprenal.00206.2005. [DOI] [PubMed] [Google Scholar]
- 16.Lacroix L, Mian C, Caillou B, Talbot M, Filetti S, Schlumberger M, Bidart JM. Na+/I- symporter and Pendred syndrome gene and protein expressions in human extra-thyroidal tissues. Eur J Endocrinol. 2001;144:297–302. doi: 10.1530/eje.0.1440297. [DOI] [PubMed] [Google Scholar]
- 17.Marcus DC, Liu J, Wangemann P. Transepithelial voltage and resistance of vestibular dark cell epithelium from the gerbil ampulla. Hear Res. 1994;73:101–108. doi: 10.1016/0378-5955(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 18.Marcus DC, Wu T, Wangemann P, Kofuji P. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol Cell Physiol. 2002;282:C403–C407. doi: 10.1152/ajpcell.00312.2001. [DOI] [PubMed] [Google Scholar]
- 19.Marcus NY, Marcus DC. Potassium secretion by nonsensory region of gerbil utricle in vitro. Am J Physiol. 1987;253:F613–F621. doi: 10.1152/ajprenal.1987.253.4.F613. [DOI] [PubMed] [Google Scholar]
- 20.Marquis RE, Hudspeth AJ. Effects of extracellular Ca2+ concentration on hair-bundle stiffness and gating-spring integrity in hair cells. Proc Natl Acad Sci U S A. 1997;94:11923–11928. doi: 10.1073/pnas.94.22.11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pendred V. Deaf-mutism and Goitre. Lancet II. 1896:532–535. [Google Scholar]
- 22.Peng JB, Brown EM, Hediger MA. Apical entry channels in calcium-transporting epithelia. News Physiol Sci. 2003;18:158–163. doi: 10.1152/nips.01440.2003. [DOI] [PubMed] [Google Scholar]
- 23.Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem. 1999;274:22739–22746. doi: 10.1074/jbc.274.32.22739. [DOI] [PubMed] [Google Scholar]
- 24.Raber G, Willems PH, Lang F, Nitschke R, Van Os CH, Bindels RJ. Co-ordinated control of apical calcium influx and basolateral calcium efflux in rabbit cortical collecting system. Cell Calcium. 1997;22:157–166. doi: 10.1016/s0143-4160(97)90009-9. [DOI] [PubMed] [Google Scholar]
- 25.Reardon W, Coffey R, Phelps PD, Luxon LM, Stephens D, Kendall-Taylor P, Britton KE, Grossman A, Trembath R. Pendred syndrome--100 years of underascertainment? QJM. 1997;90:443–447. doi: 10.1093/qjmed/90.7.443. [DOI] [PubMed] [Google Scholar]
- 26.Rillema JA, Hill MA. Prolactin regulation of the pendrin-iodide transporter in the mammary gland. Am J Physiol Endocrinol Metab. 2003;284:E25–E28. doi: 10.1152/ajpendo.00383.2002. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal R, Strauss O. Ca2+-channels in the RPE. Adv Exp Med Biol. 2002;514:225–235. [PubMed] [Google Scholar]
- 28.Royaux IE, Belyantseva IA, Wu T, Kachar B, Everett LA, Marcus DC, Green ED. Localization and functional studies of pendrin in the mouse inner ear provide insight about the etiology of deafness in pendred syndrome. J Assoc Res Otolaryngol. 2003;4:394–404. doi: 10.1007/s10162-002-3052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA. 2001;98:4221–4226. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott DA, Karniski LP. Human pendrin expressed in Xenopus laevis oocytes mediates chloride/formate exchange. Am J Physiol Cell Physiol. 2000;278:C207–C211. doi: 10.1152/ajpcell.2000.278.1.C207. [DOI] [PubMed] [Google Scholar]
- 31.Scott DA, Wang R, Kreman TM, Sheffield VC, Karniski LP. The Pendred syndrome gene encodes a chloride-iodide transport protein. Nature Genet. 1999;21:440–443. doi: 10.1038/7783. [DOI] [PubMed] [Google Scholar]
- 32.Shinomori Y, Spack DS, Jones DD, Kimura RS. Volumetric and dimensional analysis of the guinea pig inner ear. Ann Otol Rhinol Laryngol. 2001;110:91–98. doi: 10.1177/000348940111000117. [DOI] [PubMed] [Google Scholar]
- 33.Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE. Pendrin: an apical Cl-/OH-/ HCO3- exchanger in the kidney cortex. Am J Physiol Renal Physiol. 2001;280:F356–F364. doi: 10.1152/ajprenal.2001.280.2.F356. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki K, Royaux IE, Everett LA, Mori-Aoki A, Suzuki S, Nakamura K, Sakai T, Katoh R, Toda S, Green ED, Kohn LD. Expression of PDS/Pds, the Pendred syndrome gene, in endometrium. J Clin Endocrinol Metab. 2002;87:938. doi: 10.1210/jcem.87.2.8390. [DOI] [PubMed] [Google Scholar]
- 35.Ugawa S, Inagaki A, Yamamura H, Ueda T, Ishida Y, Kajita K, Shimizu H, Shimada S. Acid-sensing ion channel-1b in the stereocilia of mammalian cochlear hair cells. Neuroreport. 2006;17:1235–1239. doi: 10.1097/01.wnr.0000233093.67289.66. [DOI] [PubMed] [Google Scholar]
- 36.Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci U S A. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Eerden BC, Hoenderop JG, de Vries TJ, Schoenmaker T, Buurman CJ, Uitterlinden AG, Pols HA, Bindels RJ, van Leeuwen JP. The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc Natl Acad Sci U S A. 2005;102:17507–17512. doi: 10.1073/pnas.0505789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vennekens R, Prenen J, Hoenderop JG, Bindels RJ, Droogmans G, Nilius B. Pore properties and ionic block of the rabbit epithelial calcium channel expressed in HEK 293 cells. J Physiol. 2001;530:183–191. doi: 10.1111/j.1469-7793.2001.0183l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wada Y, Suzuki H, Watanabe S. Changes of ampulla pressure in the semicircular canal of pigeons by caloric stimulation. Acta Astronaut. 1994;33:15–18. doi: 10.1016/0094-5765(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 40.Wangemann P, Itza EM, Albrecht B, Wu T, Jabba SV, Maganti RJ, Lee JH, Everett LA, Wall SM, Royaux IE, Green ED, Marcus DC. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med. 2004;2:30. doi: 10.1186/1741-7015-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wangemann P, Marcus DC. The membrane potential of vestibular dark cells is controlled by a large Cl- conductance. Hear Res. 1992;62:149–156. doi: 10.1016/0378-5955(92)90180-u. [DOI] [PubMed] [Google Scholar]
- 42.Wangemann P, Schacht J. Homeostatic mechanisms in the cochlea. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea. New York: Springer-Verlag; 1996. pp. 130–185. [Google Scholar]
- 43.Wood JD, Muchinsky SJ, Filoteo AG, Penniston JT, Tempel BL. Low endolymph calcium concentrations in deafwaddler2J mice suggest that PMCA2 contributes to endolymph calcium maintenance. J Assoc Res Otolaryngol. 2004;5:99–110. doi: 10.1007/s10162-003-4022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamauchi D, Raveendran NN, Pondugula SR, Kampalli SB, Sanneman JD, Harbidge DG, Marcus DC. Vitamin D upregulates expression of ECaC1 mRNA in semicircular canal. Biochem Biophys Res Commun. 2005;331:1353–1357. doi: 10.1016/j.bbrc.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 45.Yeh BI, Kim YK, Jabbar W, Huang CL. Conformational changes of pore helix coupled to gating of TRPV5 by protons. EMBO J. 2005;24:3224–3234. doi: 10.1038/sj.emboj.7600795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeh BI, Sun TJ, Lee JZ, Chen HH, Huang CL. Mechanism and molecular determinant for regulation of rabbit transient receptor potential type 5 (TRPV5) channel by extracellular pH. J Biol Chem. 2003;278:51044–51052. doi: 10.1074/jbc.M306326200. [DOI] [PubMed] [Google Scholar]


