Abstract
Background
Listeria monocytogenes is a well-characterized food-borne pathogen that infects pregnant women and immunocompromised individuals. Listeriolysin O (LLO) is the major virulence factor of the pathogen and is often used as a diagnostic marker for detection of L. monocytogenes. In addition, LLO represents a potent antigen driving T cell-mediated immunity during infection. In the present work, Lactococcus lactis NZ9000 was used as an expression host to hyper-produce LLO under inducible conditions using the NICE (NIsin Controlled Expression) system. We created a modified pNZ8048 vector encoding a six-His-tagged LLO downstream of the strong inducible PnisA promoter.
Results
The constructed vector (pNZPnisA:CYTO-LLO) was expressed in L. lactis NZ9000 and was best induced at mid-log phase with 0.2% v/v nisin for 4 h statically at 30°C. Purification of the His-tagged LLO was accomplished by Ni-NTA affinity chromatography and functionality was confirmed through haemolytic assays. Total LLO yield (measured as total protein content) was 4.43–5.9 mg per litre culture and the haemolytic activity was still detectable after 8 months of storage at 4°C.
Conclusion
The LLO production method described in this work provides an approach to efficient LLO production in the Gram-positive Lactococcus bacterium to yield a significant source of the protein for research and diagnostic applications. Expression of LLO in L. lactis has a number of benefits over E. coli which may facilitate both in vivo and in vitro applications of this system.
Background
Listeria monocytogenes is the causative agent of listeriosis, a food-borne disease generally associated with the consumption of contaminated ready-to-eat food products. Listeriosis affects mainly immunocompromised individuals and the outcome of infection includes spontaneous miscarriage in pregnant women, and meningitis in the newborn [1]. L. monocytogenes is common in the environment and can be found in the gastrointestinal tract of approximately 5% of healthy persons. Symptomatic infection occurs in individuals with immunosuppression including patients with AIDS and organ transplant recipients [2]. Listeriosis is usually a severe disease with a mean mortality rate in humans of 20 to 30% [3]. Several common-source outbreaks of listeriosis have been linked to consumption of Listeria-contaminated foods (frankfurters, pâté, pasteurised milk and soft-cheese) [2]. Controlled good manufacturing procedures and early detection of Listeria contamination in food are of great importance in preventing such outbreaks.
The major virulence factor of L. monocytogenes is the haemolysin listeriolysin O (LLO). Upon internalisation of the bacterium within the host cell phagolysosome, rapid acidification (~pH 5.5) activates LLO. The protein then interacts with vacuolar membrane cholesterol, oligomerizes and produces pores in the phagolysosomal membrane permitting bacterial escape to the cytoplasm [3]. LLO is therefore critical to L. monocytogenes infection as it facilitates intracellular pathogenesis and subsequent spread of the infection to other tissues.
Enhanced expression systems for in vitro production of LLO have a number of potential applications. Since LLO is associated solely with the pathogenic strain L. monocytogenes, the use of anti-LLO antibodies to identify the pathogen in diagnostic or food samples has demonstrated significant potential [4]. Pure LLO could also find an application in assays to determine prior exposure and immunity to the pathogen in humans or animals [5]. Finally, LLO is recognised as an immunodominant antigen by T cells generated during infection [6]. Modified strains expressing LLO may therefore have applications as vaccines for delivering this Listeria-specific antigen to the host immune system.
We have chosen to utilise Lactococcus lactis as a vector for hyper-expressing LLO. L. lactis is a GRAS (generally regarded as safe) microorganism widely used in the food industry. Recent advances in the molecular characterization of L. lactis and the development of L. lactis-compatible genetic engineering tools have increased the versatility of this organism as a means of protein production [7-9]. Moreover, the use of lactococci has been extended by others as a live antigen and therapeutic drug delivery system [10,11]. Several protein expression systems, both constitutive and inducible, have been developed in lactococci [8]. However, constitutive systems have a potential disadvantage in that the protein may be more liable to lactococcal degradation, or may prove toxic to the cell if produced in high amounts for extended periods.
One of the best characterized inducible expression systems for use in lactococci is the NICE (NIsin Controlled Expression) system [12,13]. In the present work, we describe high level production of the L. monocytogenes LLO protein in Lactococcus lactis NZ9000 using the NICE system. The His-tagged LLO was purified by Ni-NTA affinity chromatography to give a considerable yield and high functional haemolytic activity compared to other published methods [14,15]. The significant yield and purity obtained for LLO using the NICE-expression system in Lactococcus demonstrate the benefits of this approach for the production of LLO for research and diagnostic purposes. Moreover, the constructed LLO-expressing L. lactis strain has the potential to act as a safe live vaccine candidate against listeriosis.
Methods
Culture media, antibiotics and incubation conditions
For L. lactis, GM17 broth (M17 broth (Oxoid) supplemented with 0.5% glucose) was used as a standard culture medium. Luria-Bertani (LB) broth (10 g peptone from casein, 5 g yeast extract and 5 g sodium chloride per litre) was used for Escherichia coli cultures. Solid media were prepared by adding technical agar (Merck) to the corresponding broth with a final concentration of 1.5% w/v. L. lactis cultures were incubated statically at 30°C while E. coli was incubated at 37°C with shaking when applicable. When required, ampicillin (Amp) was used at a concentration of 100 μg/ml for E. coli while chloramphenicol (Cm) was used at 10 μg/ml for both E. coli and L. lactis.
Construction of plasmid vector for expression of LLO in L. lactis NZ9000
Bacterial strains and vectors used in the present study are described in Table 1 while PCR primers are summarized in Table 2. High fidelity KOD hot start DNA polymerase (Novagen) was used in all PCR reactions throughout the whole procedures following manufacturer's instructions. Restriction enzymes and T4 DNA ligase were purchased from Roche Diagnostics (Mannheim, Germany). The gene encoding listeriolysin O, hly, of L. monocytogenes EGD-e was PCR-amplified without the signal peptide coding sequence from the chromosomal DNA [GenBank: AL591824] using primers 1 and 2. The resulting PCR product was sequentially digested by BamHI and PstI respectively. Ligation to a similarly digested pQE30 vector (Qiagen) was successfully performed using T4 DNA ligase. The ligation reaction mixture was transformed into chemically-competent E. coli BL21 (Novagen) by heat shock following manufacturer's instructions and plated onto LB agar containing 100 μg/ml ampicillin. Positive colonies were identified by PCR and plasmid was extracted from BL21 using Qiagen Miniprep Kit (Qiagen). The resulting plasmid (pQE30/hly) had an N-terminus six-histidine tagged hly gene and the correct nucleotide sequence was confirmed by DNA sequencing (Lark Technologies Inc., UK). pQE30/hly was utilised as a template for further cloning steps outlined below.
Table 1.
Bacterial strains and plasmid vectors used in the present study
|
Strain or plasmid name |
Description |
Reference or source |
| E. coli Top10 | Chemically-competent intermediate host, plasmid free | Invitrogen |
| E. coli BL21 | Chemically-competent E. coli, used in this study as an intermediate host for pQE30, plasmid free | Novagen |
| Listeria monocytogenes EGD-e serovar 1/2a | Wild type Listeria monocytogenes | [32] |
| Lactococcus lactis NZ9700 | Nisin producer strain | [13] |
| Lactococcus lactis NZ9000 | L. lactis subsp. Cremoris MG1363 carrying nisRK on the chromosome | [13] |
| L. lactis NZ9000 (pNZPnisA:CYTO-LLO) | Lactococcus lactis NZ9000 harbouring pNZPnisA:CYTO-LLO plasmid and over-expressing LLO upon nisin induction | This study |
| pQE30 | Expression vector using phage T5 promoter and adding an N-terminus six-His tag to the expressed protein, AmpR (ampicillin resistant) | Qiagen |
| pQE30/hly | pQE30 vector with hly gene (without signal sequence) inserted between BamHI and PstI restriction sites. | This study |
| pNZ8048 | E. coli-L. lactis shuttle vector containing PnisA promoter and start codon in NcoI site, CmR (chloramphenicol resistant) | [13] |
| pNZPnisA:CYTO-LLO | Modified pNZ8048 containing PnisA promoter (NcoI site eliminated) with downstream His-tagged hly gene, CmR | This study |
Table 2.
Oligonucleotide primers used in this study.
|
Primer code number |
Primer name |
Primer sequence (5'-3') (restriction enzyme site or overhang)a |
| 1 | pQE30 forward primer |
GAAGGATCCGATGCATCTGCATTCAATAAAG (BamHI site) |
| 2 | pQE30 reverse primer |
ACGCCTGCAGTTCGATTGGATTATCTACTTTATTA (PstI site) |
| 3 | PnisA forward primer |
CCAAGATCTAGTCTTATAACTATACTG (BglII site) |
| 4 | PnisA reverse primer (hly overhang) |
GGTGATGTCCCATTTTGAGTGCCTCCTTATAATTTATTTTG (hly overhang) |
| 5 |
hly forward primer (PnisA overhang) |
AGGCACTCAAAATGGGACATCACCATCACCATCACGGA (PnisA overhang) |
| 6 |
hly reverse primer |
AGTCGGTACCTTATTCGATTGGATTATCTAC (KpnI site) |
(a) Restriction enzyme recognition sites are italicized and underlined while SOE overhangs are underlined.
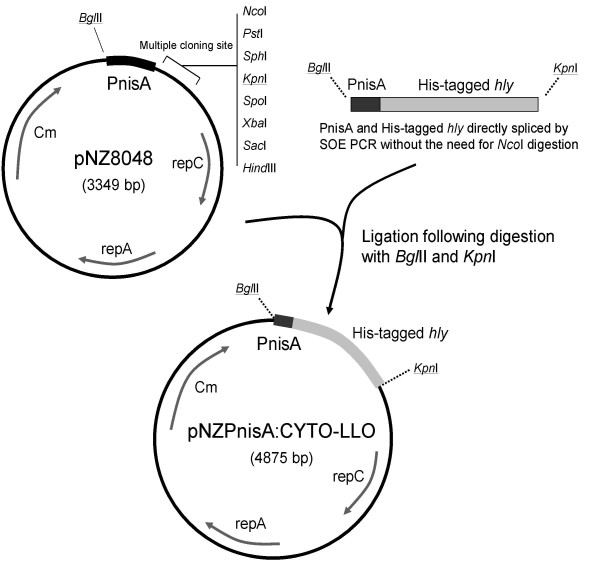
The lactococcal plasmid pNZ8048 [13] was manipulated to allow insertion of the His-tagged hly gene. Plasmid pNZ8048 contains the PnisA promoter (nisin inducible) with a downstream start codon (ATG) inside an NcoI restriction site (CCATGG). However, since the hly gene contains an internal NcoI restriction site, we modified pNZ8048 to eliminate this NcoI site and added a new start codon in frame with hly gene. The Splicing by Overlap Extension (SOE) technique [16] was used to construct an insert containing the His-tagged hly gene spliced directly to PnisA promoter without the need for NcoI digestion. Two PCR reactions were initially performed: primers 3 and 4 were used to amplify the PnisA promoter from pNZ8048 while primers 5 and 6 were used to amplify hly (along with the His-tag) from the constructed pQE30/hly plasmid. The hybrid primers 4 and 5 had overhangs (Table 2) which allow the overlap of the previous two PCR products upon combination for the third splicing PCR reaction. This third PCR reaction was done by combining the initial two PCR products in a molar ratio of 1:1 and again PCR-amplified using primers 3 and 6. The resulting PCR spliced product (PnisA with downstream His-tagged hly) was sequentially digested by BglII and KpnI respectively. pNZ8048 was similarly digested with those two restriction enzymes, to remove the PnisA promoter along with the NcoI site, and the digested plasmid was agarose gel purified using the Qiagen gel extraction kit (Qiagen). The spliced PCR product was ligated into the digested pNZ8048 using T4 DNA ligase. The ligation reaction was transformed into chemically-competent E. coli TOP10 (Invitrogen) following the manufacturer's instructions and plated onto LB agar containing 10 μg/ml chloramphenicol. After incubation at 37°C for 24–48 h, positive colonies were detected by colony PCR and plasmid (designated pNZPnisA:CYTO-LLO) was extracted and correct DNA sequence was confirmed (Lark Technologies Inc., UK). Plasmid pNZPnisA:CYTO-LLO was transformed to electrocompetent L. lactis NZ9000, prepared as previously described [17], using Gene Pulser (Biorad) and plated onto GM17 agar containing 10 μg/ml chloramphenicol. After 24 h incubation at 30°C, colonies were checked by colony PCR and one positive colony was stocked for protein induction and expression. Figures 1 and 2 show an overview of the plasmid vector construction and the sequence of DNA and amino acids of the final His-tagged LLO respectively.
Figure 1.
Schematic representation of the construction of the pNZPnisA:CYTO-LLO vector.
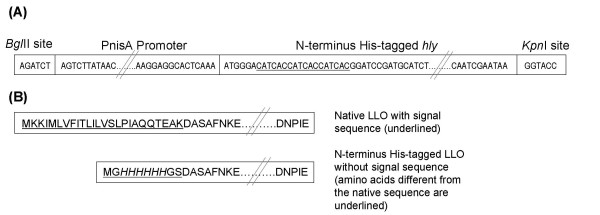
Figure 2.
Illustrative partial DNA and amino acid sequence of the constructed vector and LLO respectively. (A) Illustrative DNA sequence of the SOE (splicing by overlap extension) PCR product consisting of the PnisA promoter spliced to downstream six-His-tagged hly gene of L. monocytogenes EGD-e. Flanking restriction sites of BglII and KpnI exist on the PnisA and hly sides respectively. The six-His-tag codons are underlined. (B) Comparison between the amino acid sequence of native LLO (L. monocytogenes EGD-e) with the signal sequence and the constructed six-His-tagged LLO produced in this study.
Protein induction
Filter-sterilized culture supernatant of the nisin-secreting strain L. lactis NZ9700 was used as a source of nisin [18]. Sterile culture supernatant of L. lactis NZ9700 was stored in small aliquots at -20°C and one aliquot was thawed and used as required. The use of the same filter-sterilized batch of supernatant throughout the study ensured consistency of the nisin content between induction experiments. Overnight culture of L. lactis NZ9000 (pNZPnisA:CYTO-LLO) was subcultured into fresh GM17 broth (Cm 10 μg/ml) and incubated statically at 30°C. Nisin was added when the optical density at 600 nm (OD600) reached 0.5. Induction was conducted statically at 30°C after which cells were pelleted (3200 × g for 10 min) and washed once with buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8). Pellets were frozen at -80°C prior to sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE) or protein purification.
SDS-PAGE and Western blot
Frozen induced pellets from 500 ml cultures were thawed on ice and resuspended in 10–15 ml ice-cold lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8) containing 30 mg/ml lysozyme (Sigma) and kept for 30 min on ice. This was followed by sonication at maximum amplitude (Soniprep 150, MSE UK Ltd.) using eight ten-second pulses with intervening ten-second pauses on ice. For small volume cultures (10–50 ml), pellets were resuspended in 500 μl lysis buffer in eppendorf tubes and acid-washed glass beads (Sigma) were added followed by beating the tubes in a bead beater (Mini beadbeater-8, Biospec products) for three one-minute beats with one-minute pauses in between on ice. Clear supernatant was obtained after centrifugation at 10000 × g for 30 min at 4°C.
For SDS-PAGE, 12% SDS-polyacrylamide separating gel and 4% stacking gel were used and gels were stained with coomassie blue stain followed by destaining and gel imaging. For Western blot, pre-stained protein marker was used (Amersham Biosciences UK, Ltd) and gels were blotted against a nitrocellulose membrane (Hybond-ECL™, Amersham Biosciences UK, Ltd.) using a semi-dry Western transfer apparatus. Membranes were blocked overnight at 4°C in 5% skimmed milk in TBS buffer (0.8% NaCl, 20 mM Tris-HCl, pH 7.6). Primary rabbit anti-LLO antibody (Diatheva, Italy) and secondary anti-rabbit antibody (Amersham ECL Western Blotting System) were used at 1/1000 and 1/1500 dilutions in 5% and 10% skimmed milk in TBS buffer respectively. Western blot detection was done using Amersham ECL Western Blotting System (Amersham Biosciences UK, Ltd.) using the protocol recommended by the manufacturer.
Protein purification and quantitation
Clear supernatant obtained after cell lysis (performed as described in the previous section) was passed through a disposable chromatography column (Biorad) containing 2 ml Ni-NTA affinity gel (Qiagen). Column was washed twice with wash buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, pH 8) then eluted in fractions of 0.5 ml with elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, pH 8). Elutions were checked by SDS-PAGE and combined together and dialysed overnight at 4°C against 2 l of dialysis buffer (500 mM NaCl, 10 mM Na2HPO4, 0.5 mM EDTA, 0.02% NaN3, pH 7.0). Listeriolysin O concentration was measured as total protein concentration by Micro Lowry total protein kit (Sigma) and also by absorbance at 280 nm using the molar extinction coefficient for His-tagged LLO as 71,950 cm-1 (calculated based on the actual sequence of the His-tagged LLO) [19]. The purified protein was stored at 4°C.
Measurement of haemolytic activity of purified LLO
Haemolytic activity of LLO was measured according to the method described by Khoda et al [20] with slight modification. Briefly, defibrinated sheep blood was centrifuged at 800 × g for 10 min at 4°C, then washed twice with phosphate buffered saline (PBS) (Gibco) pH 5.5. The pelleted red blood cells (RBCs) were diluted with PBS (pH 5.5) to obtain 0.5% RBCs volume/volume (v/v). Aliquots of 100 μl of the RBCs suspension were distributed in 1.5 ml tubes and twofold serial dilutions of LLO (in PBS pH 5.5) was added to each tube to a final volume of 1 ml. LLO final concentrations covered the range from 8 μg ml-1 to 29 pg ml-1. A positive control (distilled water, 100% haemolysis) and a negative control (PBS pH 5.5) were also included. Tubes were incubated statically at 37°C for 45 min after which they were centrifuged at 1700 × g for 5 min and supernatants were collected. Absorbance was measured colorimetrically at 415 nm and haemolytic units were calculated. One haemolytic unit (HU) was defined as the amount of protein required to cause 50% haemoglobin release from sheep RBCs as compared to the 100% haemoglobin release of the positive control (distilled water) [20].
Results
DNA sequence of the six His-tagged hly gene
Native LLO secreted by Listeria monocytogenes EGD-e is composed of a secretory signal peptide (25 amino acids) and the actual active portion of LLO (504 amino acids). In the cloning described in this paper we omitted the native secretory signal sequence (coding for the first 25 amino acids) of LLO and added six histidine amino acids (a His-tag) at the N-terminus (Figure 2). DNA sequencing of the constructed pNZPnisA:CYTO-LLO confirmed the addition of the six-His-tag upstream of the hly gene and the integrity of the remainder of the hly gene sequence. Overall we added a total of 10 amino acids (including the 6 histidine residues) at the N-terminus of LLO, in place of the signal sequence, to facilitate tagging and purification of the protein (Figure 2). These few amino acids did not affect protein activity as shown below.
Nisin-induced LLO production in L. lactis NZ9000 (pNZPnisA:CYTO-LLO)
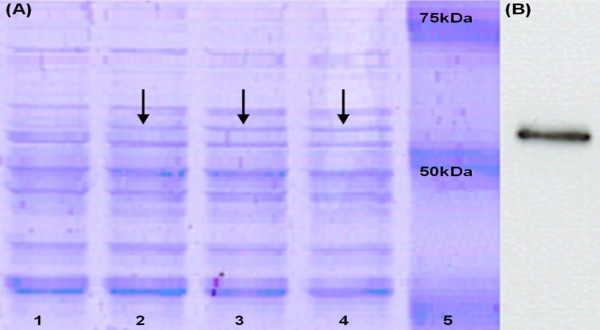
We performed a series of induction experiments on L. lactis NZ9000 (pNZPnisA:CYTO-LLO) to determine the optimum conditions for protein expression. Preliminary induction time-course experiments showed the optimum duration for nisin-induction to be 3–4 h (data not shown). Consequently, induction was performed at mid-log phase (OD600 = 0.5) for three hours. Initially, different nisin concentrations were examined: 0.02%, 0.1%, and 0.2% (v/v), to determine the optimum concentration for induction and SDS-PAGE was performed to assess the amount of LLO produced (Figure 3A). Subsequently, for protein over-production experiments, 0.2% v/v nisin concentration was chosen for induction to maximize the amount of LLO produced. Western blot was also performed and confirmed the production of pure non-degraded LLO (Figure 3B). It is noteworthy that we detected minimal basal expression of LLO in the uninduced L. lactis NZ9000 (pNZPnisA:CYTO-LLO) with no apparent toxicity on bacterial growth (data not shown). The inducer of basal PnisA activity in our experiments in the absence of nisin is not clear although other inducers such as lactose and galactose have been previously reported to induce this promoter at low levels [21]. In our experiments the basal LLO expression was negligible compared to the amount of LLO produced upon nisin induction.
Figure 3.
Optimization of nisin concentration for induction experiments. (A) SDS-PAGE of the induction experiment for 3 h statically at 30°C without nisin addition (lane 1), and using nisin at concentrations of 0.02% (lane 2), 0.1% (lane 3) and 0.2% (lane 4) (v/v). Lane 5 represents the protein marker. (B) Western blot showing LLO at the expected size of about 57 kDa using rabbit anti-LLO as primary antibody.
Purification of LLO and total protein quantitation
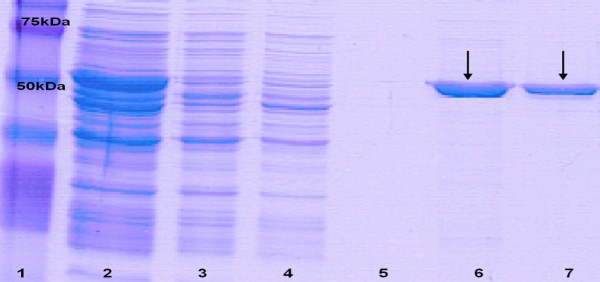
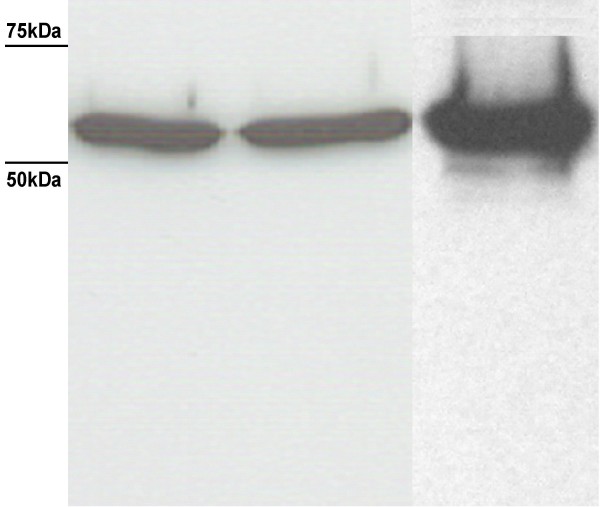
To obtain rapid and reproducible purification of LLO, we used the Ni-NTA technology to capture and purify the six-His-tagged protein by affinity chromatography under native purification conditions. Figure 4 shows a representative purification procedure of the His-tagged LLO (about 57 kDa) checked by SDS-PAGE. The average yield of pure LLO (measured as total protein) after 3 h-induction at 30°C using a concentration of 0.2% v/v of nisin was 3 mg per litre culture as measured by both Lowry and A280 absorbance methods. When the induction time was increased to 4 h at 30°C, the yield ranged from 4.43–5.9 mg per litre culture. However, to increase the total yield of LLO, we modified the LLO extraction method by applying double sonication to the 4 h-induced pellets of L. lactis NZ9000 (pNZPnisA:CYTO-LLO). In brief, after the first sonication was completed (as described in the Methods section), the supernatant was collected for Ni-NTA purification and the remaining pellets were frozen again at -20°C for 24 hours then thawed, resuspended in lysis buffer and sonicated again. Supernatant was collected and then Ni-NTA-gel-purified. This second sonication of the same pellets simply extracted LLO which had not been released from Lactococcus cells upon the first treatment. Eluted proteins from the two sonication treatments were tested by SDS-PAGE for purity (Figure 5) then combined and dialysed as mentioned earlier. This double sonication approach increased the total combined yield of LLO to the range of 9.3–12.9 mg per litre culture (average 11.6 ± 1.56 mg per litre culture). However, when the resulting LLO was examined by Western blot, minor lower molecular weight bands appeared below the major LLO band. This indicated slight degradation of LLO upon the double sonication procedure (Figure 6).
Figure 4.
SDS-PAGE of purified LLO using Ni-NTA affinity chromatography. Lane 1 is the protein marker, lane 2 is culture lysate of L. lactis NZ9000 (pNZPnisA:CYTO-LLO) before passing through the column, lane 3 is the flow-through of the column, lanes 4 and 5 are two successive washes of the columns while lanes 6 and 7 are two successive elutions showing the purified His-tagged LLO.
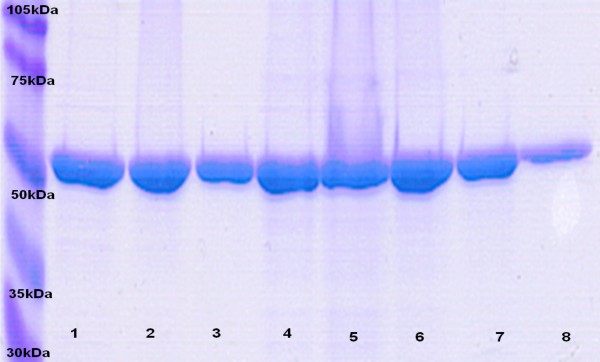
Figure 5.
SDS-PAGE showing pure LLO elutions (500 ml bacterial culture) using the double sonication approach. Pure LLO was eluted from the Ni-NTA affinity gel after 4 h-0.2% nisin induction at 30°C and double sonication treatment of the induced cells as described in the Methods section. Lanes 1–3: elutions after first sonication treatment. Lanes 4–8: elutions after second sonication treatment.
Figure 6.
Western blot showing LLO level and stability after 0.2% nisin induction. Left lane, LLO after 3 h induction (single sonication); Middle lane, LLO after 4 h induction (single sonication); Right lane, LLO after 4 h induction followed by the double sonication treatment (few degradation bands are observed at slightly lower molecular weights).
To check the functionality of LLO, the haemolytic titre test was carried out and specific LLO haemolytic activity ranged from 5 × 105 to 5 × 107 HU per mg total protein. This haemolytic activity was still detectable after at least 8 months of storage at 4°C.
Discussion
In the present study, we describe the over-expression of the haemolysin listeriolysin O (LLO) of L. monocytogenes by the GRAS microorganism L. lactis NZ9000. We used a well-defined strong promoter system (the NICE system) in which addition of subinhibitory nisin induces the PnisA promoter for over-expression of downstream genes [12,13]. In the present work, we utilised a nisin concentration of 0.2% v/v for induction which maximized LLO production and was far below the minimum inhibitory concentration (MIC) of nisin against the producing strain (MIC was found to be 3.1% v/v).
The NICE system has been used extensively in the literature to over-produce proteins in lactococci. For instance, Brucella abortus GroEL protein was produced in L. lactis NZ9000 although it was found that only the secreted rather than the intracellular form was stably produced suggesting a detrimental effect of GroEL protein on Lactococcus [22]. In contrast, Giardia lamblia cyst wall protein 2 (CWP2) was successfully produced in different cell compartments (intracellular, secreted, and cell-surface anchored) by use of appropriately designed vectors without any detrimental effects on the Lactococcus producer. Lactococci expressing CWP2 on their surface could elicit CWP2-specific IgA antibodies and reduced cyst shedding in a murine Giardia challenge model [23].
Here, we demonstrate the use of the NICE system to successfully over-express LLO of L. monocytogenes in the intracellular compartment of L. lactis NZ9000. Several previous studies examined the over-production of LLO in different host cells and expression systems (summarized in Table 3). Earlier studies that purified native LLO directly from L. monocytogenes culture supernatants had poor LLO yields (0.022–0.25 mg per litre culture) and utilised laborious multi-step procedures requiring large culture volumes [24,25]. Dealing with such large culture volumes of pathogenic bacteria is potentially hazardous and necessitates special precautions if scaling-up is required. When LLO was constitutively over-expressed and secreted by the non-pathogenic Listeria innocua, an improved yield was obtained (1.6 mg LLO per litre culture) [26]. However, purification of LLO from L. innocua involved multiple steps involving preliminary concentration by filtration, followed by two sequential chromatography purification steps [26].
Table 3.
Comparative results of LLO production and purification by different investigators.
|
Source of LLO |
Starter Culture volume (litre) |
LLO yield (mg l-1) {Total yield (mg)} |
Specific LLO haemolytic activity (HU per mg protein) pH 5.5 |
Expression host |
Plasmid expression system/inducer (If applicable) |
Reference |
| Culture supernatant | 27 | 0.022 {0.6} | 106 (pH 6) | Listeria monocytogenesa | N/A | [24] |
| Culture supernatant | 3 | 1.6 {4.8} | 1.02 × 106 (pH 5.7) | Listeria innocuab | pERL3-503 (constitutive) |
[26] |
| Culture supernatant | 6 | 0.25 {1.5} | 2.6 × 105 | LLO-hypersecretor Listeria monocytogenesa | N/A | [25] |
| Cell lysate | 1 | 4.5 {4.5} | 1.25 × 106 | Escherichia colic | pET system/IPTG | [15] |
| Cell lysate | 0.6 | 3.5 – 8 {2.1 – 4.8} | 1.8 × 106 | Escherichia colid | pQE31 system/IPTG | [14] |
| Cell lysate | 0.6 | 2.5 {1.5} | 2.16 × 106 | Escherichia colie | pQE70 system/IPTG | [14] |
| Cell lysate | 0.5 | -Single sonication treatment: 4.43 – 5.9 {2.215 – 2.95} -Double sonication approach: 9.3 – 12.9 {4.65 – 6.45} | 5 × 105 – 5 × 107 | Lactococcus lactis NZ9000d | NICE system (pNZPnisA:CYTO-LLO)/nisin | This study |
(N/A) not applicable; IPTG: Isopropyl β-D-1-thiogalactopyranoside; a untagged native full-length LLO; b untagged full-length LLO; c untagged LLO without signal sequence; d N-terminus His-tagged LLO without signal sequence; e C-terminus His-tagged LLO without signal sequence
Giammarini and coworkers could inducibly over-express LLO in E. coli obtaining a relatively good yield (4.5 mg per litre culture) [15]. However, two sequential purification steps were applied with haemolytic activity assessment on blood agar and SDS-PAGE performed between the first hydroxyapatite purification step and the second ammonium sulphate precipitation step. Further concentration steps were applied before the effluent fractions containing LLO were concentrated by ultrafiltration [15]. Although the LLO yield was good, those multi-step procedures are relatively time-consuming. To the best of our knowledge, Churchill et al were able to obtain the highest yield of haemolytically active LLO [14]. In their study, several expression systems have been attempted in E. coli among which the N-terminus His-tagged LLO was the most successful using the pQE31/IPTG (Isopropyl β-D-1-thiogalactopyranoside)-inducible system (Qiagen). This system makes use of the well-characterized strong T5 promoter/lac operator transcription-translation system to control LLO expression in E. coli. A significant LLO yield (3.5–8 mg per litre culture) was obtained with proven haemolytic activity (1.8 × 106 HU per mg protein) and stability up to one year upon storage at 4°C [14].
The current L. lactis-based LLO production method resembles to some extent the N-terminus His-tagged LLO prepared by Churchill et al [14]. As regards LLO yield, the single sonication treatment gave an appreciably good pure yield (4.43–5.9 mg per litre culture) though the highest value (i.e. 5.9 mg per litre culture) is less than the highest yield obtained by Churchill and coworkers (8 mg per litre culture) [14]. Although the double sonication treatment of the same culture resulted in some minor degradation of LLO (Figure 6), the total yield almost doubled (up to 12.9 mg per litre culture) without using any additional culture. This minor degradation may be tolerable for experimental scale production (but not for large commercial scale production). We also assessed the haemolytic activity of the purified LLO at intervals up to 8 months and found it to be within the same range (5 × 105 to 5 × 107 HU per mg total protein).
We consider the most important innovation in the current work is the use of L. lactis as an expression host. L. lactis is a GRAS, Gram-positive lactic acid bacterium widely used in the food industry. More recently L. lactis has demonstrated significant promise as a means of producing heterologous proteins for experimental or commercial applications [8]. Lactic acid bacteria expressing heterologous proteins have been used in the food industry [27], in experimental vaccine antigen delivery [11,28], and for the targeted delivery of therapeutic bioactive proteins (cytokines and trefoil factors) [10,29]. In producing bioactive proteins for human or animal use, lactococci have a significant advantage over E. coli in that they do not produce endotoxins. Moreover, L. lactis is known to produce very low amounts of native exoproteins and the lactococcal genome is generally about half the size of the E. coli genome [9,18,30]. This is an advantage both for in vitro heterologous protein production and for the use of L. lactis as a vaccine delivery vehicle as there are fewer contaminating proteins when L. lactis is used as a heterologous host [18,31].
Conclusion
The present study offers a convenient method for LLO production in an advantageous host providing a good protein yield, high haemolytic activity and significant stability that compares well with previously published studies. Purified LLO from L. lactis may find significant applications in basic research into listerial pathogenesis including the stimulation and development of improved monoclonal antibodies against LLO. This L. monocytogenes-specific protein may also have applications in the development of improved food testing protocols. Finally, L. lactis production of LLO may have applications in vaccine development.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MB carried out the molecular genetic and protein studies and drafted the manuscript. BTG participated in the design and coordination of the study. CGMG conceived of the study, and participated in its design and follow-up and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
Mohammed Bahey-El-Din is a recipient of a postgraduate scholarship from the Egyptian government administered through the Education and Culture Bureau of the Egyptian Embassy, London. The authors would like to acknowledge the funding received from the Irish Government under the National Development Plan 2000–2006 and the funding of the Alimentary Pharmabiotic Centre by the Science Foundation of Ireland Centres for Science Engineering and Technology (CSET) programme.
We thank Abdelhamid Abbas for helpful advice and information.
Contributor Information
Mohammed Bahey-El-Din, Email: mohammedbahey@student.ucc.ie.
Brendan T Griffin, Email: brendan.griffin@ucc.ie.
Cormac GM Gahan, Email: c.gahan@ucc.ie.
References
- Meng J, Doyle MP. Emerging issues in microbiological food safety. Annu Rev Nutr. 1997;17:255–275. doi: 10.1146/annurev.nutr.17.1.255. [DOI] [PubMed] [Google Scholar]
- Ramaswamy V, Cresence VM, Rejitha JS, Lekshmi MU, Dharsana KS, Prasad SP, Vijila HM. Listeria--review of epidemiology and pathogenesis. J Microbiol Immunol Infect. 2007;40:4–13. [PubMed] [Google Scholar]
- Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill RL, Lee H, Hall JC. Detection of Listeria monocytogenes and the toxin listeriolysin O in food. J Microbiol Methods. 2006;64:141–170. doi: 10.1016/j.mimet.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kalorey DR, Kurkure NV, Warke SR, Barbuddhe SB. Evaluation of indirect and avidin-biotin enzyme linked immunosorbent assays for detection of anti-listeriolysin O antibodies in bovine milk samples. Zoonoses Public Health. 2007;54:301–306. doi: 10.1111/j.1863-2378.2007.01066.x. [DOI] [PubMed] [Google Scholar]
- Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- Mills S, McAuliffe OE, Coffey A, Fitzgerald GF, Ross RP. Plasmids of lactococci - genetic accessories or genetic necessities? FEMS Microbiol Rev. 2006;30:243–273. doi: 10.1111/j.1574-6976.2005.00011.x. [DOI] [PubMed] [Google Scholar]
- Nouaille S, Ribeiro LA, Miyoshi A, Pontes D, Le Loir Y, Oliveira SC, Langella P, Azevedo V. Heterologous protein production and delivery systems for Lactococcus lactis. Genet Mol Res. 2003;2:102–111. [PubMed] [Google Scholar]
- Wegmann U, O'Connell-Motherway M, Zomer A, Buist G, Shearman C, Canchaya C, Ventura M, Goesmann A, Gasson MJ, Kuipers OP, van Sinderen D, Kok J. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol. 2007;189:3256–3270. doi: 10.1128/JB.01768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, van Deventer SJ, Neirynck S, Peppelenbosch MP, Steidler L. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:754–759. doi: 10.1016/j.cgh.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Hanniffy SB, Carter AT, Hitchin E, Wells JM. Mucosal delivery of a pneumococcal vaccine using Lactococcus lactis affords protection against respiratory infection. J Infect Dis. 2007;195:185–193. doi: 10.1086/509807. [DOI] [PubMed] [Google Scholar]
- Kuipers OP, Beerthuyzen MM, de Ruyter PG, Luesink EJ, de Vos WM. Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J Biol Chem. 1995;270:27299–27304. doi: 10.1074/jbc.270.45.27299. [DOI] [PubMed] [Google Scholar]
- Kuipers OP, de Ruyter P, Kleerebezem M, de Vos WM. Quorum sensing-controlled gene expression in lactic acid bacteria. Journal of Biotechnology. 1998;64:15–21. doi: 10.1016/S0168-1656(98)00100-X. [DOI] [Google Scholar]
- Churchill RL, Lee H, Hall JC. Rapid purification of recombinant listeriolysin O (LLO) from Escherichia coli. J Ind Microbiol Biotechnol. 2005;32:355–363. doi: 10.1007/s10295-005-0002-2. [DOI] [PubMed] [Google Scholar]
- Giammarini C, Andreoni F, Amagliani G, Casiere A, Barocci S, Magnani M. High-level expression of the Listeria monocytogenes listeriolysin O in Escherichia coli and preliminary characterization of the purified protein. Protein Expr Purif. 2003;28:78–85. doi: 10.1016/S1046-5928(02)00682-4. [DOI] [PubMed] [Google Scholar]
- Horton RM, Cai ZL, Ho SN, Pease LR. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990;8:528–535. [PubMed] [Google Scholar]
- Holo H, Nes IF. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunji ER, Slotboom DJ, Poolman B. Lactococcus lactis as host for overproduction of functional membrane proteins. Biochim Biophys Acta. 2003;1610:97–108. doi: 10.1016/S0005-2736(02)00712-5. [DOI] [PubMed] [Google Scholar]
- Chazan A. Peptide property calculator, center for biotechnology, Northwestern University http://www.basic.northwestern.edu/biotools/proteincalc.html
- Kohda C, Kawamura I, Baba H, Nomura T, Ito Y, Kimoto T, Watanabe I, Mitsuyama M. Dissociated linkage of cytokine-inducing activity and cytotoxicity to different domains of listeriolysin O from Listeria monocytogenes. Infect Immun. 2002;70:1334–1341. doi: 10.1128/IAI.70.3.1334-1341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrapati S, O'Sullivan DJ. Nisin independent induction of the nisA promoter in Lactococcus lactis during growth in lactose or galactose. FEMS Microbiol Lett. 1999;170:191–198. doi: 10.1111/j.1574-6968.1999.tb13374.x. [DOI] [PubMed] [Google Scholar]
- Miyoshi A, Bermudez-Humaran LG, Ribeiro LA, Le Loir Y, Oliveira SC, Langella P, Azevedo V. Heterologous expression of Brucella abortus GroEL heat-shock protein in Lactococcus lactis. Microb Cell Fact. 2006;5:14. doi: 10.1186/1475-2859-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Faubert GM. Expression of the Giardia lamblia cyst wall protein 2 in Lactococcus lactis. Microbiology. 2006;152:1981–1990. doi: 10.1099/mic.0.28877-0. [DOI] [PubMed] [Google Scholar]
- Geoffroy C, Gaillard JL, Alouf JE, Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987;55:1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton CM, Wu CH, Wu GY. A method for purification of listeriolysin O from a hypersecretor strain of Listeria monocytogenes. Protein Expr Purif. 1999;15:243–245. doi: 10.1006/prep.1998.1022. [DOI] [PubMed] [Google Scholar]
- Darji A, Chakraborty T, Niebuhr K, Tsonis N, Wehland J, Weiss S. Hyperexpression of listeriolysin in the nonpathogenic species Listeria innocua and high yield purification. J Biotechnol. 1995;43:205–212. doi: 10.1016/0168-1656(95)00138-7. [DOI] [PubMed] [Google Scholar]
- Noonpakdee W, Sitthimonchai S, Panyim S, Lertsiri S. Expression of the catalase gene katA in starter culture Lactobacillus plantarum TISTR850 tolerates oxidative stress and reduces lipid oxidation in fermented meat product. Int J Food Microbiol. 2004;95:127–135. doi: 10.1016/j.ijfoodmicro.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Jun do Y, Yang CH, Kim YH. Expression of Helicobacter pylori cag12 gene in Lactococcus lactis MG1363 and its oral administration to induce systemic anti-Cag12 immune response in mice. Appl Microbiol Biotechnol. 2006;72:462–470. doi: 10.1007/s00253-005-0285-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke K, Hans W, Van Huysse J, Neirynck S, Demetter P, Remaut E, Rottiers P, Steidler L. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology. 2004;127:502–513. doi: 10.1053/j.gastro.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Blattner FR, Plunkett G, 3rd, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- Bermudez-Humaran LG, Langella P, Miyoshi A, Gruss A, Guerra RT, Montes de Oca-Luna R, Le Loir Y. Production of human papillomavirus type 16 E7 protein in Lactococcus lactis. Appl Environ Microbiol. 2002;68:917–922. doi: 10.1128/AEM.68.2.917-922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, Baquero F, Berche P, Bloecker H, Brandt P, Chakraborty T, Charbit A, Chetouani F, Couve E, de Daruvar A, Dehoux P, Domann E, Dominguez-Bernal G, Duchaud E, Durant L, Dussurget O, Entian KD, Fsihi H, Garcia-del Portillo F, Garrido P, Gautier L, Goebel W, Gomez-Lopez N, Hain T, Hauf J, Jackson D, Jones LM, Kaerst U, Kreft J, Kuhn M, Kunst F, Kurapkat G, Madueno E, Maitournam A, Vicente JM, Ng E, Nedjari H, Nordsiek G, Novella S, de Pablos B, Perez-Diaz JC, Purcell R, Remmel B, Rose M, Schlueter T, Simoes N, Tierrez A, Vazquez-Boland JA, Voss H, Wehland J, Cossart P. Comparative genomics of Listeria species. Science. 2001;294:849–852. doi: 10.1126/science.1063447. [DOI] [PubMed] [Google Scholar]